Identification and Characterization of a Novel Mannanase from Klebsiella grimontii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Expression and Purification of Beta-Endo-Mannanase Gene KgManA from K. grimontii
2.3. Activity Assay of KgManA
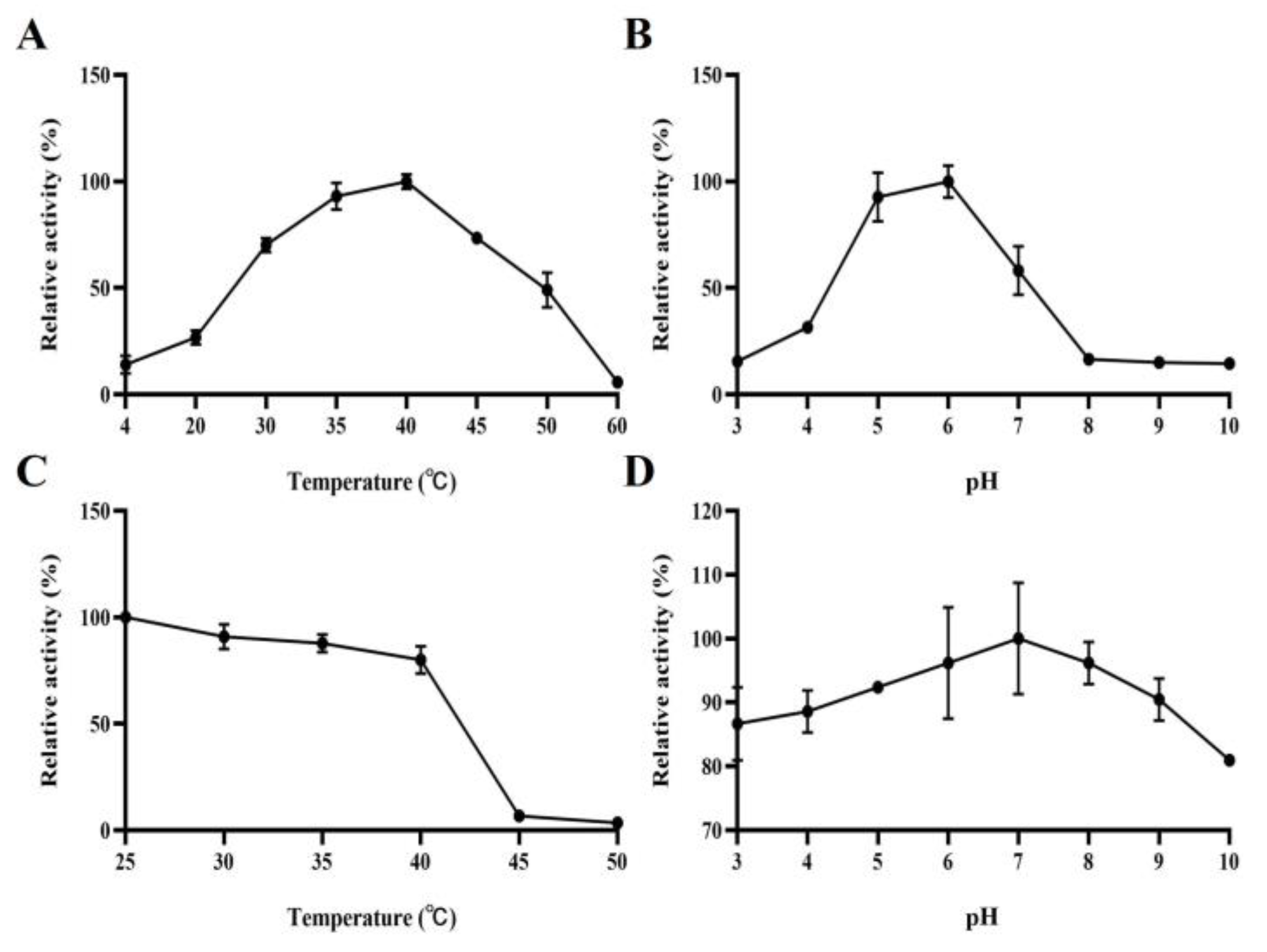
2.4. Biochemical Properties of KgManA
2.5. Hydrolysis Product Analysis of KgManA
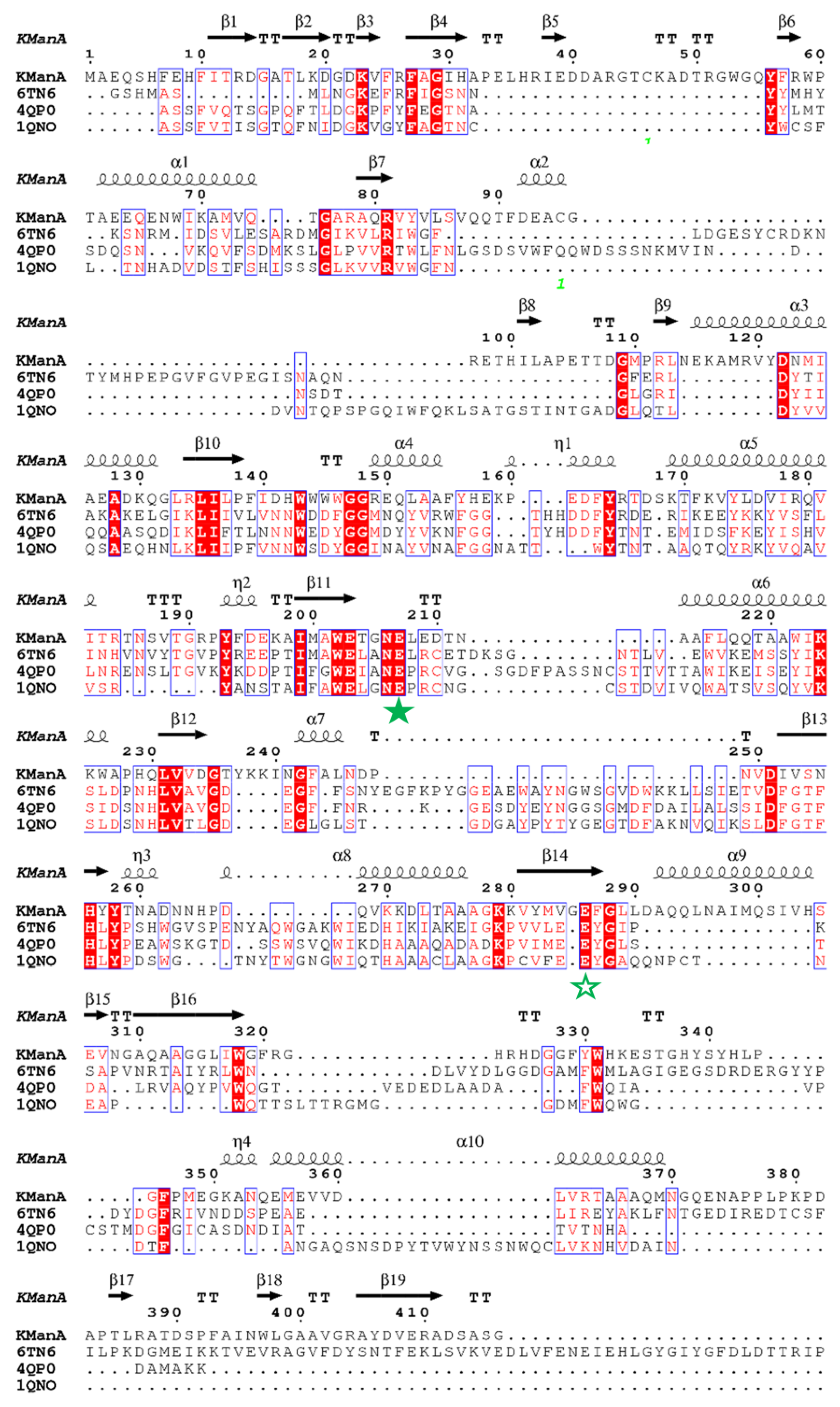
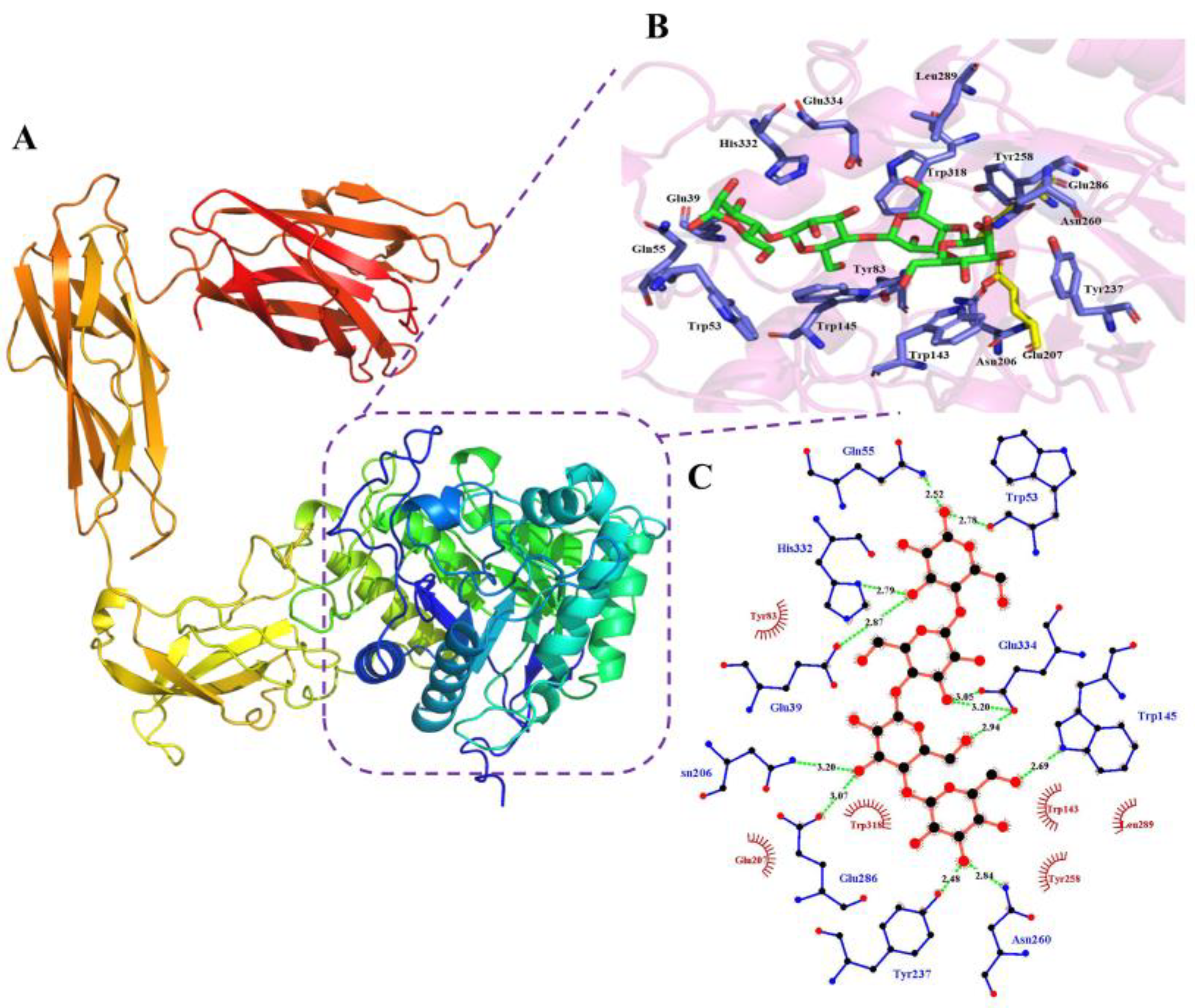
2.6. Homology Modeling and Analysis of the Degradation Mechanism
3. Results
3.1. Cloning, Expression and Purification of KgManA
3.2. Substrate Specificity of KgManA
3.3. Biochemical Properties of KgManA
3.4. Analysis of Hydrolysis Products of KgManA
3.5. In-Silico Analysis of the Mechanism of KgManA Degradation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, Y.; Zhang, S.; Sheng, L.; Ma, H.; Xu, F.; Waterhouse, G.I.N.; Sun-Waterhouse, D.; Wu, P. Food packaging films based on ionically crosslinked konjac glucomannan incorporating zein-pectin nanoparticle-stabilized corn germ oil-oregano oil Pickering emulsion. Food Chem. 2023, 429, 136874. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, Y.; Zhang, W.; Shi, S.; Zhu, W.; Wang, R.; Zhang, L.; Chen, L.; Sun, J.; Pang, J.; et al. Advanced konjac glucomannan-based films in food packaging: Classification, preparation, formation mechanism and function. LWT—Food Sci. Technol. 2021, 152, 112338. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. Int. J. Biol. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Shen, R.; Zhang, R.; Liu, L.; Yang, X. Konjac glucomannan-based edible films loaded with thyme essential oil: Physical properties and antioxidant-antibacterial activities. Food Packag. Shelf Life 2021, 29, 100700. [Google Scholar] [CrossRef]
- Du, Q.; Liu, J.; Ding, Y. Recent progress in biological activities and health benefits of konjac glucomannan and its derivatives. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100270. [Google Scholar] [CrossRef]
