Size-Dependent Variability in Flow and Viscoelastic Behavior of Levan Produced by Gluconobacter albidus TMW 2.1191
Abstract
1. Introduction
2. Material and Methods
2.1. Production and Recovery of Levan at Different pH Values
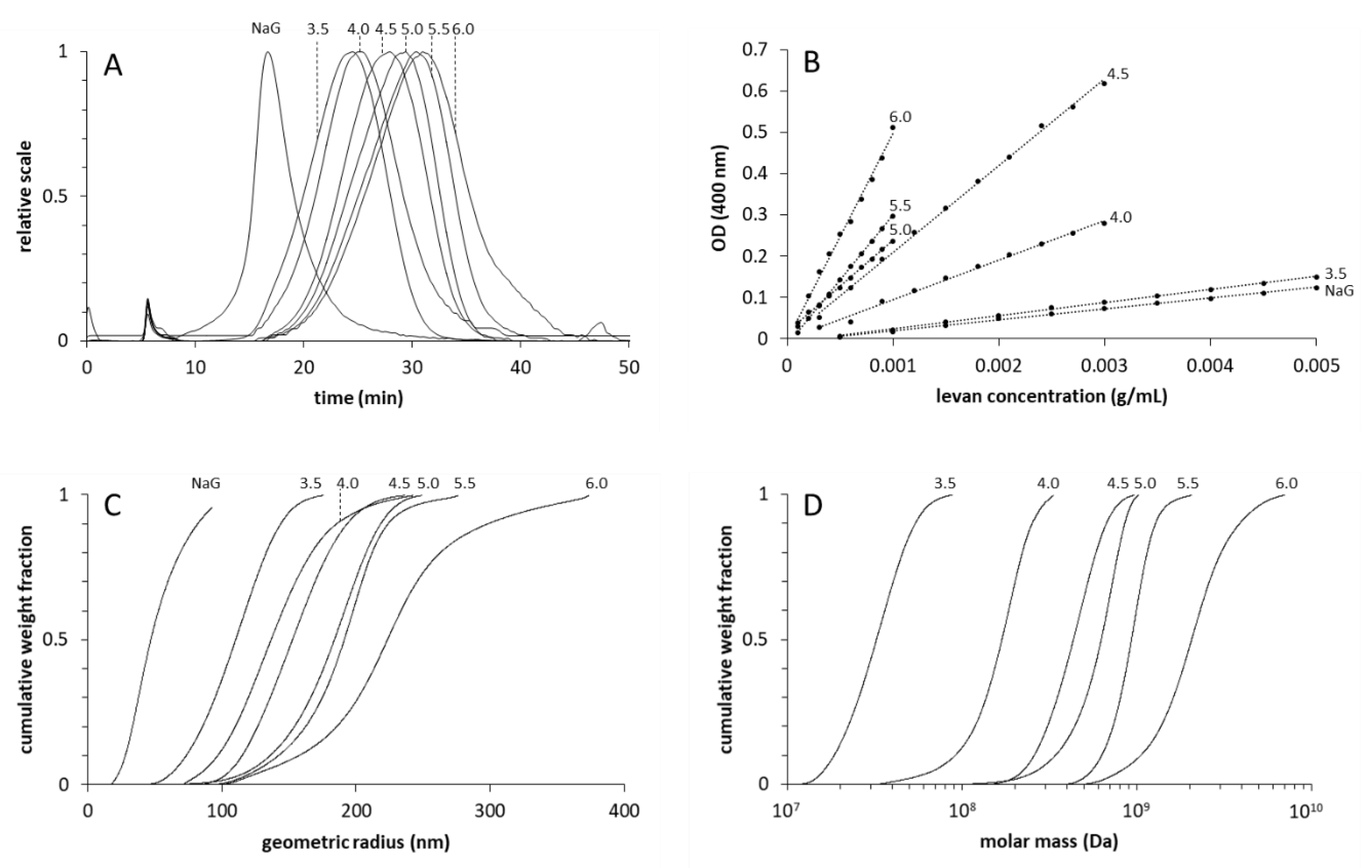
2.2. Separation and Size Determinations of Levan Fractions
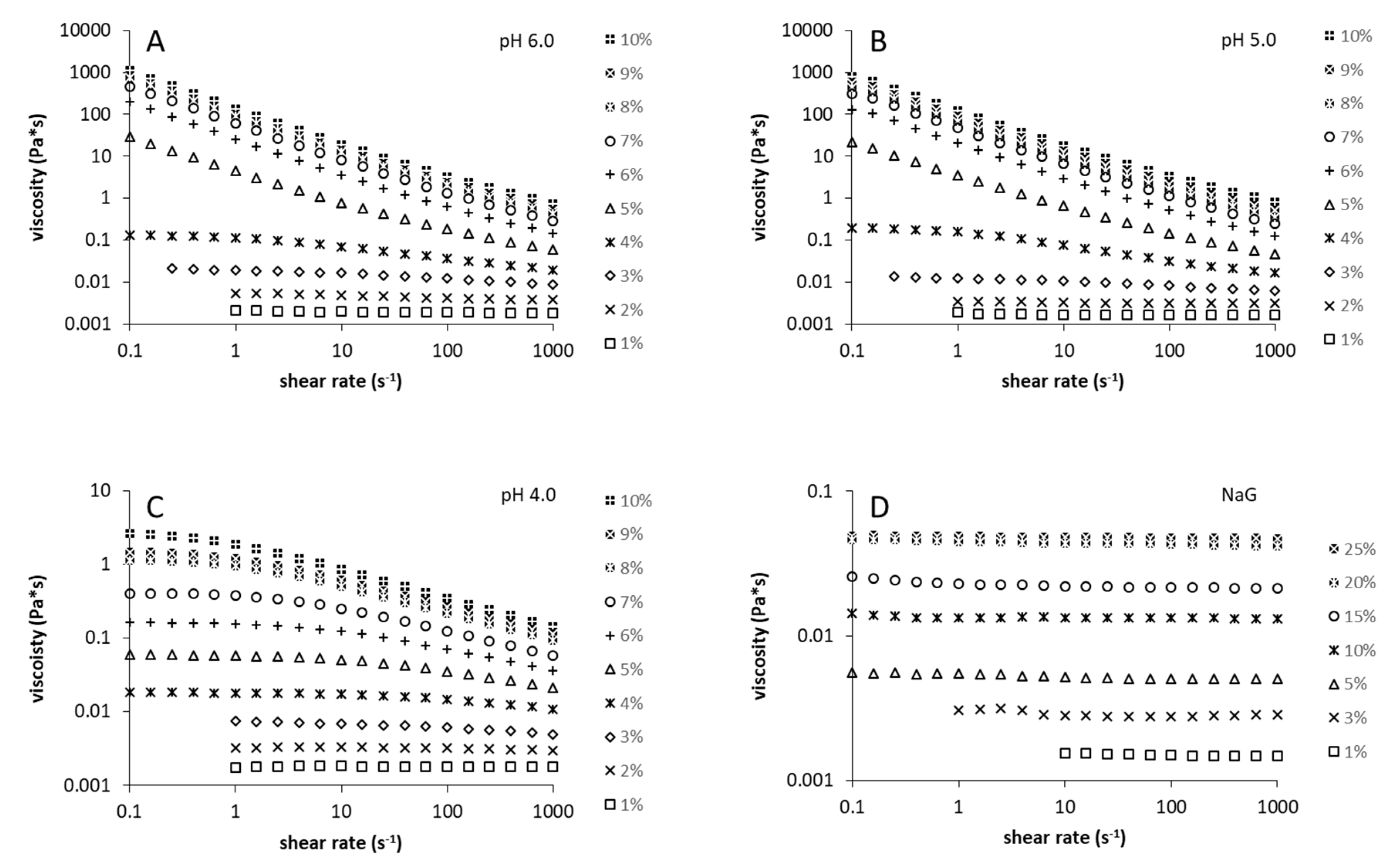
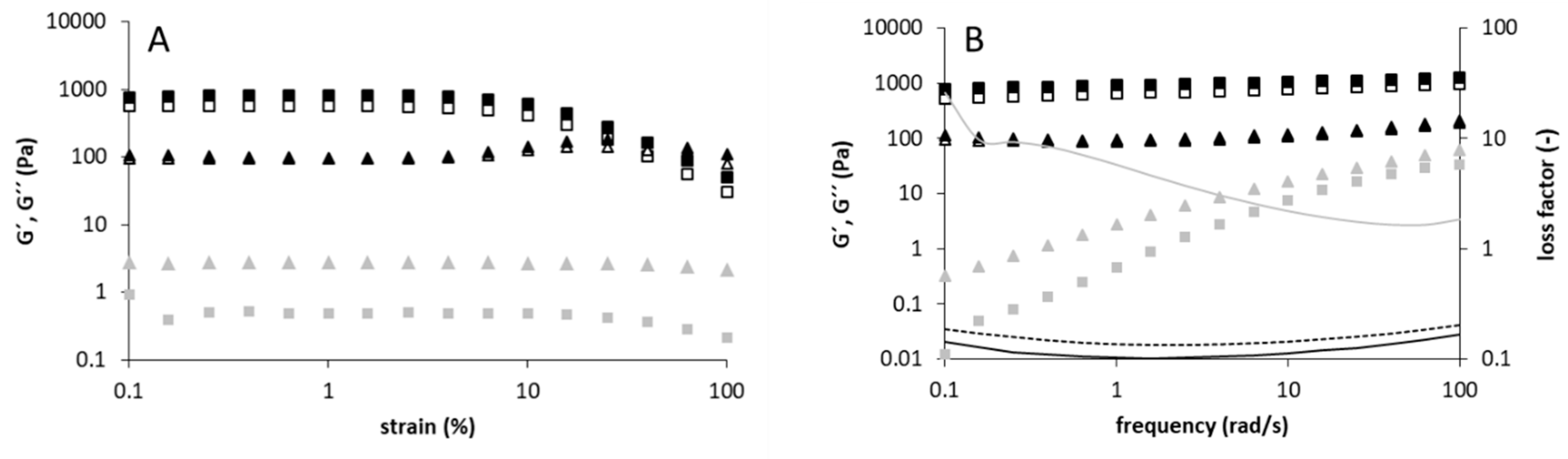
2.3. Rheological Measurements
2.4. Determination of the Degree of Branching
3. Results
3.1. Amounts and Sizes of the Produced Levans
3.2. Rheological and Structural Properties of the Produced Levans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Öner, E.T.; Hernández, L.; Combie, J. Review of levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef]
- Velázquez-Hernández, M.; Baizabal-Aguirre, V.; Bravo-Patiño, A.; Cajero-Juárez, M.; Chávez-Moctezuma, M.; Valdez-Alarcón, J. Microbial fructosyltransferases and the role of fructans. J. Appl. Microbiol. 2009, 106, 1763–1778. [Google Scholar] [CrossRef]
- Kırtel, O.; Lescrinier, E.; Van den Ende, W.; Öner, E.T. Discovery of fructans in Archaea. Carbohydr. Polym. 2019, 220, 149–156. [Google Scholar] [CrossRef]
- Van Hijum, S.A.; Bonting, K.; van der Maarel, M.J.; Dijkhuizen, L. Purification of a novel fructosyltransferase from Lactobacillus reuteri strain 121 and characterization of the levan produced. FEMS Microbiol. Lett. 2001, 205, 323–328. [Google Scholar] [CrossRef]
- Korakli, M.; Pavlovic, M.; Gänzle, M.G.; Vogel, R.F. Exopolysaccharide and kestose production by Lactobacillus sanfranciscensis LTH2590. Appl. Environ. Microbiol. 2003, 69, 2073–2079. [Google Scholar] [CrossRef]
- Tieking, M.; Ehrmann, M.A.; Vogel, R.F.; Gänzle, M.G. Molecular and functional characterization of a levansucrase from the sourdough isolate Lactobacillus sanfranciscensis TMW 1.392. Appl. Microbiol. Biotechnol. 2005, 66, 655–663. [Google Scholar] [CrossRef]
- Anwar, M.A.; Kralj, S.; Pique, A.V.; Leemhuis, H.; van der Maarel, M.J.; Dijkhuizen, L. Inulin and levan synthesis by probiotic Lactobacillus gasseri strains: Characterization of three novel fructansucrase enzymes and their fructan products. Microbiology 2010, 156, 1264–1274. [Google Scholar] [CrossRef]
