1. Introduction
The conventional municipal wastewater treatments seem to be inefficient regarding the degradation of some specific compounds [
1]. The so called emerging contaminants encompass compounds usually used on pharmaceuticals and personal care products and are progressively appearing on natural aquatic resources and on the wastewater treatment plants [
1]. Among these pollutants are the parabens. The parabens are widely used as preservatives and are antimicrobial, mainly in pharmaceutical and personal care products [
2]. However, some studies point out that these compounds present carcinogenic potential [
3]. Thus, their presence in rivers and wastewater treatment plants may constitute a problem of human health [
2]. Bearing in mind the low efficiency of the traditional municipal wastewater treatment processes on the degradation of these compounds, scientific efforts have been made to find a suitable solution to their degradation [
4,
5]. In this way, advanced oxidation processes (AOPs) appear as promising technologies.
Ozone is a powerful oxidant, with a high potential oxidation (E
o = 2.07 V), greater than oxygen and hydrogen peroxide, that can oxidize a large amount of organic compounds with high electronic density, such as aromatic compounds [
6]. Heterogeneous catalysis tends to be helpful with improving wastewater treatment by ozonation [
6]. The presence of heterogeneous catalysts on ozonation can enhance the production of hydroxyl radicals due to the decomposition of ozone onto the surface of catalysts [
7]. Besides the hydroxyl radical production, the organic contaminants can adsorb on the catalyst surface, which can suffer an attack by ozone dissolved in bulk, or ozone and organic contaminants can adsorb on the catalyst surface followed by surface chemical reactions [
7]. The higher potential of oxidation of hydroxyl radicals (E
o = 2.80 V) and their low specificity allows reactions with a wider range of organic and inorganic substances. Thus, hydroxyl radicals may improve the mineralization degree achieved by ozonation. In fact, molecular ozone reactions can be a source of refractory by-products no longer reactive with ozone, leading to low mineralization levels [
7]. The choice of a suitable catalyst can be an important factor for the catalytic ozonation performance. This choice can also be influenced by the use of UV-radiation for aiding ozone action.
Titanium dioxide is the most commonly used photocatalyst, due to low cost, easy handling and good optical and electronic properties [
8]. The photocatalytic ozonation using TiO
2 under LED irradiation was used for removal micropollutants from urban wastewater with high efficiency [
9]. The band gap is 3.2 eV for anatase TiO
2 (λ < 387 nm), therefore, for exciting an electron from the valence band to the conduction band, UV-light is required [
10]. This electron transfer generates a positive hole on the valence band of TiO
2, usually a denominated photogenerated hole. This mechanism can be seen in Equation (1):
The band gap of TiO
2 can be reduced using metal (such as, Ag, Au, Pd and Pt) or non-metal (such as N and B) dopants, which allows the production of TiO
2 photogenerated electrons and holes using lower energy, such as solar irradiation [
10,
11,
12].