- Shi, W.; Wu, J.; Pi, Y.; Yan, X.; Hu, X.; Cheng, J.; Yu, H.; Shao, Z. E7 peptide enables BMSC adhesion and promotes chondrogenic differentiation of BMSCs via the LncRNA H19/miR675 axis. Bioengineering 2023, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, Y.; Hu, M.; Liu, Y.; Jiang, C.; Wang, Q.; Jin, Q.; Zhang, D.; Yin, Z.; Zhang, J. Comparative effects of different enzymatic hydrolysates of konjac glucomannan on gut flora and constipation in rats. Food Funct. 2022, 13, 8717–8729. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Du, F.; Yuan, Q. Multifunctional sodium hyaluronate/chitosan foam used as an absorbable hemostatic material. Bioengineering 2023, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zong, X.; Wang, S.; Yin, C.; Gao, X.; Xiong, G.; Xu, X.; Qi, J.; Mei, L. Emulsified blend film based on konjac glucomannan/carrageenan/camellia oil: Physical, structural, and water barrier properties. Carbohydr. Polym. 2021, 251, 117100. [Google Scholar] [CrossRef]
- Walayat, N.; Tang, W.; Nawaz, A.; Ding, Y.; Liu, J.; Lorenzo, J.M. Influence of Konjac oligo-glucomannan as cryoprotectant on physicochemical and structural properties of silver carp surimi during fluctuated frozen storage. LWT—Food Sci. Technol. 2022, 164, 113641. [Google Scholar] [CrossRef]
- Liu, J.; Fang, C.; Luo, Y.; Ding, Y.; Liu, S. Effects of konjac oligo-glucomannan on the physicochemical properties of frozen surimi from red gurnard (Aspitrigla cuculus). Food Hydrocoll. 2019, 89, 668–673. [Google Scholar] [CrossRef]
- Hayeeawaema, F.; Wichienchot, S.; Khuituan, P. Amelioration of gut dysbiosis and gastrointestinal motility by konjac oligo-glucomannan on loperamide-induced constipation in mice. Nutrition 2020, 73, 110715. [Google Scholar] [CrossRef]
- Ye, S.; Zongo, A.W.-S.; Shah, B.R.; Li, J.; Li, B. Konjac glucomannan (KGM), deacetylated KGM (Da-KGM), and degraded KGM derivatives: A special focus on colloidal nutrition. J. Agric. Food Chem. 2021, 69, 12921–12932. [Google Scholar] [CrossRef]