- Jakob, F.; Meißner, D.; Vogel, R.F. Comparison of novel GH 68 levansucrases of levan-overproducing Gluconobacter species. Acetic Acid Bact. 2012, 1, e2. [Google Scholar] [CrossRef]
- Semjonovs, P.; Shakirova, L.; Treimane, R.; Shvirksts, K.; Auzina, L.; Cleenwerck, I.; Zikmanis, P. Production of extracellular fructans by Gluconobacter nephelii P1464. Lett. Appl. Microbiol. 2016, 62, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Jakob, F.; Quintero, Y.; Musacchio, A.; Estrada-de los Santos, P.; Hernández, L.; Vogel, R.F. Acetic acid bacteria encode two levansucrase types of different ecological relationship. Environ. Microbial. 2019, 21, 4151–4165. [Google Scholar] [CrossRef] [PubMed]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Fels, L.; Jakob, F.; Vogel, R.F.; Wefers, D. Structural characterization of the exopolysaccharides from water kefir. Carbohydr. Polym. 2018, 189, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yajima, T.; Li, W.; Saito, K.; Ohshima, Y.; Yoshikai, Y. Levan (β-2, 6-fructan), a major fraction of fermented soybean mucilage, displays immunostimulating properties via Toll-like receptor 4 signalling: Induction of interleukin-12 production and suppression of T-helper type 2 response and immunoglobulin E production. Clin. Exp. Allergy 2006, 36, 94–101. [Google Scholar] [PubMed]
- Shih, I.-L.; Yu, Y.-T.; Shieh, C.-J.; Hsieh, C.-Y. Selective production and characterization of levan by Bacillus subtilis (Natto) Takahashi. J. Agric. Food Chem. 2005, 53, 8211–8215. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.A.; Jang, K.-H.; Seo, J.-W.; Kim, K.H.; Kim, Y.H.; Rairakhwada, D.; Seo, M.Y.; Lee, J.O.; Ha, S.; Kim, C. Levan: Applications and perspectives. In Microbial Production of Biopolymers and Polymer Precursors: Applications and Perspectives; Caister Academic Press: Norfolk, UK, 2009; pp. 145–161. [Google Scholar]
- Jakob, F.; Steger, S.; Vogel, R.F. Influence of novel fructans produced by selected acetic acid bacteria on the volume and texture of wheat breads. Eur. Food Res. Technol. 2012, 234, 493–499. [Google Scholar] [CrossRef]