The presence of ozone on photocatalytic experiments with these kinds of catalysts (semicondutors) can enhance the photogeneration of electron–hole pairs because ozone adsorbed on TiO
2 surface works as an electron acceptor producing ozonide radicals (O
3•−) Equation (2) [
13,
14]:
Moreover, as can be seen in Equation (3), noble metals on the surface of titanium dioxide may work as scavengers of photogenerated electrons (e
−SC) [
15]. These two situations can reduce the recombination phenomenon of electron–hole pairs [
16,
17]:
On the other hand, the water adsorbed on the catalyst surface can react with the photogenerated holes (h
+VB) to produce hydroxyl radicals (
•OH) Equation (4) [
8,
10]. The ozonide radical at acidic conditions can also enhance the production of hydroxyl radicals [
18]. In addition, these organic contaminants (such as parabens) adsorbed on catalysts can be oxidized on the photogenerated holes Equation (5) [
17]:
Photocatalytic ozonation has been used for the degradation of emerging contaminants. The degradation of ibuprofen using photocatalytic ozonation under visible light irradiation with the catalyst WO
3 was studied and verified total removal in less than 20 min [
19]. The effect of Pt loading onto TiO
2 surface was studied for the oxidation of formaldehyde and the 0.6 wt % loading showed the highest dispersion of the dopant over the surface of the catalyst [
20]. An increase of Ag loading onto TiO
2 surface can block the UV-light thus affecting the photocatalytic activity and for Ag loading of 1.6 wt % the photogenerated hole trapping effect was negligible. Moreover, an increase of Ag loading can increase the photogenerated electron scavenging [
21]. The effect of doped TiO
2 at 0.5 wt % loading (Ag, Au, Pd and Pt) and pure TiO
2 was compared on the mixture of parabens degradation using UVA radiation coupled with ozone [
22]. Thus, the effect of metal loading onto TiO
2 must be carefully analyzed. The photodegradation using Ag
3PO
4 under simulated solar radiation to promote the degradation of ethyl paraben was studied taking into account pH, initial parabens concentration, catalyst concentration and water matrix [
23]. The photocatalytic degradation of paracetamol promoted by TiO
2 can be influenced by different features of catalysts such as surface acidity [
24].
Bearing this in mind, the aim of the present study is to verify the effect of noble metal loading onto TiO
2 surface over the efficiency of photocatalytic ozonation of a mixture of five parabens, methylparaben (MP), ethylparaben (EP), propylparaben (PP), butylparaben (BuP) and benzylparaben (BeP). For this purpose, the noble metals Ag, Pd and Pt, at loadings of 0.1, 0.5 and 1 wt %, doped onto TiO
2 were tested using ozone and UV-A irradiation. In order to understand photocatalytic ozonation promoting the mineralization, chemical oxygen demand (COD) and total organic carbon (TOC) were analyzed. Furthermore, the parabens are toxic compounds, so another goal of this work is to verify the ecological impact of raw and treated solutions. For this, the toxicity assessment over three different species
Vibrio fischeri, Corbicula fluminea and
Lepidium sativum was performed. The results achieved with the loading of 0.5 wt % onto TiO
2 were obtained from a previous work [
22]. The present study allows for understanding that the increase of noble metals load does not necessarily mean an increase in the parabens degradation, TOC and COD removal efficiency. This analysis is important due to the compromise between efficiency and cost of doped catalysts. The increase in noble metal load over titanium dioxide surface can promote a significant impact in terms of investment cost, which is not probably followed by an increase in the efficiency of the process. Moreover, to the best of our knowledge, no studies have been made for analysis of the effect of different loads of noble metals on parabens degradation through the photocatalytic ozonation process. In addition, this work also takes into account the effect of the different loadings over toxicity reduction. This analysis was performed taking into account the possibility of leading to wastewater reuse. Thus, the impact over different species (plant, clam, bacteria) gives a broader overview about the potential of real application of this methodology to remove emerging contaminants aiming water reuse.
3. Results and Discussion
3.1. Catalysts Characterization
XRD analysis reveals that the predominant phase is anatase (results not shown). However, rutile and brookite were also identified in a smaller amount. The diffractogram of pure TiO
2 was presented in a previous study [
29]. The presence of the doping metals on TiO
2 does not change the XRD pattern, independently of the loading of noble metals considered. The explanation for the absence of diffractive peak of noble metals is due to the low doping dose. For 1.5 wt % of Pt-TiO
2, the diffractive peak of Pt on XRD analysis was not found due to the low amount doping [
30]. The presence of gold, platinum and silver at 1 wt % load does not change the diffractive peaks of anatase phase from TiO
2 [
11]. These studies reveal that the lack of detection in the XRD analysis of diffractive peaks of noble metals for different loads used in our study could also be explained by the low doped dose and/or small size of noble metals’ nanoparticles.
