Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pomegranates Peels
2.2. Chemicals
2.3. Extraction Methodology
2.4. Methodology of the Determination of TPC
2.5. Determination of Antioxidant Capacity of the PPE (DPPH● Method)
2.6. Box-Behnken Design (BBD) Experiment
2.7. Data Analysis
2.8. Statistical Analysis
3. Results and Discussion
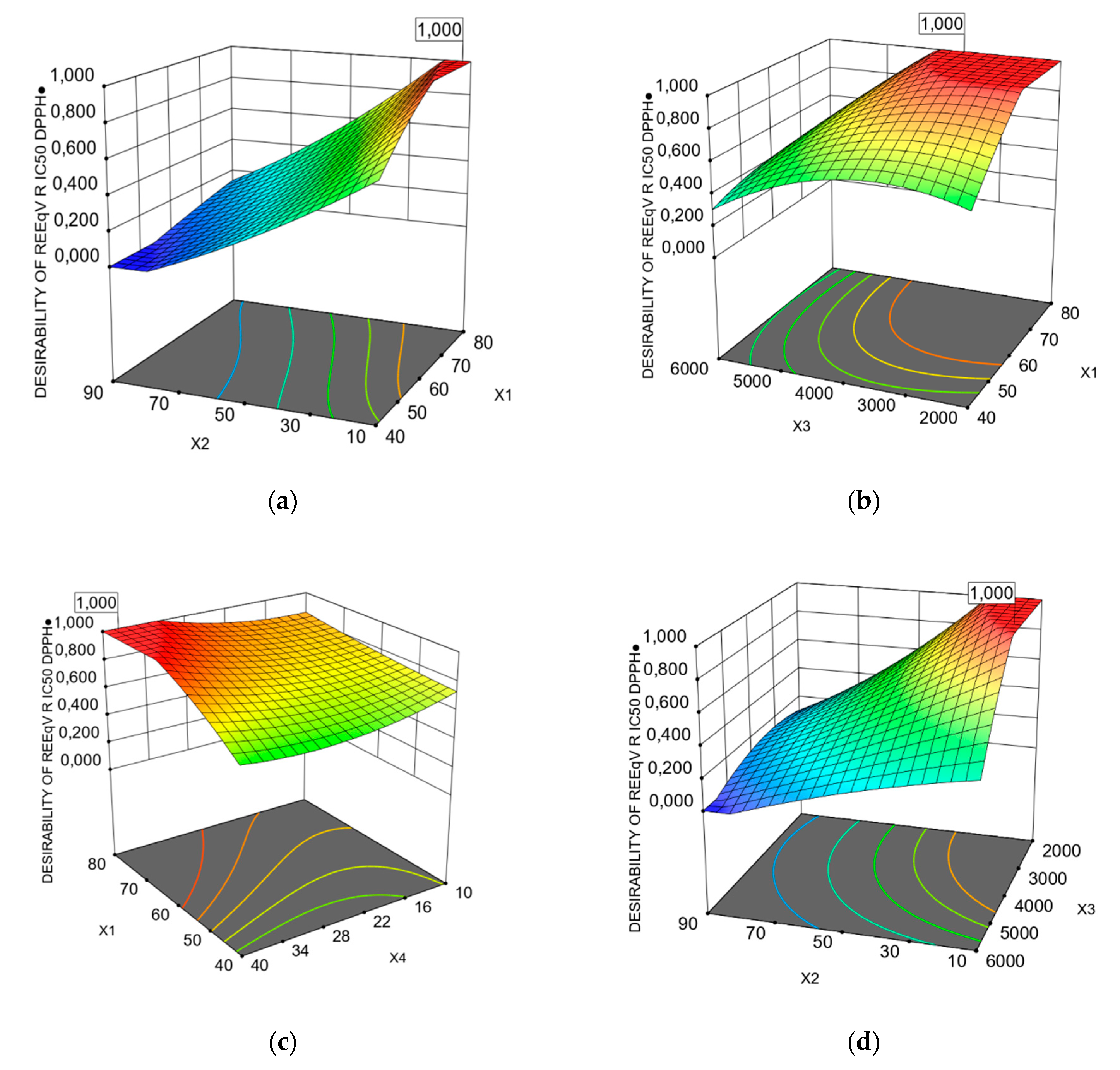
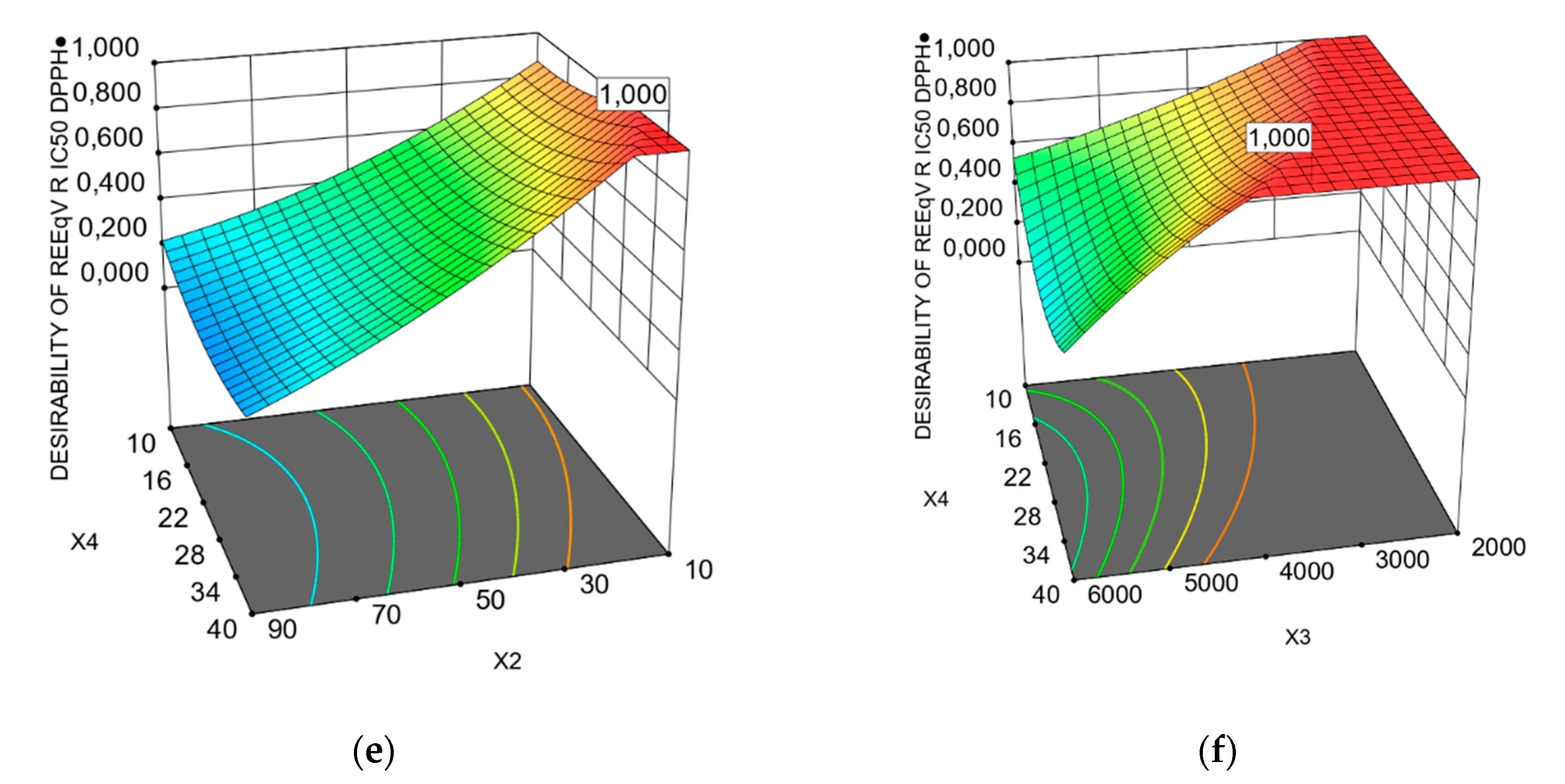
3.1. Predicted Models of Bioactivity Indices by RSM
3.2. Optimization of PP Vacuum Microwave-Assisted Aqueous Extraction
| Independent Variables | |||||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | TPC (mgGAE/g Fresh PP) | EEqVR IC50 DPPH● (L) |
| 77.060 | 10.210 | 3165.020 | 38.590 | 146.442 | |
| 77.050 | 12.480 | 2240.010 | 39.830 | 74.730 | |
X3X4 + 18.73 X12 − 4.81Χ22 − 5.35 X32 − 23.88 X42 + 12.13 X22X4 − 29.49 Χ2Χ42 − 22.30 Χ32Χ4 + 32.24 Χ22Χ42
X1X4 + 11.63 X2X3 − 8.97 X2X4 − 7.25 X3X4 + 8.49 X12 − 7.14 X22 − 0.9221 X32 + 10.11 X42 + 1.32 X12X2− 4.16
X12X3 − 3.24 X1X22 + 4.05 X2X42 − 6.44 X32X4+6.13 X3X42 − 6.74 X12X22
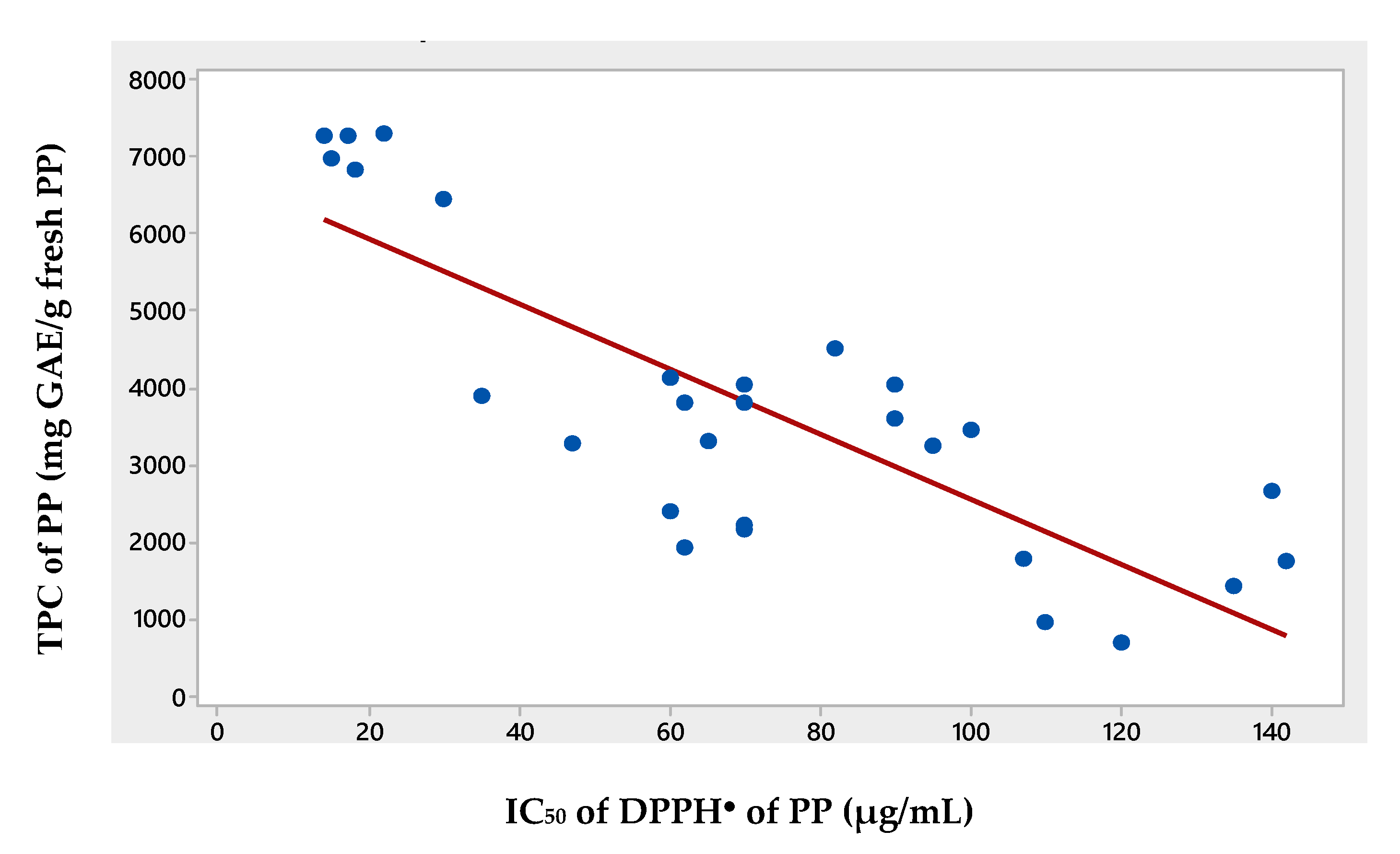
3.3. Correlation of TPC with IC50 of DPPH●
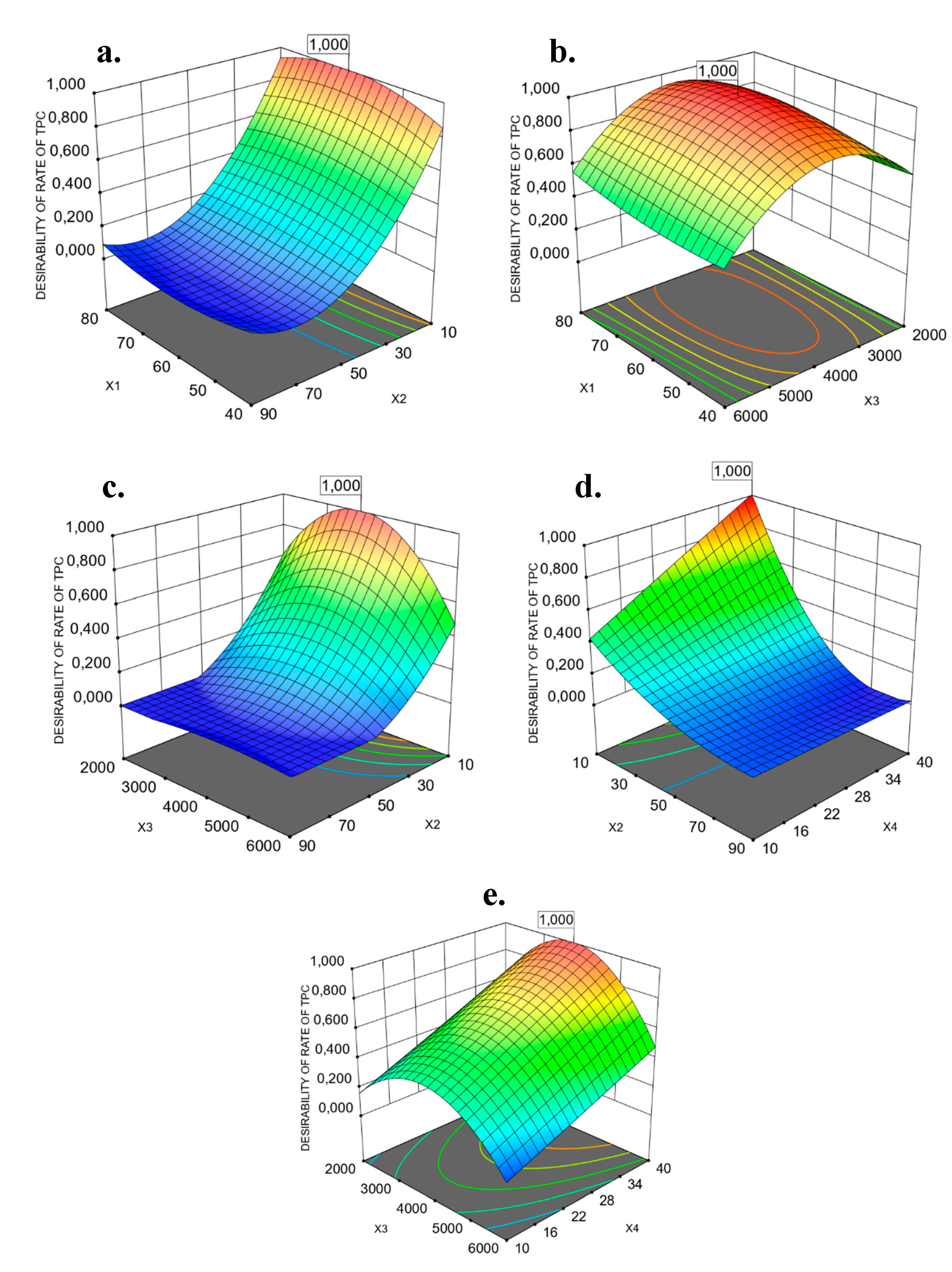
3.4. Modeling of the Extraction of PP Based on the Operational Costs
| Independent Variables | |||||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | RTPC (mgGAE/g Fresh PP)/min | REEqV R IC50 DPPH● (L/min) |
| 61.480 | 10.037 | 3797.240 | 39.924 | 5.542 | |
| 79.158 | 12.127 | 3576.470 | 38.201 | 1.813 | |
0.7728 X2X4 − 0.0365 X3X4 − 0.0994 X12 + 1.13 X22 − 0.3961 X32 + 0.4491 X12X2 + 0.0243 X12X3 − 0.0026 X1X32
− 0.0513 X22X3 + 0.6621 X22X4 + 1.06 X2X32− 0.3431 X32X4 + 0.7116 X12X32 − 0.5249 X22X32
+ 0.1471 X1X4 + 0.2941 X2X3 − 0.1362 X2X4 − 0.1116 X3X4 + 0.1306 X12 + 0.2125 X22 − 0.0138 X32 + 0.1552 X42