- Jakob, F.; Pfaff, A.; Novoa-Carballal, R.; Rübsam, H.; Becker, T.; Vogel, R.F. Structural analysis of fructans produced by acetic acid bacteria reveals a relation to hydrocolloid function. Carbohydr. Polym. 2013, 92, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Characterization of growth and exopolysaccharide production of selected acetic acid bacteria in buckwheat sourdoughs. Int. J. Food Microbiol. 2016, 239, 103–112. [Google Scholar] [CrossRef]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Influence of levan-producing acetic acid bacteria on buckwheat-sourdough breads. Food Microbiol. 2017, 65, 95–104. [Google Scholar] [CrossRef]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Fermentation pH modulates the size distributions and functional properties of Gluconobacter albidus TMW 2.1191 levan. Front. Microbiol. 2017, 8, 807. [Google Scholar] [CrossRef]
- Dogsa, I.; Brloznik, M.; Stopar, D.; Mandic-Mulec, I. Exopolymer diversity and the role of levan in Bacillus subtilis biofilms. PLoS ONE 2013, 8, e62044. [Google Scholar] [CrossRef]
- Benigar, E.; Dogsa, I.; Stopar, D.; Jamnik, A.; Cigić, I.K.; Tomšič, M. Structure and dynamics of a polysaccharide matrix: Aqueous solutions of bacterial levan. Langmuir 2014, 30, 4172–4182. [Google Scholar] [CrossRef]
- Stojković, B.; Sretenovic, S.; Dogsa, I.; Poberaj, I.; Stopar, D. Viscoelastic properties of levan-DNA mixtures important in microbial biofilm formation as determined by micro-and macrorheology. Biophys. J. 2015, 108, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Benigar, E.; Zupančič Valant, A.; Dogsa, I.; Sretenovic, S.; Stopar, D.; Jamnik, A.; Tomšič, M. Structure and dynamics of a model polymer mixture mimicking a levan-based bacterial biofilm of Bacillus subtilis. Langmuir 2016, 32, 8182–8194. [Google Scholar] [CrossRef] [PubMed]
- Laue, H.; Schenk, A.; Li, H.; Lambertsen, L.; Neu, T.R.; Molin, S.; Ullrich, M.S. Contribution of alginate and levan production to biofilm formation by Pseudomonas syringae. Microbiology 2006, 152, 2909–2918. [Google Scholar] [CrossRef]
- Koczan, J.M.; McGrath, M.J.; Zhao, Y.; Sundin, G.W. Contribution of Erwinia amylovora exopolysaccharides amylovoran and levan to biofilm formation: Implications in pathogenicity. Phytopathology 2009, 99, 1237–1244. [Google Scholar] [CrossRef]