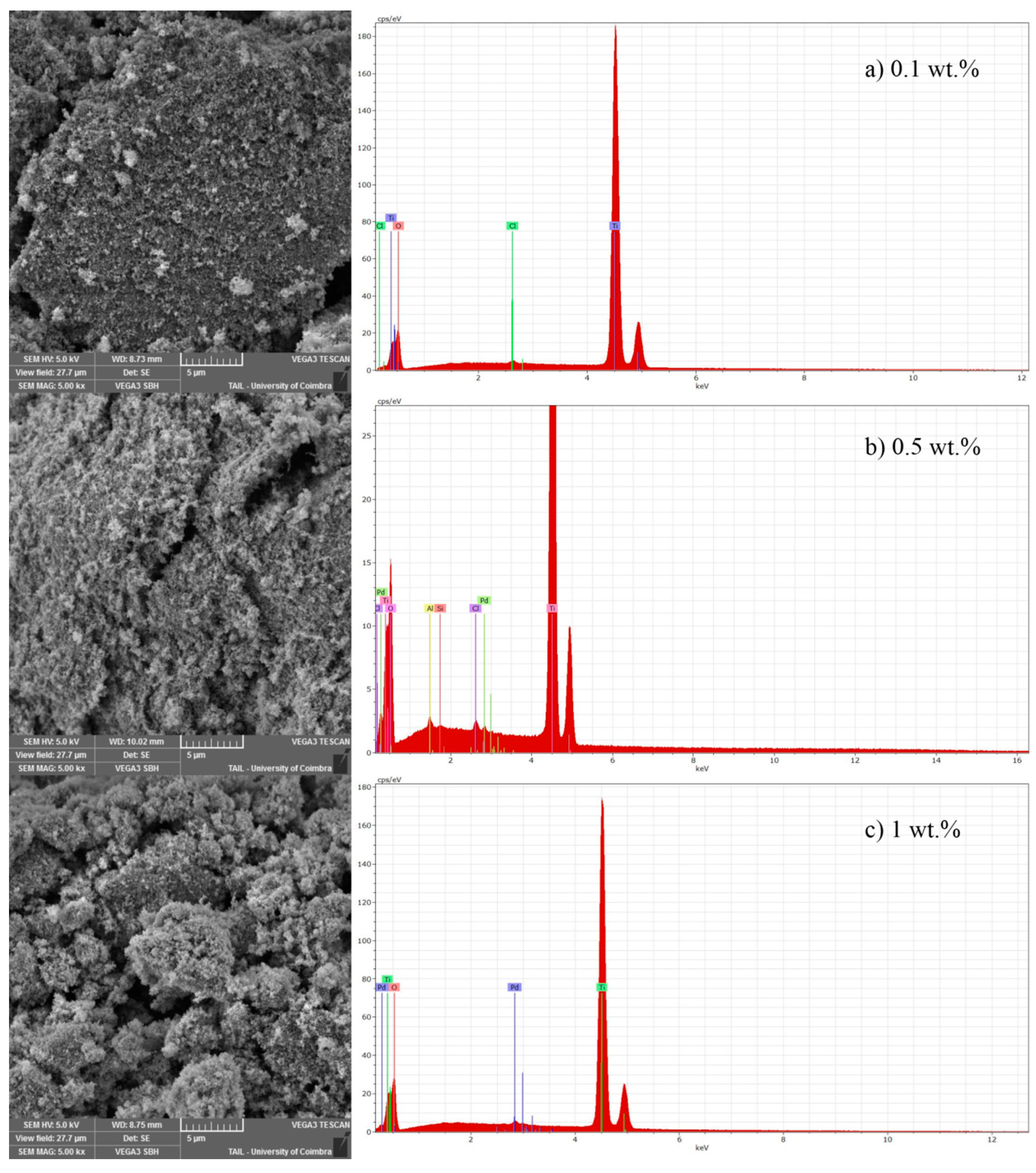
The SEM-EDS analysis was made for all catalysts used in these experiments. As an example, in
Figure 1, the results for different loads of Pd onto TiO
2 were presented.