+ 0.0601 X12X2 − 0.0753 X12X4 + 0.0157 X1X22 − 0.1170 X22X3 + 0.0685 X2X32 + 0.0000 X2X42 − 0.1786 X32X4
+ 0.1584 X3X42 − 0.1414 X12X22 − 0.0437 X22X32
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef]
- Skenderidis, P.; Petrotos, K.; Leontopoulos, S. Functional Properties of Goji Berry (Lycium barbarum) Fruit Extracts. In Phytochemicals in Goji Berries; Xingqian Ye, Y.J., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 181–224. ISBN 9780429021749. [Google Scholar]
- Ben-Ali, S.; Akermi, A.; Mabrouk, M.; Ouederni, A. Optimization of extraction process and chemical characterization of pomegranate peel extract. Chem. Pap. 2018, 72, 2087–2100. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hmid, I.; Elothmani, D.; Hanine, H.; Oukabli, A.; Mehinagic, E. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab. J. Chem. 2017, 10, S2675–S2684. [Google Scholar] [CrossRef] [Green Version]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS(n). Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical properties, fatty-acid composition, and antioxidant activity of Goji berry (Lycium barbarum L. and Lycium Chinense mill.) fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Choe, E.; Min, D.B. Chemistry and Reactions of Reactive Oxygen Species in Foods. J. Food Sci. 2005, 70, R142–R159. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Leontopoulos, S.; Skenderidis, P.; Kalorizou, H.; Petrotos, K. Bioactivity Potential of Polyphenolic Compounds in Human Health and their Effectiveness Against Various Food Borne and Plant Pathogens. A Review. J. Food Biosyst. Eng. 2017, 7, 1–19. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moure, A.; Cruz, J.M.; Franco, D.; Manuel Domínguez, J.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Carlos Parajó, J. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Giavasis, I.; Petrotos, K.; Lampakis, D.; Leontopoulos, S.; Hadjichristodoulou, C.; Tsakalof, A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019, 13, 2017–2031. [Google Scholar] [CrossRef]
