Lipid Oxidation in Emulsions Fortified with Iron-Loaded Alginate Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alginate Beads
2.3. Encapsulation Efficiency (EE) of Iron-Loaded Alginate Beads
2.4. Morphological Characterization of Alginate Beads
2.5. Preparation and Physical Characterization of Emulsions
2.6. Measurement of Lipid Oxidation and Hydrolysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Alginate Beads
3.2. Physical Properties and Free Fatty Acid Formation in Incubated Emulsions
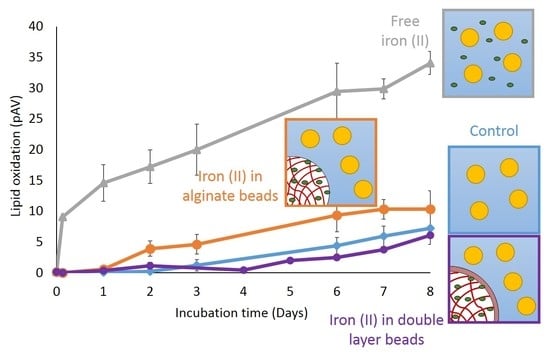
3.3. Lipid Oxidation in the Incubated Emulsions
3.4. Estimation of the Reactive Iron Fraction in Iron-Loaded Beads
3.5. Effect of a Second Alginate Layer on the Pro-Oxidant Activity of Iron
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hurrell, R.F. Preventing Iron Deficiency Through Food Fortification. Nutr. Rev. 2009, 55, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Alemán, M.; Bou, R.; Tres, A.; Polo, J.; Codony, R.; Guardiola, F. Oxidative stability of a heme iron-fortified bakery product: Effectiveness of ascorbyl palmitate and co-spray-drying of heme iron with calcium caseinate. Food Chem. 2016, 196, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, A.; Kahyaoglu, T.; Schröen, K.; Berton-Carabin, C. Oxidative stability of emulsions fortified with iron: The role of liposomal phospholipids. J. Sci. Food Agric. 2019, 99, 2957–2965. [Google Scholar] [CrossRef] [PubMed]
- Gul, O.; Dervisoglu, M. Application of multicriteria decision technique to determine optimum sodium alginate concentration for microencapsulation of Lactobacillus casei Shirota by extrusion and emulsification. J. Food Process Eng. 2017, 40, e12481. [Google Scholar] [CrossRef]
- Corstens, M.N.; Berton-Carabin, C.C.; Elichiry-Ortiz, P.T.; Hol, K.; Troost, F.J.; Masclee, A.A.M.; Schroën, K. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: Towards appetite control. J. Funct. Foods 2017, 34, 319–328. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Wang, Y.; Lin, S.C.Y.; Bostrom, T.; Bhandari, B.; Coombes, A.G.A. Novel alginate gel microspheres produced by impinging aerosols for oral delivery of proteins. J. Microencapsul. 2012, 29, 250–261. [Google Scholar] [CrossRef]
- Belšćak-Cvitanović, A.; Stojanović, R.; Manojlović, V.; Komes, D.; Cindrić, I.J.; Nedović, V.; Bugarski, B. Encapsulation of polyphenolic antioxidants from medicinal plant extracts in alginate-chitosan system enhanced with ascorbic acid by electrostatic extrusion. Food Res. Int. 2011, 44, 1094–1101. [Google Scholar] [CrossRef]
- Draget, K.I. Alginates. In Hanbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 379–395. ISBN 1-85573-501-6. [Google Scholar]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particle-A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Hassan, R.M.; Summan, A.M.; Hassan, M.K.; El-Shatoury, S.A. Kinetics and mechanism of sol-gel transformation on polyelectrolytes of some transition metal ions, especially cobalt alginate ionotropic membranes. Eur. Polym. J. 1989, 25, 1209–1212. [Google Scholar] [CrossRef]
- Gupta, C.; Chawla, P.; Arora, S. Development and evaluation of iron microencapsules for milk fortification. CYTA-J. Food 2015, 13, 116–123. [Google Scholar] [CrossRef]
- Jayalalitha, V.; Balasandaram, B. Palanidoral Naresh Kumar, C. Fortification of Encapsulated Iron in Probiotic Yoghurt. Int. J. Agric. Res. Rev. 2012, 2, 80–84. [Google Scholar]
- Mukhija, K.; Singhal, K.; Angmo, S.; Yadav, K.; Yadav, H.; Sandhir, R.; Singhal, N.K. Potential of Alginate Encapsulated Ferric Saccharate Microemulsions to Ameliorate Iron Deficiency in Mice. Biol. Trace Elem. Res. 2016, 172, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.; Hernández, V.; Morales, M.S.; Neira-Carrillo, A.; Pizarro, F. Preparation and characterization of heme iron-alginate beads. LWT-Food Sci. Technol. 2014, 59, 1283–1289. [Google Scholar] [CrossRef]
- Churio, O.; Pizarro, F.; Valenzuela, C. Preparation and characterization of iron-alginate beads with some types of iron used in supplementation and fortification strategies. Food Hydrocoll. 2018, 74, 1–10. [Google Scholar] [CrossRef]
- Perez-Moral, N.; Gonzalez, M.C.; Parker, R. Preparation of iron-loaded alginate gel beads and their release characteristics under simulated gastrointestinal conditions. Food Hydrocoll. 2013, 31, 114–120. [Google Scholar] [CrossRef]