- Jakob, F. Novel Fructans from Acetic Acid Bacteria; Technische Universität München: Munich, Germany, 2014. [Google Scholar]
- Jakob, F.; Vogel, R.F. Method for Producing Exopolysaccharides, Products and Uses Thereof; Google Patents: Mountain View, CA, USA, 2015. [Google Scholar]
- Carpita, N.C.; Housley, T.L.; Hendrix, J.E. New features of plant-fructan structure revealed by methylation analysis and carbon-13 NMR spectroscopy. Carbohydr. Res. 1991, 217, 127–136. [Google Scholar] [CrossRef]
- Sweet, D.P.; Shapiro, R.H.; Albersheim, P. Quantitative analysis by various GLC response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydr. Res. 1975, 40, 217–225. [Google Scholar] [CrossRef]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Non-Newton. Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of nonlinear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Schmid, J.; Bechtner, J.; Vogel, R.F.; Jakob, F. A systematic approach to study the pH-dependent release, productivity and product specificity of dextransucrases. Microb. Cell Fact. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Hundschell, C.S.; Wagemans, A.M. Rheology of Common Uncharged Exopolysaccharides for Food Applications. Curr. Opin. Food Sci. 2019, 27, 1–7. [Google Scholar] [CrossRef]
- Runyon, J.R.; Nilsson, L.; Ulmius, M.; Castro, A.; Ionescu, R.; Andersson, C.; Schmidt, C. Characterizing changes in levan physicochemical properties in different pH environments using asymmetric flow field-flow fractionation. Anal. Bioanal. Chem. 2014, 406, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Lorenzo, L.; Porras-Domínguez, J.R.; Raga-Carbajal, E.; Olvera, C.; Rodríguez-Alegría, M.E.; Carrillo-Nava, E.; Costas, M.; Munguia, A.L. Intrinsic levanase activity of Bacillus subtilis 168 levansucrase (SacB). PLoS ONE 2015, 10, e0143394. [Google Scholar] [CrossRef] [PubMed]
- Kasapis, S.; Morris, E.R.; Gross, M.; Rudolph, K. Solution properties of levan polysaccharide from Pseudomonas syringae pv. phaseolicola, and its possible primary role as a blocker of recognition during pathogenesis. Carbohydr. Polym. 1994, 23, 55–64. [Google Scholar] [CrossRef]