- Barathikannan, K.; Venkatadri, B.; Khusro, A.; Al-Dhabi, N.A.; Agastian, P.; Arasu, M.V.; Choi, H.S.; Kim, Y.O. Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complement. Altern. Med. 2016, 16, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, K.M.M.; Bhagwat, A.A.; Luthria, D.L. Swarm motility inhibitory and antioxidant activities of pomegranate peel processed under three drying conditions. Food Chem. 2017, 235, 145–153. [Google Scholar] [CrossRef]
- Wang, Z. Extract of Phenolics From Pomegranate Peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Ramaswamy, H.S.; Zomorodi, S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. Lebenson. Wiss. Technol. 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep eutectic solvent-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J. Sci. Food Agric. 2019, 99, 1969–1979. [Google Scholar] [CrossRef]
- Ho, K.; Ferruzzi, M.G.; Liceaga, A.; San Martin-Gonzalez, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Chanioti, S.; Siamandoura, P.; Tzia, C. Evaluation of Extracts Prepared from Olive Oil By-Products Using Microwave-Assisted Enzymatic Extraction: Effect of Encapsulation on the Stability of Final Products. Waste Biomass Valorization 2016, 7, 831–842. [Google Scholar] [CrossRef]
- Razzaghi, S.E.; Arabhosseini, A.; Turk, M.; Soubrat, T.; Cendres, A.; Kianmehr, M.H.; Perino, S.; Chemat, F. Operational efficiencies of six microwave based extraction methods for orange peel oil. J. Food Eng. 2019, 241, 26–32. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.H.; Wang, J.X.; Wang, G.; Wang, J.Y.; Li, G.K. Evaluation of vacuum microwave-assisted extraction technique for the extraction of antioxidants from plant samples. J. Chromatogr. A 2009, 1216, 8867–8873. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process Eng. 2017, 40, e12522. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Liu, B.; Li, L.; Zhu, X. Microwave-assisted extraction and antioxidant activity of total phenolic compounds from pomegranate peel. J. Med. Plants Res. 2011, 5, 1004–1011. [Google Scholar]
- Huang, J.; He, W.; Yan, C.; Du, X.; Shi, X. Microwave assisted extraction of flavonoids from pomegranate peel and its antioxidant activity. BIO Web Conf. 2017, 8, 3008. [Google Scholar] [CrossRef] [Green Version]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Lampakis, D.; Petrotos, K.; Giavasis, I. The effect of encapsulated powder of goji berry (Lycium barbarum) on growth and survival of probiotic bacteria. Microorganisms 2020, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Hu, Y. Optimization of polysaccharides extraction from Trametes robiniophila and its antioxidant activities. Carbohydr. Polym. 2014, 111, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Zhang, X.; Farooq, U.; Abbas, S.; Xia, S.; Jia, C.; Zhong, F.; Zhang, J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010, 123, 423–429. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Singh, R.; Vijayraghavan, R.; Arora, A. From waste to wealth: High recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crops Prod. 2018, 112, 790–802. [Google Scholar] [CrossRef]
- Qu, W.; Pan, Z.; Ma, H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010, 99, 16–23. [Google Scholar] [CrossRef]
