Novel Fabrication of 3-D Cell Laden Micro-Patterned Porous Structure on Cell Growth and Proliferation by Layered Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GelMA Synthesis
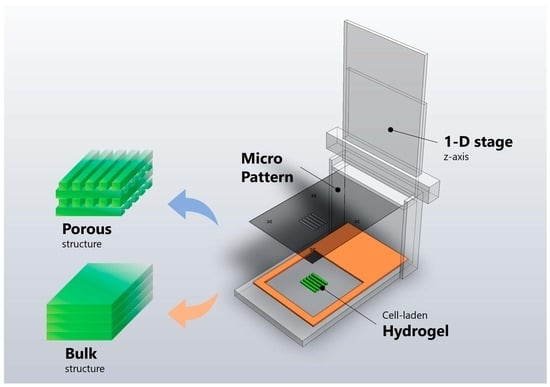
2.3. Design of Micro-Patterned Porous Structures
2.4. Hydrogel Preparation
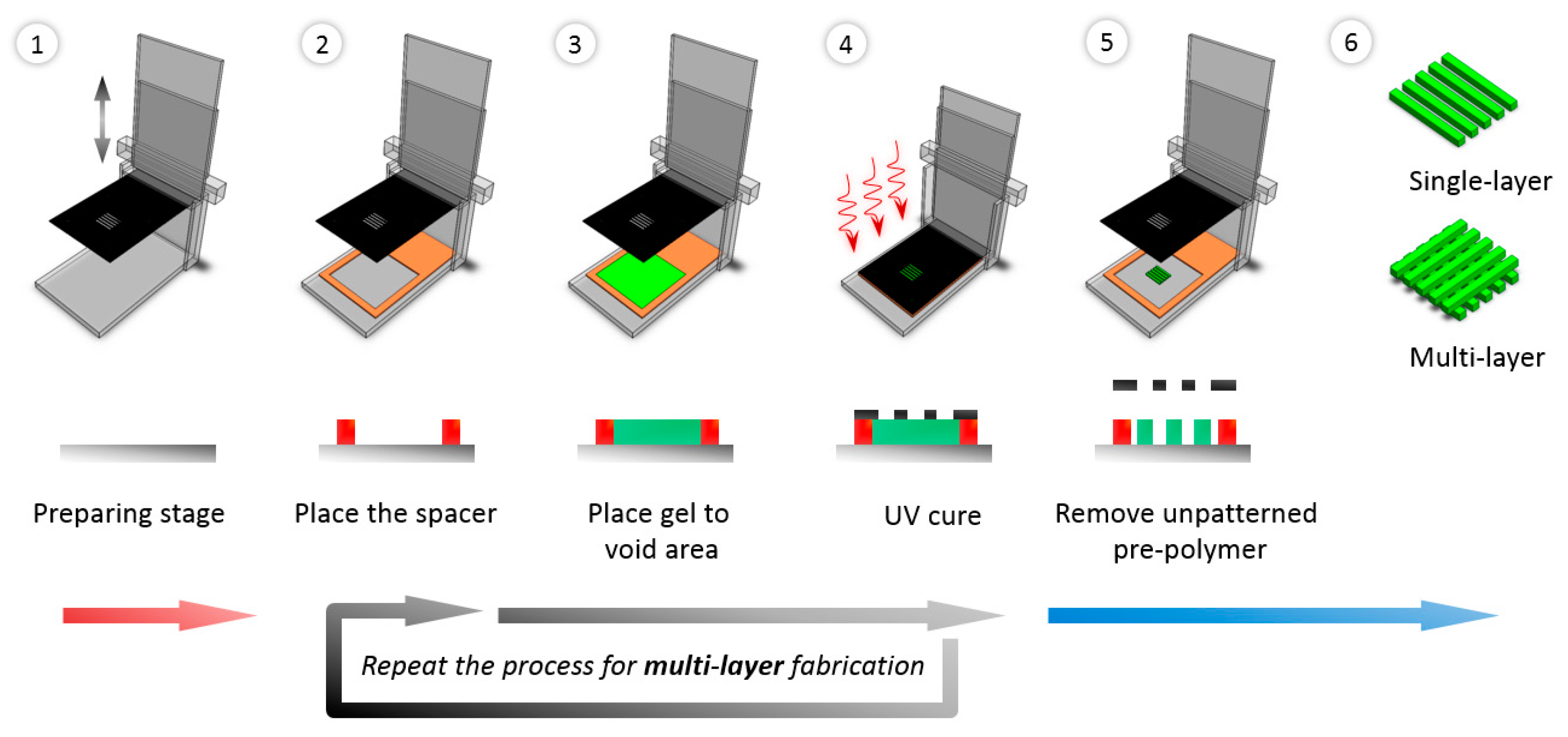
2.5. Fabrication of Micro-Patterned Porous Structures
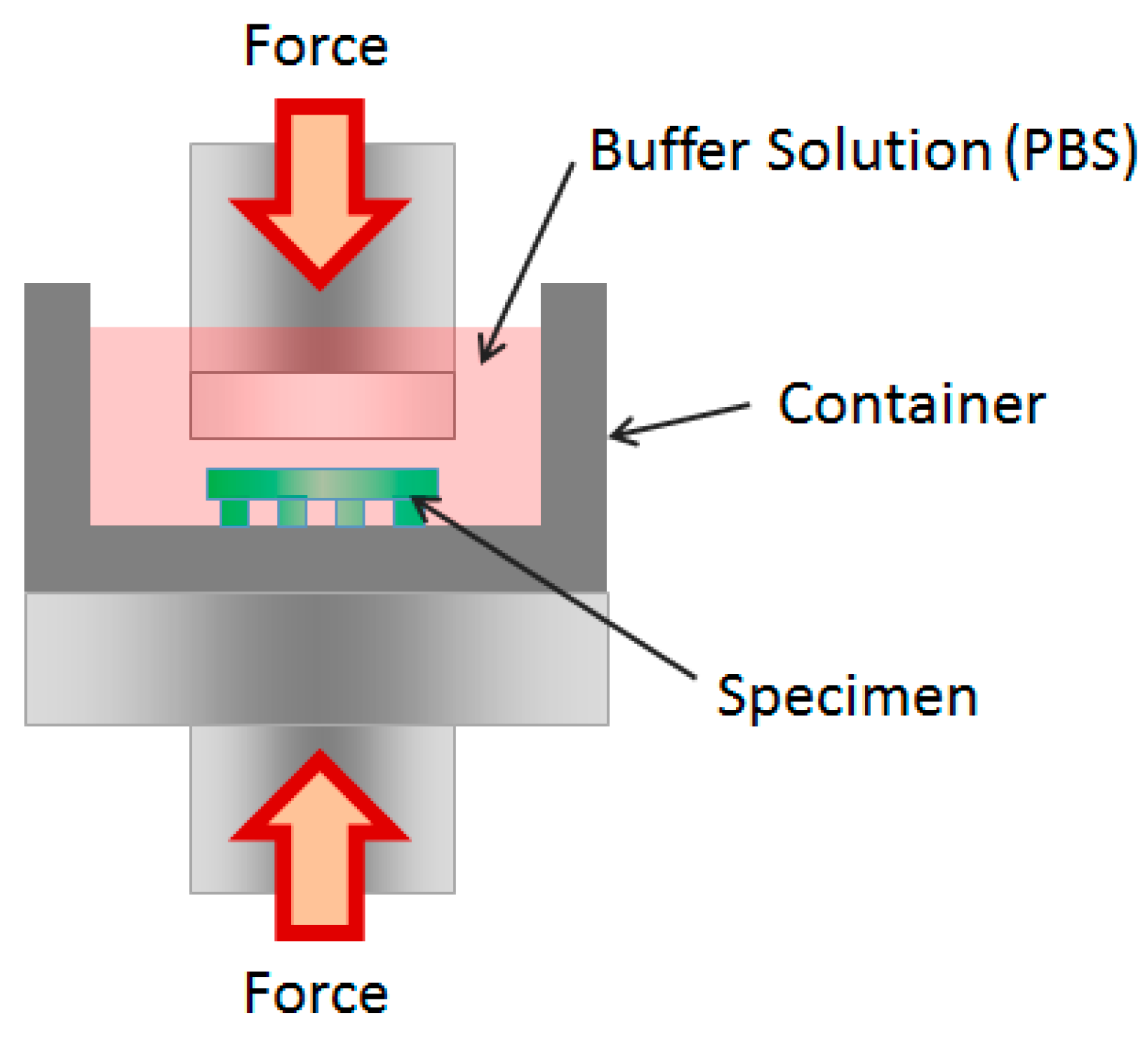
2.6. Mechanical Tests
2.7. Cell Culture and Cell Seeding
2.8. Cell Activity
2.8.1. MTT Assay
2.8.2. Alkaline Phosphatase (ALP) Activity
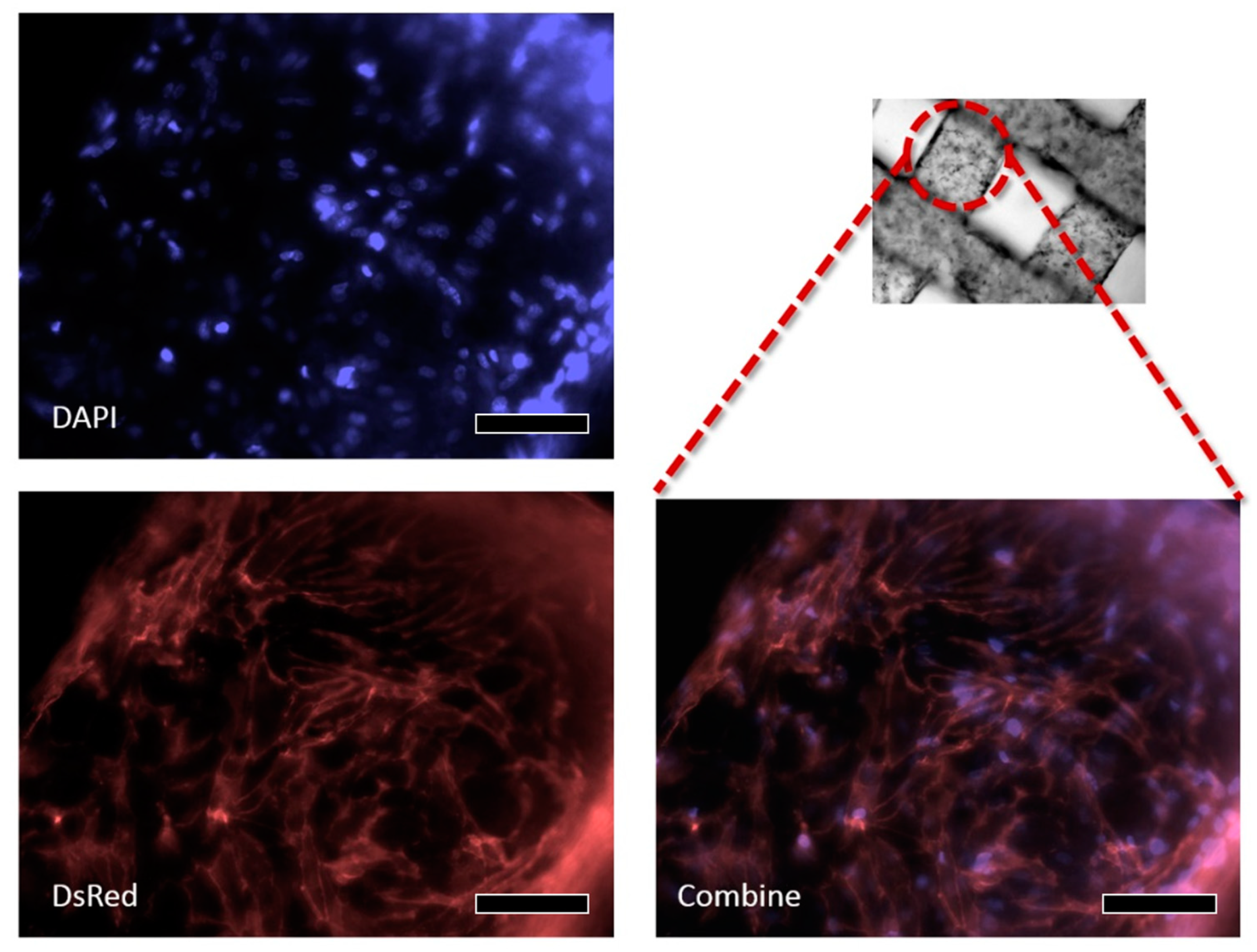
2.8.3. Immunofluorescence Staining
3. Results and Discussion
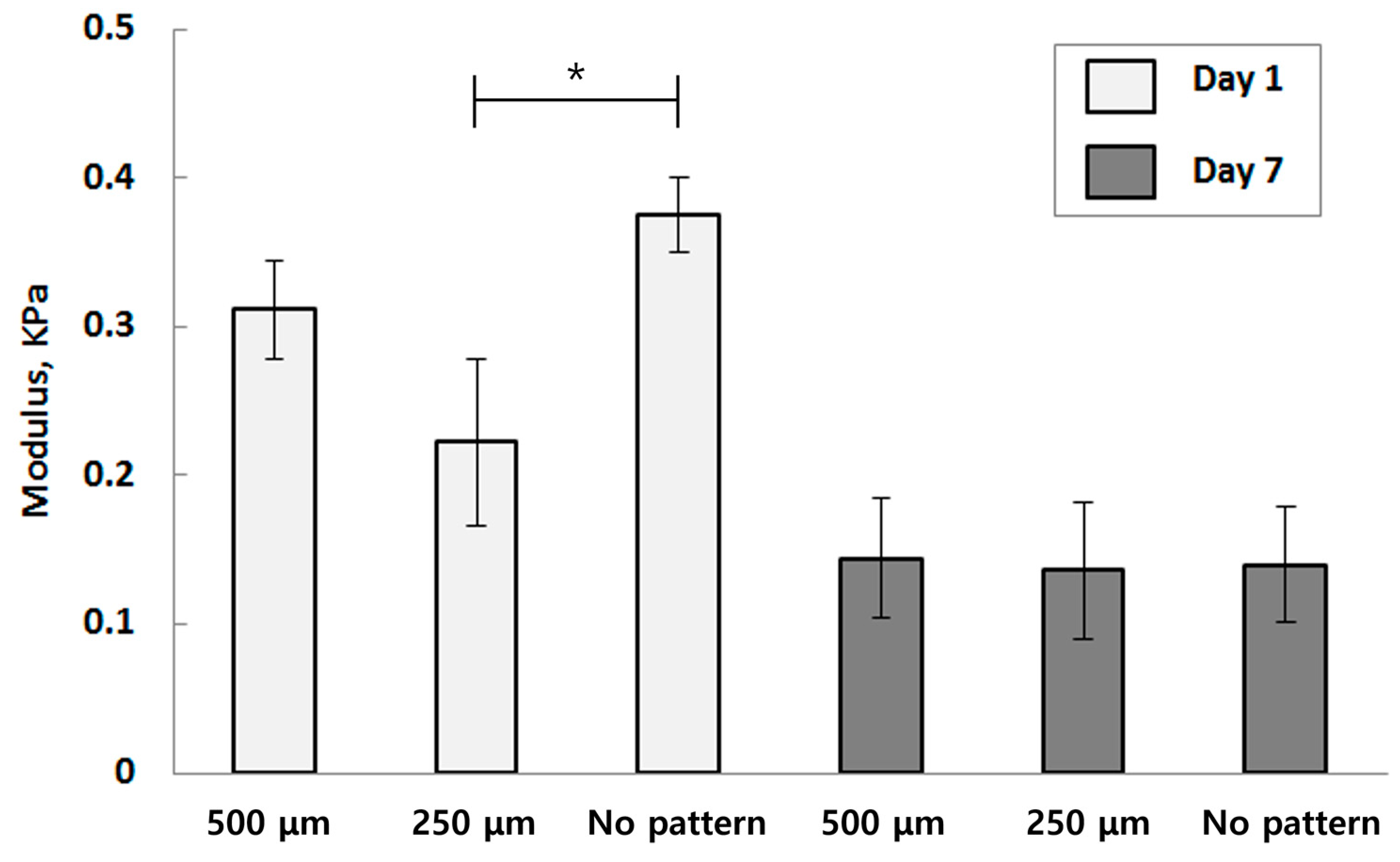
3.1. Mechanical Characterization
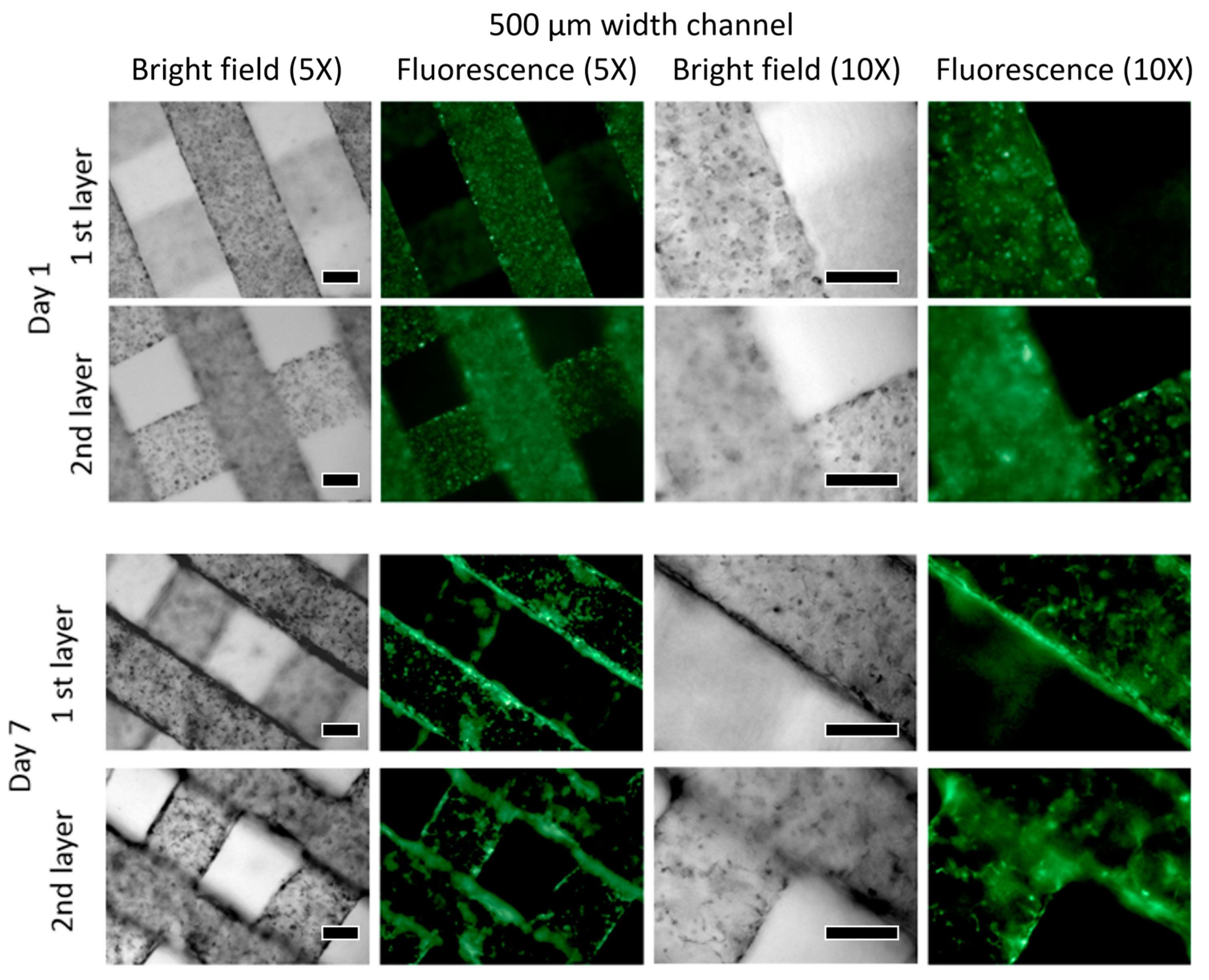
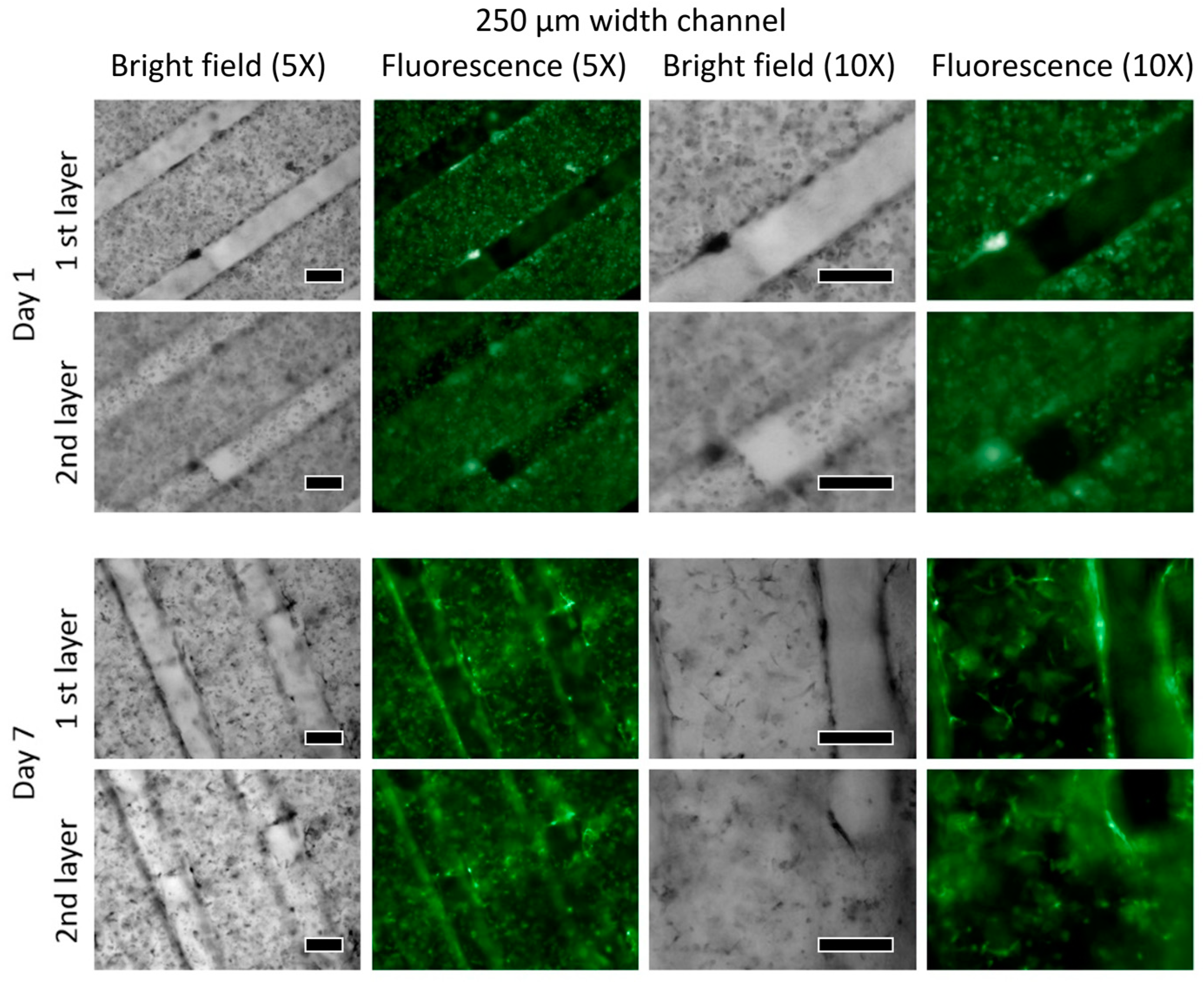
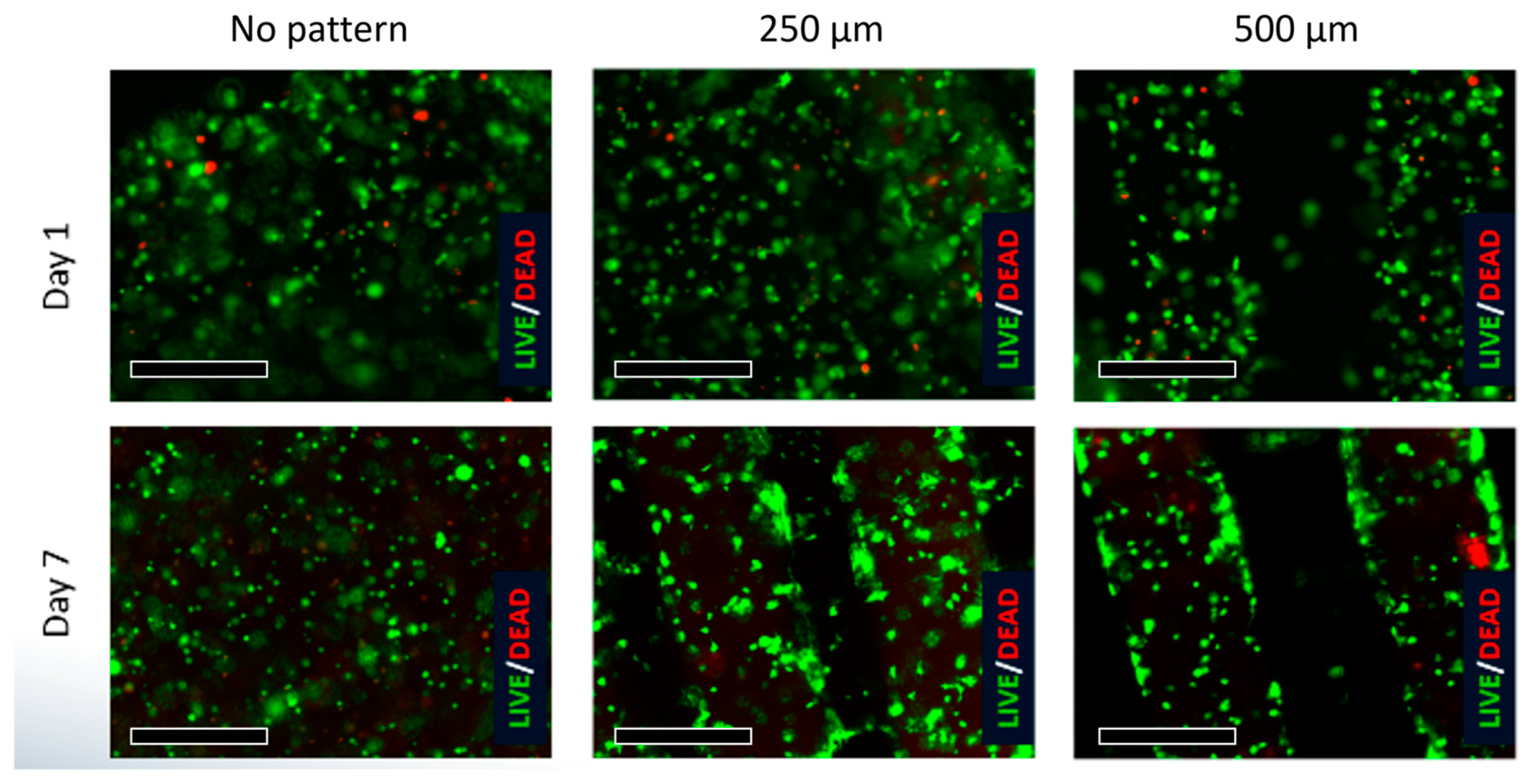
3.2. Viability
HUVEC
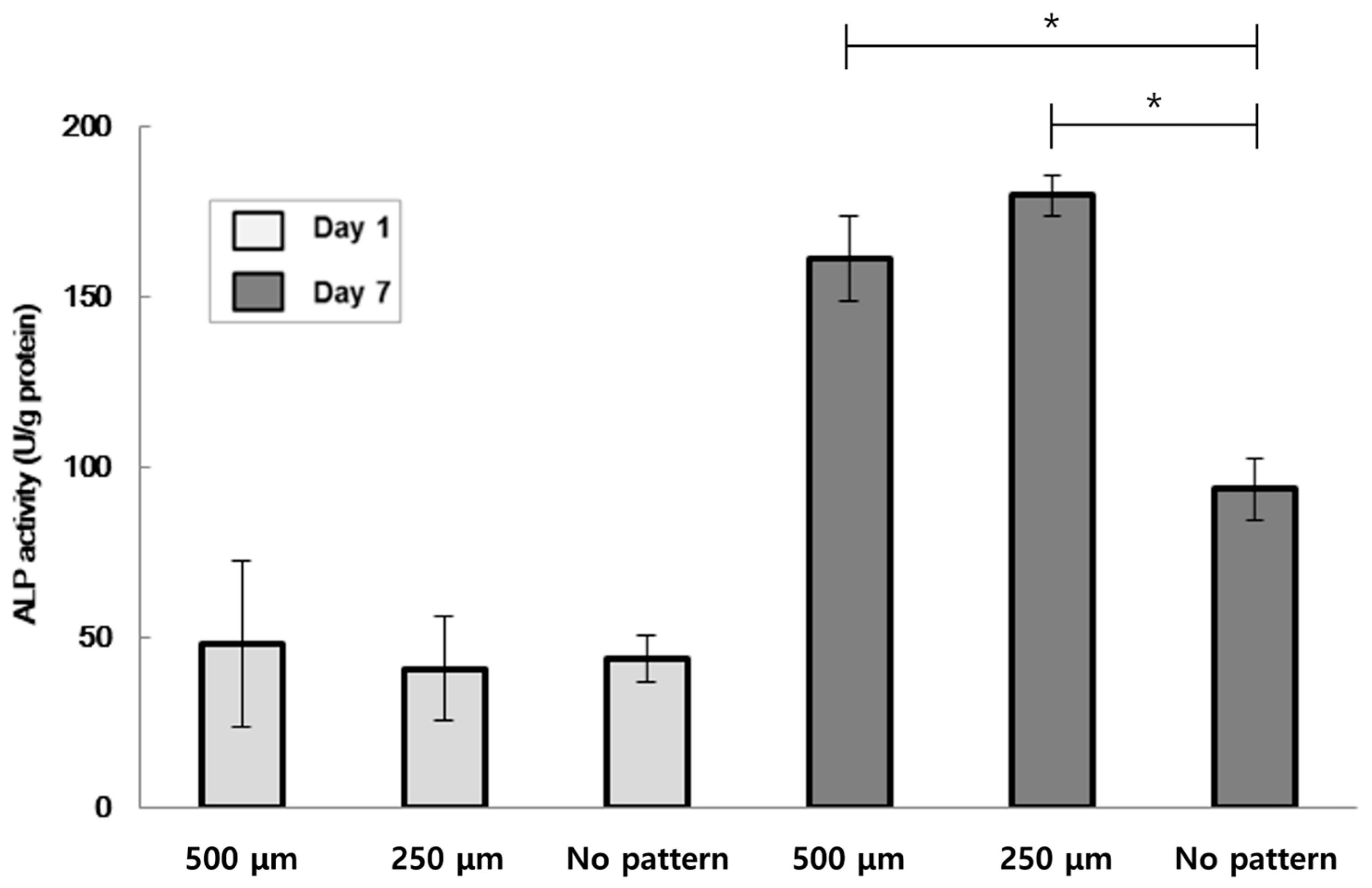
3.3. W-20-17