SEM analysis (
Figure 1) shows that catalysts prepared by photodeposition method reveal a certain roughness on their surfaces, the morphology is not very clean, or the shape is not well designed (magnification of 5000×). For all catalysts with 0.1 and 0.5 wt % loading of noble metal, the SEM analysis was similar, the shape does not change with the increase of noble metal loading (
Figure 1). On the other hand, for 1 wt % of Pd-TiO
2, the shape of catalysts changed comparatively to 0.1 and 0.5 wt % (
Figure 1), leading to agglomerates. Another aspect that seems to be relevant is related with the morphology when increasing the catalyst load. Regarding 0.1 wt % of Pd-TiO
2, it is possible to see a lower amount of white particles that can be related with palladium. When Pd load increases from 0.1 wt % to 0.5 wt %, the number of white particles also increases significantly (
Figure 1a,b). From 0.5 to 1 wt %, the same behavior occurs (
Figure 1b,c) but at lower extension comparatively to 0.1–0.5 wt %. For the first range, the noble metal load increases five times, while, from 0.5 wt % to 1 wt %, it just increases two times. The increase of one noble metal loading promotes microsphere formation. The EDS analysis made to randomly selected points of SEM image presents, for the 0.1% of Pd-TiO
2 titanium, oxygen and chlorine as the main elements, whereas the noble metal is not detected. This can be explained by the low amount of doping metal used and its good dispersion over the support. The presence of chlorine in the spectrum of 0.1% Pd-TiO
2 comes from the precursor used to prepare this catalyst. For 0.5 wt %, the presence of aluminium and silicon was also identified, which means that the sample could be contaminated from the precursor of Pd (PdCl
2) and/or TiO
2 (Titanium (IV) isopropoxide). At this loading, it is possible that the selected points from SEM to perform the EDS analysis present a low amount of Pd. This can be related with poor dispersion of noble metal onto TiO
2 surface for the 0.5 wt % loading. The weight percentage values determined with EDS analysis are very small due to the low loading tested. Only for 1 wt % Pd loading was it possible to quantify about 0.5 wt % in the randomly selected point of EDS analysis. This reinforces the possible poor dispersion of noble metal onto TiO
2 for higher loadings. The XPS analysis was conducted for the lowest load of Pt doped TiO
2 to prove the presence of noble metal at this load (
Figure S1). In this analysis, 0.08 wt % of Pt onto TiO
2 surface was identified (
Table S1). This value is near the expected value.
Thermogravimetric analysis was conducted to infer the thermal resistance of catalysts when exposed to high temperatures. The weight loss of catalysts for different noble metal loads as functions of temperature was analyzed.