- Yang, J.; Vittori, N.; Wang, W.; Shi, Y.-C.; Hoeflinger, J.L.; Miller, M.J.; Pan, Y. Molecular weight distribution and fermentation of mechanically pre-treated konjac enzymatic hydrolysates. Carbohydr. Polym. 2017, 159, 58–65. [Google Scholar] [CrossRef]
- Song, Q.; Li, T.; Xue, W.; Li, N.; Chen, L.; Dai, S.; Zhu, Z. Preparation, structure analysis and ACE inhibitory activity of konjac oligosaccharide. Ind. Crop. Prod. 2018, 124, 812–821. [Google Scholar] [CrossRef]
- Duan, Z.; Wang, Y.; Yu, X.; Wu, N.; Pang, J.; Bai, Y. Effect of konjac oligo-glucomannan on emulsifying properties of myofibrillar protein. J. Sci. Food Agric. 2023, 103, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Xie, Y.; Shabani, K.I.; Liu, X. Preparation, characterization and physicochemical properties of Konjac glucomannan depolymerized by ozone assisted with microwave treatment. Food Hydrocoll. 2021, 119, 106878. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Yang, Y.; Ding, J.; Pang, J. Effect of γ-irradiation on some physiochemical properties of konjac glucomannan. Carbohydr. Polym. 2007, 70, 444–450. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Wang, H.; Tian, H.; Feng, X.; Tan, L.; Liu, X. Synergistic effects of oxidized konjac glucomannan on rheological, thermal and structural properties of gluten protein. Int. J. Biol. Macromol. 2023, 248, 125598. [Google Scholar] [CrossRef]
- Bangoria, P.; Patel, A.; Shah, A.R. Thermotolerant and protease-resistant GH5 family β-mannanase with CBM1 from Penicillium aculeatum APS1: Purification and characterization. 3 Biotech 2023, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Calley, J.N.; Ebert, P.J.; Helmes, E.B. Paenibacillus lentus sp. nov., a β-mannanolytic bacterium isolated from mixed soil samples in a selective enrichment using guar gum as the sole carbon source. Int. J. Syst. Evol. Microbio. 2014, 64, 1166–1172. [Google Scholar] [CrossRef]
- Saeed, M.; Tian, X.-L. Circulatory System. In Encyclopedia of Gerontology and Population Aging; Gu, D., Dupre, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–8. [Google Scholar]