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A. The triad of nanotechnology, cell signalling, and scaffold implantation for the successful repair of damaged organs: An overview on soft-tissue engineering. J. Control Release 2021, 332, 460–492. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Nanotechnology, and scaffold implantation for the effective repair of injured organs: An overview on hard tissue engineering. J. Control Release 2021, 333, 391–417. [Google Scholar] [CrossRef]
- Sipe, J.D. Tissue engineering and reparative medicine. Ann. N. Y. Acad. Sci. 2002, 961, 1–9. [Google Scholar] [CrossRef]
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef]
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue engineering: Current strategies and future directions. Chonnam Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, E.M.; Yamamoto, M.; Park, H.; Shin, H. Engineering multi-cellular spheroids for tissue engineering and regenerative medicine. Adv. Healthc. Mater. 2020, 9, 2000608. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials 2019, 209, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, A.; Chen, S.; Zhang, X.; Chen, L.; Zhu, Y.; Xiao, Z.; Sun, J.; Luo, H.; Fan, H. Cell-laden electroconductive hydrogel simulating nerve matrix to deliver electrical cues and promote neurogenesis. ACS Appl. Mater. Interfaces 2019, 11, 22152–22163. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, A.; Rayat Pisheh, H.; Ansari, M.; Salati, A. Progress in bioprinting technology for tissue regeneration. J. Artif. Organs 2023, 26, 1–20. [Google Scholar] [CrossRef]
- Li, H.; Dai, J.; Wang, Z.; Zheng, H.; Li, W.; Wang, M.; Cheng, F. Digital light processing (DLP)-based (bio) printing strategies for tissue modeling and regeneration. Aggregate 2023, 4, e270. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, L.; Lin, F.; Tang, Y.; Cui, W. 3D bioprinting of emulating homeostasis regulation for regenerative medicine applications. J. Control. Release 2023, 353, 147–165. [Google Scholar] [CrossRef]