- Yin, G.; Dang, Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken Statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Mansour, E.; Ben Khaled, A.; Lachiheb, B.; Abid, M.; Bachar, K.; Ferchichi, A. Phenolic compounds, antioxidant, and antibacterial activities of peel extract from Tunisian pomegranate. J. Agric. Sci. Technol. 2013, 15, 1393–1403. [Google Scholar]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Durgaç, C.; Serçe, S.; Kaya, C. Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey. Food Chem. 2008, 111, 703–706. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Wu, P.; Gu, Y.; Zhao, R.; Liu, Y.; Wang, Y.; Lv, G.; Li, Z.; Bao, Y. Residual pomegranate affecting the nonspecific immunity of juvenile Darkbarbel catfish. Fish Shellfish Immunol. 2019, 95, 190–194. [Google Scholar] [CrossRef]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2012, 19, 365–372. [Google Scholar] [CrossRef]
- Xie, J.H.; Dong, C.J.; Nie, S.P.; Li, F.; Wang, Z.J.; Shen, M.Y.; Xie, M.Y. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef]
- Wang, Y.; You, J.; Yu, Y.; Qu, C.; Zhang, H.; Ding, L.; Zhang, H.; Li, X. Analysis of ginsenosides in Panax ginseng in high pressure microwave-assisted extraction. Food Chem. 2008, 110, 161–167. [Google Scholar] [CrossRef]
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Spigno, G.; De Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A. 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Muralidhar, R.V.; Chirumamila, R.R.; Marchant, R.; Nigam, P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. J. 2001, 9, 17–23. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: Boston, MA, USA, 2013; pp. 15–52. ISBN 978-1-4614-4830-3. [Google Scholar]
- Li, J.; Zu, Y.G.; Fu, Y.J.; Yang, Y.C.; Li, S.M.; Li, Z.N.; Wink, M. Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Khajeh, M.; Akbari Moghaddam, A.R.; Sanchooli, E. Application of Doehlert design in the Optimization of microwave-assisted extraction for determination of zinc and copper in cereal samples using FAAS. Food Anal. Methods 2010, 3, 133–137. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015, 123, 67–71. [Google Scholar] [CrossRef]
| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Extraction temperature (°C) | X1 | 40 | 60 | 80 |
| Extraction time (min) | X2 | 10 | 50 | 90 |
| Microwave power (W) | X3 | 2000 | 4000 | 6000 |
| Ratio of PP to water (%) | X4 | 10 | 25 | 40 |
| Design Point | Independent Variables in Coded Values | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| TPC (mgGAE/g Fresh PP) | EEqV R IC50 DPPH● (L) | |||||||
| X1 | X2 | X3 | X4 | Measured | Predicted | Measured | Predicted | |
| 1 | −1 | 0 | 1 | 0 | 82.29 ± 0.52 | 74.13 | 53.19 ± 0.17 | 53.44 |
| 2 | 1 | 0 | 0 | 1 | 77.00 ± 0.31 | 77.00 | 64.52 ± 1.81 | 64.54 |
| 3 | 0 | 0 | 0 | 0 | 55.83 ± 0.08 | 67.45 | 35.71 ± 1.46 | 37.45 |
| 4 | 0 | 0 | −1 | 1 | 38.00 ± 0.01 | 38.00 | 36.36 ± 0.44 | 36.36 |
| 5 | 0 | 0 | 0 | 0 | 82.92 ± 0.28 | 67.45 | 38.46 ± 1.12 | 37.45 |
| 6 | −1 | 0 | 0 | 1 | 70.67 ± 0.20 | 70.67 | 37.38 ± 0.22 | 36.86 |
| 7 | 0 | 0 | 0 | 0 | 54.06 ± 0.61 | 67.45 | 35.71 ± 1.46 | 37.45 |
| 8 | 0 | 0 | 1 | 1 | 28.17 ± 0.41 | 28.17 | 33.33 ± 0.11 | 33.33 |
| 9 | 0 | 1 | 0 | 1 | 57.00 ± 0.38 | 60.49 | 29.63 ± 0.22 | 29.88 |
| 10 | −1 | 1 | 0 | 0 | 95.52 ± 0.54 | 89.81 | 35.71 ± 0.46 | 35.71 |
| 11 | 1 | 0 | 1 | 0 | 101.46 ± 0.68 | 82.87 | 27.78 ± 1.00 | 27.48 |
| 12 | 0 | −1 | 0 | −1 | 64.42 ± 0.08 | 60.16 | 33.33 ± 1.07 | 33.03 |
| 13 | −1 | 0 | −1 | 0 | 90.42 ± 0.84 | 85.79 | 27.78 ± 0.09 | 28.03 |
| 14 | 0 | 0 | 0 | 0 | 101.25 ± 0.84 | 67.45 | 35.71 ± 0.25 | 37.45 |
| 15 | 1 | −1 | 0 | 0 | 95.31 ± 0.57 | 130.31 | 35.71 ± 1.11 | 35.71 |
| 16 | 0 | −1 | −1 | 0 | 59.90 ± 0.87 | 60.32 | 41.67 ± 0.01 | 41.69 |
| 17 | 0 | 1 | 0 | −1 | 72.67 ± 0.03 | 80.84 | 58.82 ± 1.35 | 58.52 |
