Evaluation of Morlet Wavelet Analysis for Artifact Detection in Low-Frequency Commercial Near-Infrared Spectroscopy Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Data Collection
2.2. Physiologic Signal Acquisition
2.3. Signal Processing
2.4. Signal Analysis—Methods Applied to Identify Artifact Segments
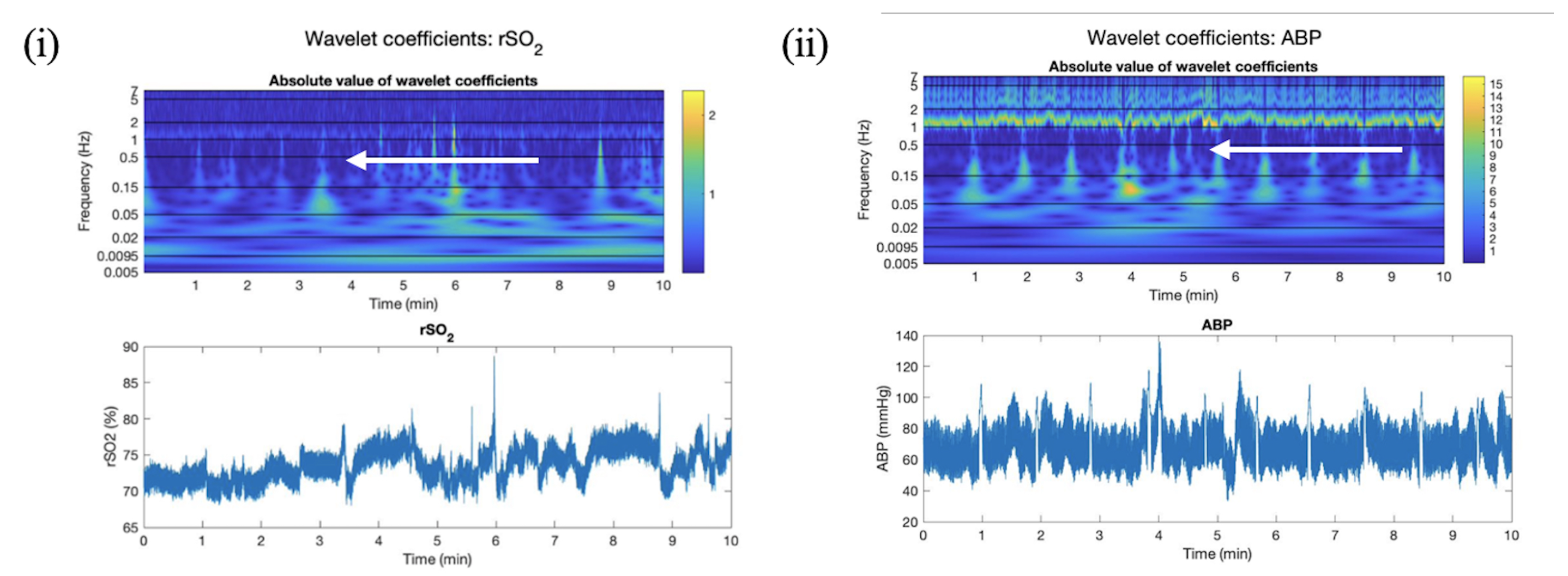
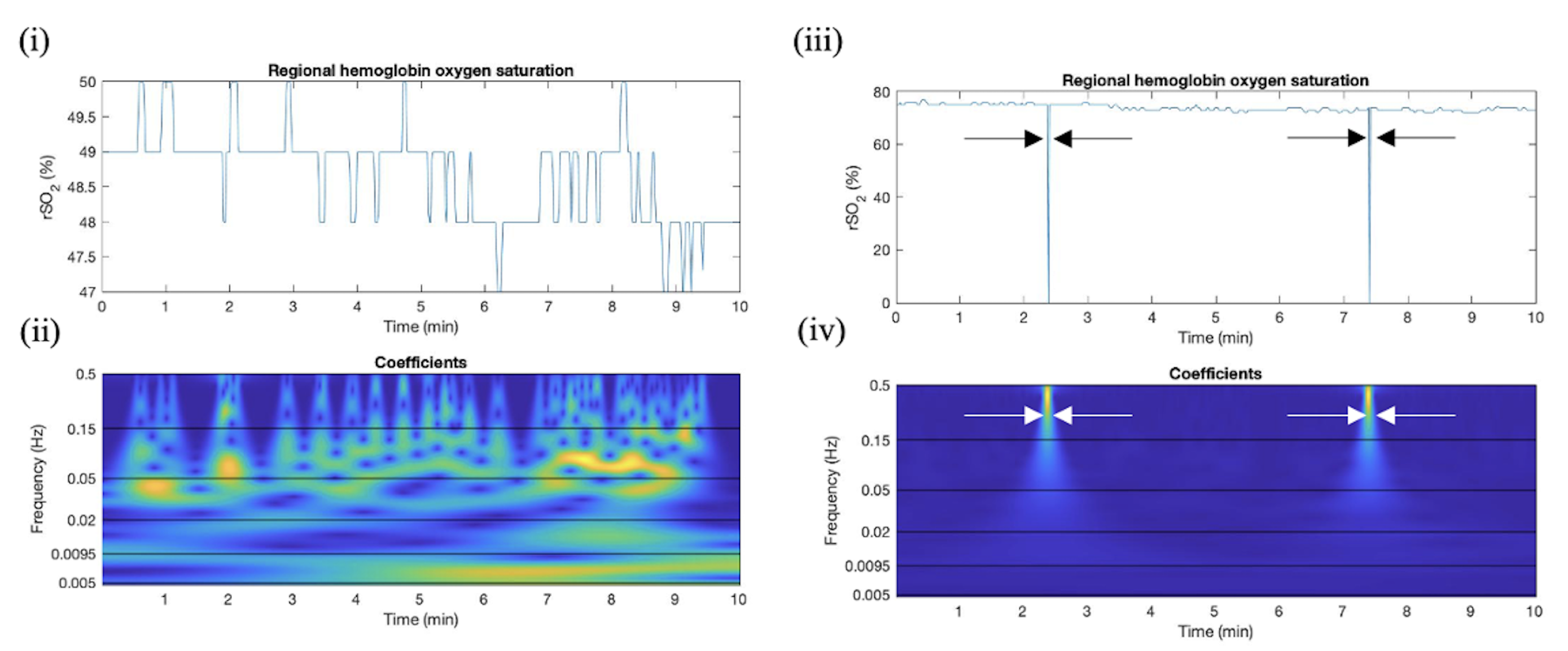
2.4.1. Overview of CWT Artifact Detection for rSO2
2.4.2. Absolute Wavelet Coefficients Applied in Artifact Clearance for rSO2 Across Populations
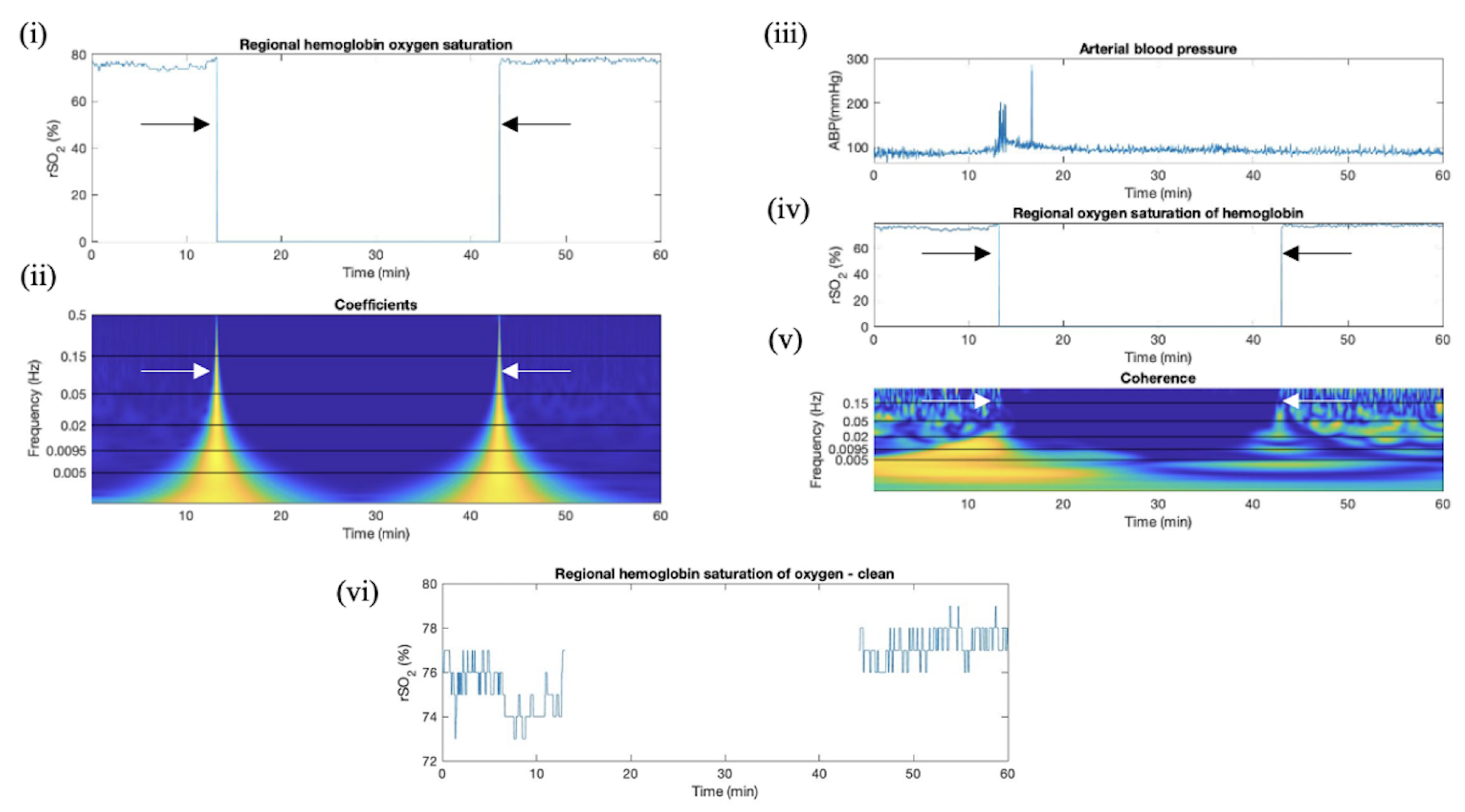
2.4.3. Wavelet Coherence between ABP and rSO2 Applied in Artifact Clearance for rSO2 Across Populations
2.4.4. Wavelet Semblance between ABP and rSO2 Applied in Artifact Clearance for rSO2 Across Populations
2.4.5. Proposed Method of CWT Artifact Clearance for rSO2
- 1.