- Pi, Q.; Maharjan, S.; Yan, X.; Liu, X.; Singh, B. Digitally tunable microfluidic Bioprinting of Multi-layered cannular tissues. Adv. Mater. 2018, 30, 1706913. [Google Scholar] [CrossRef]
- Das, S.; Kumar, V.A.; Tiwari, R.; Singh, L.; Singh, S. Recent advances in hydrogels for biomedical applications. Asian J. Pharm. Clin Res. 2018, 11, 62–68. [Google Scholar] [CrossRef]
- Ling, Y.; Rubin, J.; Deng, Y.; Huang, C.; Demirci, U.; Karp, J.M.; Khademhosseini, A. A cell-laden microfluidic hydrogel. Lap Chip 2007, 7, 756–762. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printin go fcomplex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-Laden Hydrogels for Osteochondral and Cartilage Tissue Engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Fares, M.; Sani, E.S.; Lara, R.P.; Oliveira, R.V.B.; Khademhosseini, A.; Annabi, N. Interpenetrating network gelatin methoacryloyl (GelMA) and pectin-g-PCL hydrogels with tunable properties for tissue engineering. Biomater. Sci. 2018, 6, 2938. [Google Scholar] [CrossRef]
- Blaeser, A.; Campos, D.F.D.; Fischer, H. 3D bioprinting of cell-laden hydrogels for advanced tissue engineering. Bio. Med. Eng. 2017, 2, 58–66. [Google Scholar] [CrossRef]
- Samourides, A.; Browning, L.; Hearnden, V.; Chen, B. The effect of porous structure on the cell proliferation, tissue ingrowth and angiogenic properties of poly(glycerol sebacate urethane) scaffolds. Mater. Sci. Eng. C 2020, 108, 110384. [Google Scholar] [CrossRef]
- Joukhdar, H.; Seifert, A.; Jüngst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice Templating Soft Matter: Fundamental Principles and Fabrication Approaches to Tailor Pore Structure and Morphology and Their Biomedical Applications. Adv. Mater. 2021, 33, 2100091. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.H.; Kim, T.H.; Park, S.H. Soft Biomimetic 3D Free-Form Artificial Vascular Graft Using a Highly Uniform Microspherical