| 18 | 0 | 1 | −1 | 0 | 66.56 ± 0.37 | 67.17 | 17.86 ± 0.37 | 17.88 |
| 19 | 1 | 1 | 0 | 0 | 113.02 ± 0.46 | 105.92 | 30.49 ± 0.01 | 30.49 |
| 20 | −1 | −1 | 0 | 0 | 80.94 ± 0.95 | 104.58 | 26.32 ± 1.46 | 26.32 |
| 21 | 0 | 1 | 1 | 0 | 95.52 ± 0.64 | 119.07 | 40.32 ± 0.66 | 40.35 |
| 22 | 1 | 0 | 0 | −1 | 68.50 ± 0.13 | 68.50 | 55.56 ± 1.12 | 56.13 |
| 23 | 1 | 0 | −1 | 0 | 97.19 ± 0.98 | 99.74 | 71.43 ± 1.41 | 71.13 |
| 24 | 0 | −1 | 0 | 1 | 137.97 ± 0.99 | 108.87 | 40.00 ± 0.60 | 40.25 |
| 25 | 0 | −1 | 1 | 0 | 43.96 ± 0.97 | 45.33 | 17.61 ± 0.59 | 17.63 |
| 26 | 0 | 0 | 0 | 0 | 103.23 ± 0.08 | 67.45 | 41.67 ± 1.95 | 37.45 |
| 27 | 0 | 0 | −1 | −1 | 73.17 ± 0.27 | 73.17 | 45.45 ± 0.21 | 45.45 |
| 28 | −1 | 0 | 0 | −1 | 69.79 ± 0.03 | 69.79 | 66.67 ± 0.05 | 66.69 |
| 29 | 0 | 0 | 1 | −1 | 72.83 ± 0.09 | 72.83 | 71.43 ± 0.27 | 71.43 |
| TPC (mg GAE/g Fresh PP) | EEqV R IC50 DPPH● (L) | ||
|---|---|---|---|
| p Value of the Model | p Value of the Model | ||
| Model | <0.0066 * | Model | <0.0001 * |
| Variables | p Value | Variables | p Value |
| X1 | 0.2296 | X1 | 0.0006 * |
| X2 | 0.0445 * | X2 | 0.7964 |
| X3 | 0.9843 | X3 | 0.7071 |
| X4 | 0.7498 | X4 | 0.0001 * |
| Χ2Χ3 | 0.1428 | X1X2 | 0.0089 * |
| X2X4 | 0.0085 * | X1X3 | <0.0001 * |
| X3X4 | 0.7466 | X1X4 | <0.0001 * |
| X12 | 0.0083 * | X2X3 | <0.0001 * |
| X22 | 0.5051 | X2X4 | <0.0001 * |
| X33 | 0.3903 | X3X4 | 0.0002 * |
| X42 | 0.0047 * | X12 | <0.0001 * |
| X22X4 | 0.2547 | X22 | 0.0002 * |
| X2X42 | 0.0053 * | X32 | 0.3153 |
| X32X4 | 0.0472 * | X42 | <0.0001 * |
| X22X42 | 0.0226 * | X12X2 | 0.3914 |
| X12X3 | 0.0234 * | ||
| X1X22 | 0.0356 * | ||
| X2X42 | 0.0260 * | ||
| X32X4 | 0.0013 * | ||
| X3X42 | 0.0038 * | ||
| X12X22 | 0.0066* | ||
| Lack of fitting | 0.9987 Not significant | 0.9788 Not significant | |