Thermal analysis reveals that catalysts are very stable thermally, since the highest weight loss was just 3%. The presence of different loads of noble metals does not affect significantly the weight loss. For example, in the case of Pd, the maximum weight loss was verified with 0.5 wt % (about 1.5%). This catalyst presents some water on its composition because the greatest weight loss occurs from 25 °C to 100 °C. At 350 °C, another negative variation was verified, which means that the organic contaminants partially identified by SEM-EDS analysis were eliminated at those conditions. The difference in the noble metal load does not change the preparation method. This means that the organic compounds and water present at the catalysts are the same for the different loads. Therefore, the weight loss profile during the TGA is similar
3.2. Effect of Noble Metal Loading on the Parabens Degradation
Photocatalytic ozonation with Ag, Pd and Pt doped onto TiO
2 catalysts was used to promote the degradation of the mixture of parabens. The effect of different noble metals loads on parabens degradation was analysed as a function of TOD. The UVA radiation is not able to effectively degrade parabens, and just 3% of removal was achieved [
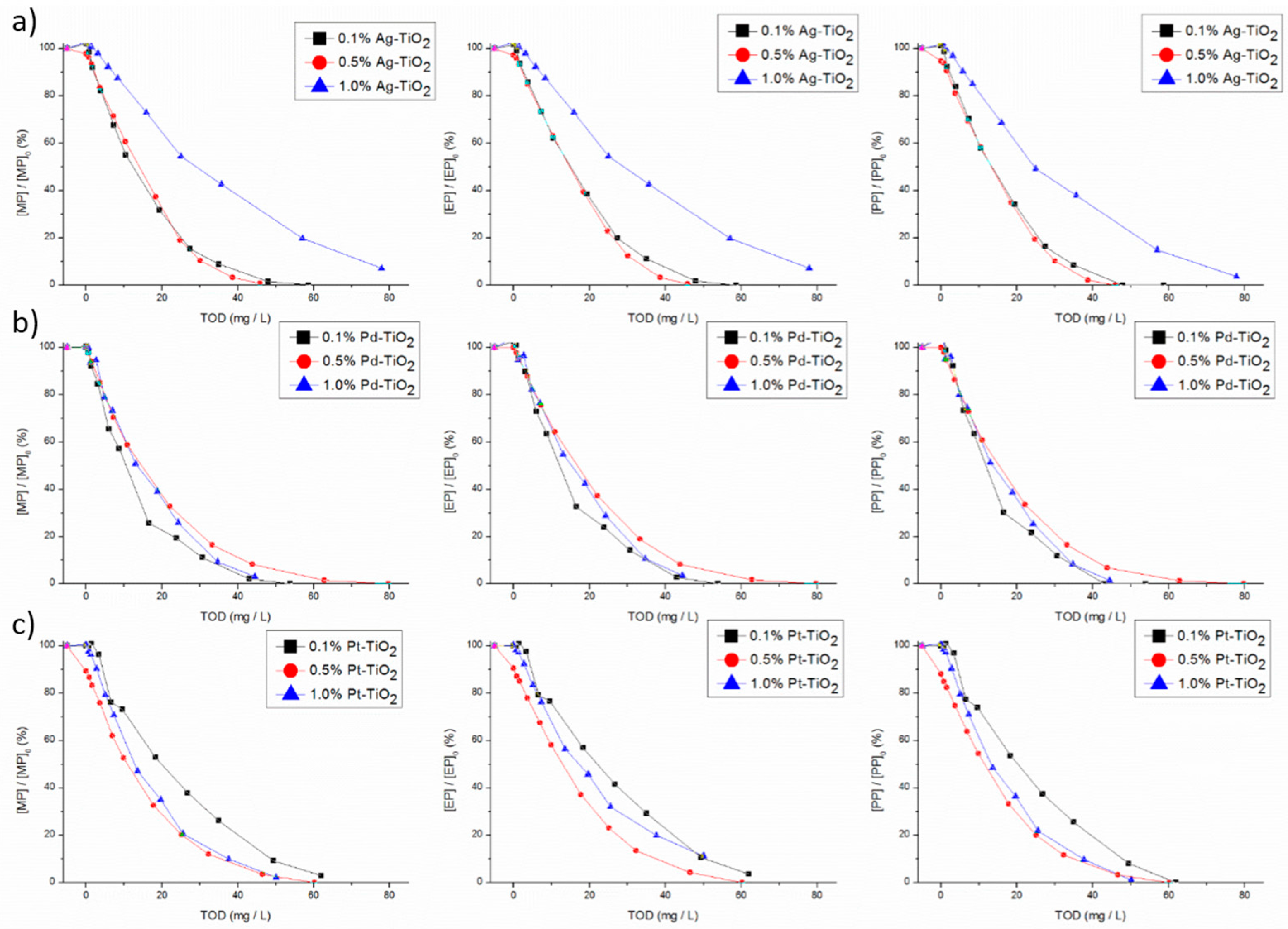
29]. On the other hand, for ozone alone, total parabens degradation needed an increase on TOD of 3-fold compared with photocatalytic ozonation. Therefore, the coupling of these two technologies substantially increases the efficiency of parabens degradation comparatively to the contribution of each one alone. As it can be seen, the parabens were all almost totally removed independently of the conditions tested (
Figure 2). Except for 1 wt % of Ag using a TOD of 78 mg/L, the total degradation for MP, EP and PP was not achieved, contrarily, to what happens for BuP and BeP. In the same way, for 0.1 wt % Pt, total degradation of MP and EP was also not observed. The results for BeP and BuP are not shown. The higher molecular weight compounds (BuP and BeP) were the first ones to be totally degraded using a low amount of TOD (below 30 mg/L for BeP), whichever the catalyst. This may be explained by ozone reactive preference for high electronic density groups such as benzenic rings. The high molecular weight of these compounds leads to a larger number of sites available for reaction. On the other hand, for the parabens with lower molecular weight, the degradation rate was slower, so a higher amount of ozone was necessary for total removal.