Porous Structure. ACS Appl. Mater. Interfaces 2022, 14, 29588–29598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y. A review of preparation methods of porous skin tissue engineering scaffolds. Mater. Today Commun. 2022, 32, 104109. [Google Scholar] [CrossRef]
- Abar, B.; Alonso-Calleja, A.; Kelly, A.; Kelly, C.; Gall, K.; West, J.L. 3D printing of high-strength, porous, elastomeric structures to promote tissue integration of implants. J. Biomed. Mater. Res. 2021, 109, 54–63. [Google Scholar] [CrossRef]
- Coulter, F.B.; Levey, R.E.; Robinson, S.T.; Dolan, E.B.; Deotti, S.; Monaghan, M.; Dockery, P.; Coulter, B.S.; Burke, L.P.; Lowery, A.J.; et al. Additive Manufacturing of Multi-Scale Porous Soft Tissue Implants That Encourage Vascularization and Tissue Ingrowth. Adv. Healthcare Mater. 2021, 10, 2100229. [Google Scholar] [CrossRef] [PubMed]
- Godau, B.; Stefanek, E.; Gharaie, S.S.; Amereh, M.; Pagan, E.; Marvdashti, Z.; Libert-Scott, E.; Ahadian, S.; Akbari, M. Non-destructive mechanical assessment for optimization of 3D bioprinted soft tissue scaffolds. iScience 2022, 25, 104251. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze NSchacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methatcrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31. [Google Scholar] [CrossRef]
- Müller, B.; Lang, S.; Dominietto, M.; Rudin, M.; Schulz, G.; Deyhle, H.; Germann, M.; Pfeiffer, F.; David, C.; Weitkamp, T. High-resolution tomographic imaging of microvessels. In Proceedings of the SPIE—The International Society for Optical Engineering, San Diego, CA, USA, 18 September 2008; Volume 7078, p. 70780B. [Google Scholar]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef]
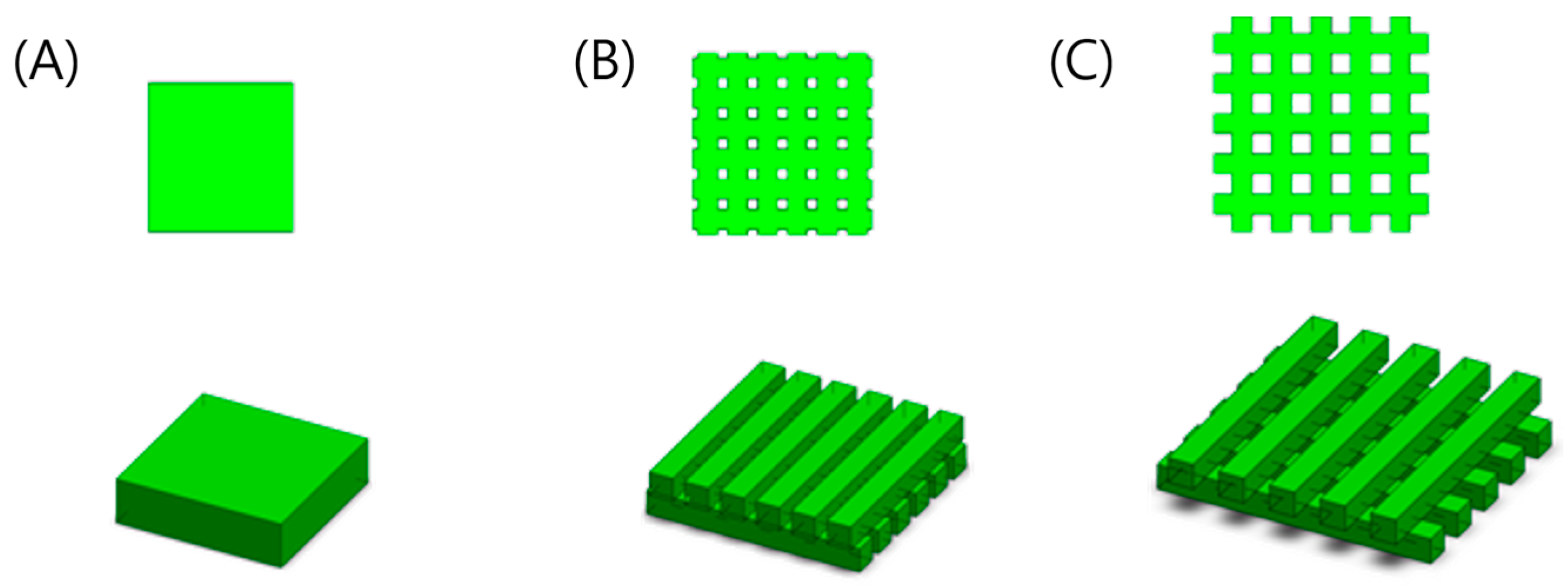



| Structure Size (Length × Width × Height × No. of Structures) | Channel Size (mm) Stand-Off Distance (SD) | Porosity (%) | Cell Number | Surface Area (mm2) | |
|---|---|---|---|---|---|