| R2 | 0.8286 | 0.9951 | |
| Adj. R2 | 0.6309 | 0.9805 | |
| RTPC (mg GAE/g Fresh PP)/min | REEqVR IC50 DPPH●(L/min) | ||
|---|---|---|---|
| p Value of the Model | p Value of the Model | ||
| Model | 0.0003 * Significant | Model | <0.0001 * Significant |
| Variables | p Value | Variables | p Value |
| X1 | 0.3535 | X1 | 0.0038 * |
| X2 | <0.0001 * | X2 | <0.0001 * |
| X3 | 0.7962 | X3 | 0.0123 * |
| X4 | 0.8117 | X4 | 0.8830 |
| X1X2 | 0.5063 | X1X2 | 0.0021 * |
| X1X3 | 0.7532 | X1X3 | <0.0001 * |
| X2X3 | 0.1612 | X1X4 | 0.0005 * |
| X2X4 | 0.0011 * | X2X3 | <0.0001 * |
| X3X4 | 0.8093 | X2X4 | 0.0007 * |
| X12 | 0.5063 | X3X4 | 0.0017 * |
| X22 | 0.0001 * | X12 | 0.0008 * |
| X32 | 0.0709 | X22 | 0.0004 * |
| X12X2 | 0.0657 | X32 | 0.4749 |
| X12X3 | 0.9095 | X42 | 0.0003 * |
| X1X32 | 0.9886 | X12X2 | 0.0686 |
| X22X3 | 0.8105 | X12X4 | 0.0337 * |
| X22X4 | 0.0148 * | X22X3 | 0.5170 |
| X2X32 | 0.0013 * | X2X32 | 0.0064 * |
| X32X4 | 0.1399 | X2X42 | 0.0459 * |
| X12X32 | 0.0249 * | X32X4 | - |
| X22X32 | 0.0741 | X3X42 | 0.0010 * |
| X12X22 | 0.0017 * | ||
| X22X32 | 0.0120 * | ||
| Lack of fitting | 0.9228 Not significant | 0.7813 Not significant | |
| R2 | 0.9823 | 0.9985 | |
| Adj. R2 | 0.9293 | 0.9915 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Giavasis, I. Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity. Foods 2020, 9, 1655. https://doi.org/10.3390/foods9111655
Skenderidis P, Leontopoulos S, Petrotos K, Giavasis I. Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity. Foods. 2020; 9(11):1655. https://doi.org/10.3390/foods9111655
Chicago/Turabian StyleSkenderidis, Prodromos, Stefanos Leontopoulos, Konstantinos Petrotos, and Ioannis Giavasis. 2020. "Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity" Foods 9, no. 11: 1655. https://doi.org/10.3390/foods9111655