Tay et al. (2010) proved that the ozonation degradation constant rates increased with the molecular chain length for the different parabens (MP, EP, PP, BuP). The degradation of our mixture follows this behaviour, namely degradation happens first for BeP/BuP and finally MP [
4]. The kinetics of degradation reactions may be highly dependent upon the matrix [
31,
32]. A study with an emerging pollutant mixture of Tetracycline, Caffeine, Paracetamol and Atenolol verified that the effect of using a mixture was negligible on the decrease of mineralization efficiency [
31]. Therefore, comparing with our results, the synergistic effect of mixture could be minimal on the parabens degradation. When real water is used, the inorganic salts present in the matrix, especially carbonates, could decrease the removal efficiency [
32]. On the other hand, some inorganic compounds such as sulfate and chlorine can improve the reverse effect, increasing the efficiency of emerging contaminants removal [
33].
The results clearly show that, for photocatalytic ozonation, the worst catalyst was 1% Ag-TiO
2. For example, for achieving 95% of MP degradation with this catalyst, a TOD of 78 mg/L was necessary, while, for the best catalyst (0.1 and 0.5% Ag-TiO
2), total MP degradation just needed a TOD of 44 and 39 mg/L, respectively. Thus, for Ag-TiO
2, the increase in the metal load above 0.5 wt % leads to lower efficiency towards parabens removal. However, for Pt-TiO
2 on MP and PP degradation, the decrease in metal loading to 0.1 wt % leads to a process needing a higher amount of ozone compared to 0.5 and 1 wt % of Pt that present similar behavior. Regarding to Pd-TiO
2,, in a general way, its efficiency seems to be independent of Pd loading (
Figure 2).
The effect of Pt loading (0.01 wt % to 1 wt %) onto TiO
2 surface was studied for the oxidation of formaldehyde and concluded that 0.6 wt % was the most active material due to the highest dispersion of the dopant over the surface of the catalyst [
20]. This is in accordance with the results obtained in this study, where 0.5 wt % is the most active load for Pt and Ag. The lowest presence of metal loading on TiO
2 surface may reduce the scavenging effect of photogenerated electrons, so the recombination phenomenon of electron–hole pairs can be more recurrent, which can explain the low efficiency of 0.1 wt % in Pt-TiO
2. Therefore, less electrons are available for ozone reduction and few holes may be available to react with adsorbed water to produce hydroxyl radicals, thus reducing the pollutants’ oxidation efficiency.