- Khosroyar, S.; Akbarzade, A.; Arjoman, M.; Safekordi, A.A.; Mortazavi, S.A. Ferric–Saccharate capsulation with alginate coating using the emulsification method. Afr. J. Microbiol. Res. 2012, 6, 2455–2461. [Google Scholar] [CrossRef]
- Campañone, L.; Bruno, E.; Martino, M. Effect of microwave treatment on metal-alginate beads. J. Food Eng. 2014, 135, 26–30. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Alginate nanoparticles protect ferrous from oxidation: Potential iron delivery system. Int. J. Pharm. 2016, 513, 404–409. [Google Scholar] [CrossRef]
- Cengiz, A. Ferrous Sulfate Nanoencapsulation with Liposome and Emulsification Methods and The Usage in Cocoa Containing Nut Paste. Master’s Thesis, Ondokuz Mayis University, Samsun, Turkey, Unpublished work. 2013. [Google Scholar]
- Berton, C.; Ropers, M.H.; Viau, M.; Genot, C. Contribution of the interfacial layer to the protection of emulsified lipids against oxidation. J. Agric. Food Chem. 2011, 59, 5052–5061. [Google Scholar] [CrossRef]
- Cameron, F.K. The Solubility of Ferrous Sulphate. J. Phys. Chem. 1929, 34, 692–710. [Google Scholar] [CrossRef]
- Lethuaut, L.; Métro, F.; Genot, C. Effect of droplet size on lipid oxidation rates of oil-in-water emulsions stabilized by protein. J. Am. Oil Chem. Soc. 2002, 79, 425. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Llanes, F.; Ryan, D.H.; Marchessault, R.H. Magnetic nanostructured composites using alginates of different M/G ratios as polymeric matrix. Int. J. Biol. Macromol. 2000, 27, 35–40. [Google Scholar] [CrossRef]
- Kroll, E.; Winnik, F.M.; Ziolo, R.F. In Situ Preparation of Nanocrystalline γ-Fe2O3 in Iron(II) Cross-Linked Alginate Gels. Chem. Mater. 2002, 8, 1594–1596. [Google Scholar] [CrossRef]
- Berner, L.A.; Hood, L.F. Iron Binding by Sodium Alginate. J. Food Sci. 1983, 48, 75–758. [Google Scholar] [CrossRef]
- Elias, H.R.G. Wilkins: Kinetics and Mechanism of Reactions of Transition Metal Complexes, 2nd Thoroughly Revised Edition; VCH: Weinheim, Germany, 1991; ISBN 3-527-28389-7. [Google Scholar]
- Herman, C.J.; Groves, M.J. The influence of free fatty acid formation on the pH of phospholipid-stabilized triglyceride emulsions. Pharm. Res. 1993, 10, 774–776. [Google Scholar] [CrossRef]
- Chen, B.; Mcclements, D.J.; Decker, E.A. Minor components in food oils: A critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit. Rev. Food Sci. Nutr. 2011, 51, 901–916. [Google Scholar] [CrossRef]
- Schaich, K.M. Lipid Oxidation: Theoretical Aspects. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons: Hoboken, NJ, USA, 5 July 2005. [Google Scholar]
- Ben Hammouda, S.; Fourcade, F.; Assadi, A.; Soutrel, I.; Adhoum, N.; Amrane, A.; Monser, L. Effective heterogeneous electro-Fenton process for the degradation of a malodorous compound, indole, using iron loaded alginate beads as a reusable catalyst. Appl. Catal. B Environ. 2016, 182, 47–58. [Google Scholar] [CrossRef]
- Titouhi, H.; Belgaied, J.E. Heterogeneous Fenton oxidation of ofloxacin drug by iron alginate support. Environ. Technol. UK 2016, 37, 2003–2015. [Google Scholar] [CrossRef]






| Sample Name | d[4,3] (mm) | Specific Surface Area (m2/kg Bead) |
|---|---|---|
| Very large bead (0.2 bar) | 2.05 ± 0.11 a | 2.9 |
| Large bead (1 bar) | 1.51 ± 0.04 b | 4.0 |
| Medium bead (2 bar) | 0.82 ± 0.01 c | 7.3 |
| Small bead (3 bar) | 0.62 ± 0.02 d | 9.7 |
| Empty medium bead | 0.76 ± 0.02 e | 7.9 |
| Sample | mg KOH/g Oil |
|---|---|
| Stripped oil | 0.02 ± 0.005 a |
| Control emulsion (0th day) | 0.06 ± 0.008 b |
| Control emulsion (8th day) | 0.65 ± 0.011 c |
| Free iron emulsion (8th day) | 1.02 ± 0.006 d |
| Iron-loaded bead emulsion (8th day) | 0.94 ± 0.009 e |
| Element | O | Na | S | Cl | Ca | Fe |
|---|---|---|---|---|---|---|
| Iron-loaded beads | 59.54 | 0.88 | 0.48 | 17.61 | 18.51 | 2.98 |
| Empty beads | 59.30 | 1.00 | - | 19.86 | 19.85 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cengiz, A.; Schroën, K.; Berton-Carabin, C. Lipid Oxidation in Emulsions Fortified with Iron-Loaded Alginate Beads. Foods 2019, 8, 361. https://doi.org/10.3390/foods8090361
Cengiz A, Schroën K, Berton-Carabin C. Lipid Oxidation in Emulsions Fortified with Iron-Loaded Alginate Beads. Foods. 2019; 8(9):361. https://doi.org/10.3390/foods8090361
Chicago/Turabian StyleCengiz, Alime, Karin Schroën, and Claire Berton-Carabin. 2019. "Lipid Oxidation in Emulsions Fortified with Iron-Loaded Alginate Beads" Foods 8, no. 9: 361. https://doi.org/10.3390/foods8090361