- Chen, C.Y.; Huang, Y.C.; Yang, T.Y.; Jian, J.Y.; Chen, W.L.; Yang, C.H. Degradation of konjac glucomannan by Thermobifida fusca thermostable β-mannanase from yeast transformant. Int. J. Biol. Macromol. 2016, 82, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2013, 42, D490–D495. [Google Scholar] [CrossRef]
- Gilbert, H. The biochemistry and structural biology of plant cell wall deconstruction. Plant Physiol. 2010, 153, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, J.; Li, C.; Wang, T.-F.; Qin, H.-M. Biochemical and structural characterization of a novel thermophilic and acidophilic β-mannanase from Aspergillus calidoustus. Enzym. Microb. Technol. 2021, 150, 109891. [Google Scholar] [CrossRef] [PubMed]
- von Freiesleben, P.; Spodsberg, N.; Blicher, T.H.; Anderson, L.; Jørgensen, H.; Stålbrand, H.; Meyer, A.S.; Krogh, K.B.R.M. An Aspergillus nidulans GH26 endo-β-mannanase with a novel degradation pattern on highly substituted galactomannans. Enzym. Microb. Technol. 2016, 83, 68–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, J.; Peng, H.; Gao, F.; Zhou, C.; Zeng, Y.; Xue, Y.; Li, Y.; Henrissat, B.; Gao, G.F.; et al. Biochemical and structural characterization of the intracellular mannanase AaManA of Alicyclobacillus acidocaldarius reveals a novel glycoside hydrolase family belonging to clan GH-A*. J. Biol. Chem. 2008, 283, 31551–31558. [Google Scholar] [CrossRef]
- Dhawan, S.; Kaur, J. Microbial mannanases: An overview of production and applications. Crit. Rev. Biotechnol. 2007, 27, 197–216. [Google Scholar] [CrossRef]
- Dawood, A.; Ma, K. Applications of Microbial β-Mannanases. Front. Bioeng. Biotechnol. 2020, 8, 598630. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, R.; Liu, X.; Shi, J.; Xu, Z.; Rao, Z. Improving the acidic stability of a β-mannanase from Bacillus subtilis by site-directed mutagenesis. Process Biochem. 2013, 48, 1166–1173. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef]