Regarding Ag based catalysts, the effect of metal loading until 0.5 wt % was not so sharp in what concerns parabens degradation. The increase of Ag loading from 0.1 wt % to 0.5 wt % allows for reducing about 10 mg/L of TOD needed for total parabens degradation. On the other hand, an increase in the metal load to 1 wt % promotes a decrease of the efficiency regarding parabens degradation, resulting in the increase of 30 mg/L of TOD required for parabens depletion. Ag presents a lower electronegativity compared with Pt and Pd (1.93 to 2.28 and 2.20 according to the Pauling scale, respectively). In this way, lower amounts of electrons can be scavenged by Ag. However, when the metal load increases to 1 wt %, the trap of electrons could be higher and the electrons trapped by Ag were not available for reaction. This can be an explanation for the reduction of efficiency on parabens degradation with this load of silver. For 0.1 and 0.5 wt % of Ag load, the photogenerated electrons are still available to reduce ozone to produce ozonide and hydroxyl radicals. This larger amount of hydroxyl radicals may be helpful for parabens degradation, reducing the amount of TOD needed. Moreover, another explanation for this efficiency reduction can be related to the photohole trapping promoted by a higher load of Ag onto TiO
2 [
34]. However, Mohamed and Khairou say that, up to 1.6 wt % of Ag, this trapping effect was negligible [
21]. On the other hand, the excess of Ag may have led to lower noble metal dispersion over titanium dioxide or even to a shielding effect, not allowing TiO
2 to efficiently absorb radiation so that less photo-electrons and holes were generated.
The recombination phenomenon can be an explanation for the reduction in the efficiency for a lower load of Pt. The noble metal doped onto TiO
2 can reduce the energy band gap [
11,
35], which is the energy needed to move an electron from the valence band to the conduction band of the catalyst, producing photogenerated holes on the surface of the catalyst [
8,
10]. In addition, the presence of noble metals can reduce the recombination phenomenon between electron–hole pairs due to the scavenging of photogenerated electrons [
35]. In this way, the lower amount of Pt minimizes the effect of scavenging electrons, which means an increase of the recombination phenomenon. Therefore, the ozone reduced and the water oxidation are lower and consequently less production of ozonide and hydroxyl radicals is possible.
The global analysis of the parabens decay results points out that the most efficient catalysts regarding parabens degradation were 0.1, 0.5% Ag-TiO2.
3.3. COD and TOC Removal
Table 1 summarizes COD and TOC removal after 120 min of photocatalytic ozonation for different catalysts. The catalysts lead to slightly different COD and TOC removals. The poorest catalyst on the COD removal, without considering that the TOD used was 0.1% Pd-TiO
2 with only 32%. In the same way, TOC removal presents a similar result. Even if this catalyst presents a good performance regarding parabens degradation, the reaction follows the oxidation pathway leading to the formation of organic intermediates instead of carbon dioxide and water.
Taking TOD into consideration, the worst catalyst on the COD removal was 1% Ag-TiO2. The higher COD removal was attained with 1% Pd-TiO2 using lower TOD (44 mg/L). In addition, for the 1% Pt-TiO2, a similar COD removal occurs, but a higher TOD was necessary. Therefore, generally high COD removal can be achieved for all catalysts if TOD increases. Thus, the effect of the amount of ozone is not negligible. Nevertheless, TOC removal does not necessarily follow the same trend since, for some cases where TOD is high (for example for 1% Ag-TiO2, TOD = 78 mg O3/L), the mineralization of treated solution is reduced (only 31% of TOC removal). According to this, it is important to analyze the TOC removal besides COD. In terms of TOC removal as a function of TOD, the best result was achieved with 0.5% Ag-TiO2 (46 mg O3/L and 37% of mineralization).
In the case of silver, the analysis of the effect of metal load on TOC and COD removal reflects the fact that the increasing amount of Ag decreases TOC and COD removal even using higher TOD (
Table 1). For palladium, the increase in metal load promotes a higher TOC and COD removal, which differs from what happens regarding parabens degradation. For platinum in terms of TOC removal, the differences could be explained by the different TODs. On the other hand, an increase of metal load promotes a slight improvement in the COD removal, even using lower TOD, which is in accordance with the results achieved for parabens degradation. For the global analysis, the best results in terms of COD and TOC removal were 0.5% Ag-TiO
2 and 1% Pd-TiO
2.