- Observationally establish thresholds for absolute value of wavelet coefficients of rSO2 to indicate when signal is lost or regained;
- 2.
- Observationally establish thresholds in frequency bands to indicate when the rSO2 signal is not oscillating (lost for a prolonged period).
2.4.6. Sub-Group Analyses—Cerebral Autoregulation State
2.4.7. Sub-Group Analyses—Cerebral Injury Patterns
3. Results
3.1. Absolute Wavelet Coefficients Applied in Artifact Clearance for rSO2 Across Populations
3.2. Wavelet Coherence between ABP and rSO2 Applied in Artifact Clearance for rSO2 Across Populations
3.3. Proposed Method of CWT Artifact Clearance for rSO2
3.4. Sub-Group Analyses—Cerebral Autoregulation State
3.5. Sub-Group Analyses—Cerebral Injury Patterns
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| SP | Elective spinal surgery patients |
| HC | Healthy controls |
| COx | Cerebral oxygenation index |
| TOx | Tissue oxygenation index |
Appendix A. Demographics from Elective Spinal Surgery Population
| Demographics | Median (Interquartile Range) or Number of Patients |
|---|---|
| Number of Patients | 27 |
| Male Sex | 22 (81.5%) |
| Age | 57 (52–65.5) |
| Weight (kg) | 84.6 (69.2–100) |
| O2 Inhalation (%) | 68 (49–100) |
| End-Tidal CO2 | 35 (18–37) |
| Arterial CO2 | 44 (37–47) |
| Arterial O2 | 240 (211–303) |
| Procedure Type | |
| ACDF | 22 (81.5%) |
| Male Sex | 6 (22%) |
| PCDF | 14 (52%) |
| ACDF and PCDF | 3 (9%) |
| Cervical Incision and Drainage | 1 (3%) |
| Corpectomy | 1 (3%) |
| Laminectomy | 1 (3%) |
| Thoracic Decompression | 1 (3%) |
| Duration of Recording (min) | 185 (166–203) |
| rSO2_L (%) | 66 (59–73) |
| rSO2_R (%) | 67 (60–73) |
| MAP (mmHg) | 83 (79–86) |