- Mirdita, M.; Schutze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, Y.; Yan, Q.; Chen, Z.; Qin, Z.; Jiang, Z. Structural insights into the substrate specificity and transglycosylation activity of a fungal glycoside hydrolase family 5 beta-mannosidase. Acta Crystallogr. Sect. D-Struct. Biol. 2014, 70, 2970–2982. [Google Scholar] [CrossRef]
- Yang, J.K.; Chen, Q.C.; Zhou, B.; Wang, X.J.; Liu, S.Q. Manno-oligosaccharide preparation by the hydrolysis of konjac flour with a thermostable endo-mannanase from Talaromyces cellulolyticus. J. Appl. Microbiol. 2019, 127, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Lu, H.; Xia, M.; Cui, Y.; Bai, Y.; Qian, L.; Shi, P.; Luo, H.; Yao, B. A novel glycoside hydrolase family 113 Endo-β-1,4-mannanase from Alicyclobacillus sp. strain A4 and insight into the substrate recognition and catalytic mechanism of this family. Appl Env. Microbiol 2016, 82, 2718–2727. [Google Scholar] [CrossRef]
- Liu, W.; Ma, C.; Liu, W.; Zheng, Y.; Chen, C.-C.; Liang, A.; Luo, X.; Li, Z.; Ma, W.; Song, Y.; et al. Functional and structural investigation of a novel β-mannanase BaMan113A from Bacillus sp. N16-5. Int. J. Biol. Macromol. 2021, 182, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Lehtovaara, B.C.; Gu, F.X. Pharmacological, Structural, and Drug Delivery Properties and Applications of 1,3-beta-Glucans. J. Agric. Food Chem. 2011, 59, 6813–6828. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of beta-glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Ozen, I.; Bahtiyari, M.I.; Haji, A.; ul Islam, S.; Wang, X. Properties of galactomannans and their textile-related applications—A concise review. Int. J. Biol. Macromol. 2023, 227, 1001–1014. [Google Scholar] [CrossRef]
- Ghebremedin, M.; Schreiber, C.; Zielbauer, B.; Dietz, N.; Vilgis, T.A. Interaction of xanthan gums with galacto- and glucomannans. Part II: Heat induced synergistic gelation mechanism and their interaction with salt. J. Phys.-Mater. 2020, 3, 034014. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications—A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A Review. J. Food Sci. Technol. -Mysore 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F.M.; Milas, M.; Rinaudo, M. Associative phenomena in galactomannan-deacetylated xanthan systems. Int. J. Biol. Macromol. 2001, 29, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yao, Y.; Li, Y.; Dong, S. Effect of cross-linking and oxidization on structure and properties of sesbania gum. Int. J. Biol. Macromol. 2018, 114, 640–648. [Google Scholar] [CrossRef]


| Purification Process | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Purification Factor | Yield (%) |
|---|---|---|---|---|---|
| Crude enzyme | 2234.93 | 502.78 | 0.22 | 1.00 | 100 |
| NTA-Ni resin | 9.60 | 2.33 | 0.24 | 1.08 | 0.46 |
| Substrate | Hydrolytic Activity (U/mg) | Relative Activity (%) |
|---|---|---|
| KGM | 0.43 ± 0.04 * | 34.40 ± 3.20 |
| β-1,4-D-mannan | 1.25 ± 0.01 * | 100.00 ± 0.80 |
| carboxymethyl cellulose | NA 1 | NA 1 |
| curdlan | NA 1 | NA 1 |
| locust bean gum | NA 1 | NA 1 |
| fenugreek gum | 0.04 ± 0.01 * | 3.20 ± 0.80 |
| guar gum | NA 1 | NA 1 |
| Sesbania gum | NA 1 | NA 1 |
| zymosan A | NA 1 | NA 1 |
| tara gum | NA 1 | NA 1 |
| Enzyme Kinetic Parameters | Value |
|---|---|
| Km | 9.04 ± 1.11 mg/mL |
| Vmax | 0.77 ± 0.18 μmol/(min·mg) |
| Kcat | 1.53 ± 0.36 min−1 |
| Kcat/Km | 0.17 ± 0.03 mg−1·mL−1·min−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Li, K.; Li, T.; Li, J.; Liu, Q.; Yin, H. Identification and Characterization of a Novel Mannanase from Klebsiella grimontii. Bioengineering 2023, 10, 1230. https://doi.org/10.3390/bioengineering10101230
Chen C, Li K, Li T, Li J, Liu Q, Yin H. Identification and Characterization of a Novel Mannanase from Klebsiella grimontii. Bioengineering. 2023; 10(10):1230. https://doi.org/10.3390/bioengineering10101230
Chicago/Turabian StyleChen, Changzheng, Kuikui Li, Tang Li, Junyan Li, Qishun Liu, and Heng Yin. 2023. "Identification and Characterization of a Novel Mannanase from Klebsiella grimontii" Bioengineering 10, no. 10: 1230. https://doi.org/10.3390/bioengineering10101230