| A | 3.7 mm × 3.7 mm × 1 mm × 1 | 0.00 | 00.0% | 67,500 | 42.18 |
| B | 4.5 mm × 0.5 mm × 0.5 mm × 12 | 0.25 | 33.3% | 67,500 | 96.00 |
| C | 5.4 mm × 0.5 mm × 0.5 mm × 10 | 0.50 | 53.7% | 67,500 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, W.-S.; Park, H.; Moon, S. Novel Fabrication of 3-D Cell Laden Micro-Patterned Porous Structure on Cell Growth and Proliferation by Layered Manufacturing. Bioengineering 2023, 10, 1092. https://doi.org/10.3390/bioengineering10091092
Chu W-S, Park H, Moon S. Novel Fabrication of 3-D Cell Laden Micro-Patterned Porous Structure on Cell Growth and Proliferation by Layered Manufacturing. Bioengineering. 2023; 10(9):1092. https://doi.org/10.3390/bioengineering10091092
Chicago/Turabian StyleChu, Won-Shik, Hyeongryool Park, and Sangjun Moon. 2023. "Novel Fabrication of 3-D Cell Laden Micro-Patterned Porous Structure on Cell Growth and Proliferation by Layered Manufacturing" Bioengineering 10, no. 9: 1092. https://doi.org/10.3390/bioengineering10091092