- Arvidson, S.A.; Rinehart, B.T.; Gadala-Maria, F. Concentration regimes of solutions of levan polysaccharide from Bacillus sp. Carbohydr. Polym. 2006, 65, 144–149. [Google Scholar] [CrossRef]
- Peng, J.; Xu, W.; Ni, D.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Preparation of a novel water-soluble gel from Erwinia amylovora levan. Int. J. Biol. Macromol. 2019, 122, 469–478. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Q.; Bai, Y.; Yu, S.; Zhang, T.; Jiang, B.; Mu, W. Physicochemical properties of a high molecular weight levan from Brenneria sp. EniD312. Int. J. Biol. Macromol. 2018, 109, 810–818. [Google Scholar] [CrossRef]
- Hundschell, C.S.; Jakob, F.; Wagemans, A.M. Molecular Weight Dependent Structure and Polymer Density of the Exopolysaccharide Levan. arXiv 2019, arXiv:1909.07737. [Google Scholar]
- Senff, H.; Richtering, W. Temperature sensitive microgel suspensions: Colloidal phase behavior and rheology of soft spheres. J. Chem. Phys. 1999, 111, 1705–1711. [Google Scholar] [CrossRef]

| Glycosidic Linkage | pH 4.0 | pH 5.0 | NaG |
|---|---|---|---|
| t-Fruf | 12.3 ± 3.4 | 13.9 ± 1.0 | 8.1 ± 0.4 |
| 2,6-Fruf | 84.6 ± 3.1 | 83.1 ± 1.1 | 88.5 ± 0.3 |
| 1,2,6-Fruf | 3.1 ± 0.3 | 3.1 ± 0.1 | 3.4 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hundschell, C.S.; Braun, A.; Wefers, D.; Vogel, R.F.; Jakob, F. Size-Dependent Variability in Flow and Viscoelastic Behavior of Levan Produced by Gluconobacter albidus TMW 2.1191. Foods 2020, 9, 192. https://doi.org/10.3390/foods9020192
Hundschell CS, Braun A, Wefers D, Vogel RF, Jakob F. Size-Dependent Variability in Flow and Viscoelastic Behavior of Levan Produced by Gluconobacter albidus TMW 2.1191. Foods. 2020; 9(2):192. https://doi.org/10.3390/foods9020192
Chicago/Turabian StyleHundschell, Christoph S., Andre Braun, Daniel Wefers, Rudi F. Vogel, and Frank Jakob. 2020. "Size-Dependent Variability in Flow and Viscoelastic Behavior of Levan Produced by Gluconobacter albidus TMW 2.1191" Foods 9, no. 2: 192. https://doi.org/10.3390/foods9020192
APA StyleHundschell, C. S., Braun, A., Wefers, D., Vogel, R. F., & Jakob, F. (2020). Size-Dependent Variability in Flow and Viscoelastic Behavior of Levan Produced by Gluconobacter albidus TMW 2.1191. Foods, 9(2), 192. https://doi.org/10.3390/foods9020192