These two parameters (COD and TOC removal) are very dependent upon TOD. The analysis for COD and TOC removal was made for the final time of reaction that necessarily corresponds to different TOD values for the different catalysts. In order to understand if the mixture of parabens degradation follows mainly partial oxidation or mineralization pathway, the ratio between COD or TOC and TOD was analyzed.
Table 2 presents the COD and TOC removal per TOD for all photocatalytic systems tested. From
Table 2, it was possible to see that higher COD removal in the function of TOD was achieved for 1 wt % of Pd doped onto TiO2. This result indicates that the parabens degradation using this catalyst progressed towards a partial oxidation pathway. On the other hand, for TOC removal, the best result was achieved for 0.5 wt % Ag-TiO2. Thus, for that catalyst, mineralization occurs to a larger extent.
The best results in terms of COD removal were achieved for higher loads of Pd and Pt. In terms of price, these catalysts present higher costs compared with the silver based materials. According to the current market values, 25–27 € is the cost per gram of palladium and platinum, while silver is obtained at 0.5 €/g of silver. This represents a high cost reduction. Therefore, accounting for the application costs, the most suitable solution for parabens degradation and COD and TOC removal would be the 0.1 and 0.5% of Ag-TiO2 catalyst.
3.4. Toxicity Assessment
Since total mineralization was not achieved during photocatalytic ozonation, it is important to analyze the toxic character of the treated samples in order to infer their potential impact over the ecosystems. Thus, the toxicity of the treated samples after 120 min of photocatalytic ozonation with the different catalysts was compared with the initial parabens mixture. Three species were tested: the luminescent bacteria Vibrio fischeri, the mollusk Corbicula fluminea and the plant Lepidium sativum.
The luminescence inhibition of
V. fischeri after 15 min in contact with treated samples decreased compared with the initial mixture of parabens (
Table 3). Miralles-Cuevas et al. verified that, for
V. fischeri light inhibition above 30%, the samples must be considered toxic [
36]. Therefore, in the present study, all of the treated samples present toxicity towards this very sensitive bacteria. The sample for 1% Ag-TiO
2, which led to the worst results in terms of parabens degradation, presents the highest light inhibition and lowest germination index, pointing out its highest toxicity. For silver and palladium, the intermediate metal load used (0.5%) leads to the lowest light inhibition. For both catalysts’ highest (1%) and lowest (0.1%) metal loads, toxicity increases compared to the intermediate metal load. In the case of platinum, the noble metal load does not affect significantly the luminescence inhibition (
Table 3).
The mortality of
C. fluminea strongly decreases when the individuals are in contact for 72 h with the treated solutions (120 min of photocatalytic ozonation) when compared with the initial mixture of parabens. In addition, 1% Pd-TiO
2 reveals the effectiveness on the parabens degradation, COD and TOC removal, but the sample presents higher toxicity towards
V. fischeri and
C. fluminea. This could be related with the by-products resulting from the photocatalytic ozonation process. The different metal loads used for Pt do not affect significantly the toxicity towards Asian clams. Contrarily, it seems that the decrease of Ag loading in the catalyst leads to a slightly more toxic treated mixture towards
C. fluminea (
Table 3).
After 48 h of contact with treated samples, the GI of
L. sativum increases, which means that the initial toxicity related with parabens was reduced. According to Trautmann and Krasny criteria, the initial parabens mixture is very phytotoxic, while the treated samples do not present phytotoxicity [
28]. The increase of metal load for Pt decreases the GI. As occurs with
V. fischeri, the intermediate load used for Ag and Pd leads to less toxic samples compared with the remaining tested loads. Moreover, the values of GI for the 0.5% of Ag and Pt are above 100%, which means that this treated solution can even improve
L. sativum germination.
In a global way, the best result in terms of toxicity removal over the different organisms tested was the one obtained by photocatalytic ozonation using 0.5% Ag-TiO
2 (
Table 3).