| COx_a_L (au) | 0.17 (0.08–0.25) |
| COx_a_R (au) | 0.2 (0.1–0.28) |
| % time COx_a_L > +0.3 (% time) | 40 (31–46) |
| % time COx_a_R > +0.3 (% time) | 41 (34–48) |
Appendix B. Demographics from Healthy Control Population
| Demographics | Median (Interquartile Range) or Number of Patients |
|---|---|
| Number of Patients | 103 |
| Male Sex | 43 (41.7%) |
| Age | 26 (22–31) |
| Hand Dominance (Right) | 89 (87%) |
| Duration of Recording (min) | 33 (29–36) |
| rSO2_L (%) | 73 (69–79) |
| rSO2_R (%) | 72 (66–79) |
| MAP (mmHg) | 101 (86–114) |
| COx_a_L (au) | 0.14 (0.01–0.22) |
| COx_a_R (au) | 0.12 (0.03–0.22) |
| % time COx_a_L > +0.3 (% time) | 27 (13–37) |
| % time COx_a_R > +0.3 (% time) | 25 (15–38) |
Appendix C. Wavelet Semblance between ABP and rSO2
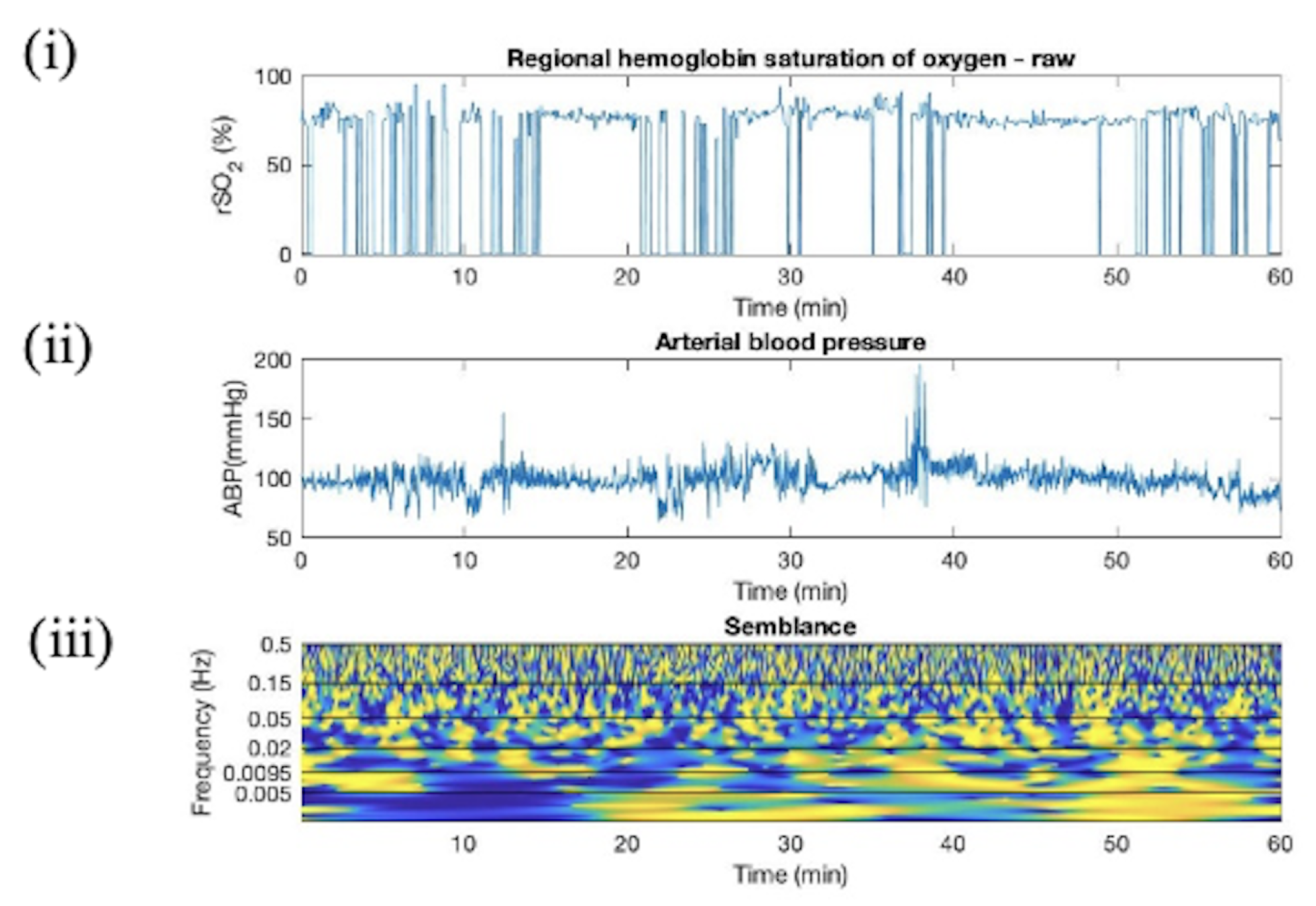

Appendix D. Artifact Detection in HC Additional Plots
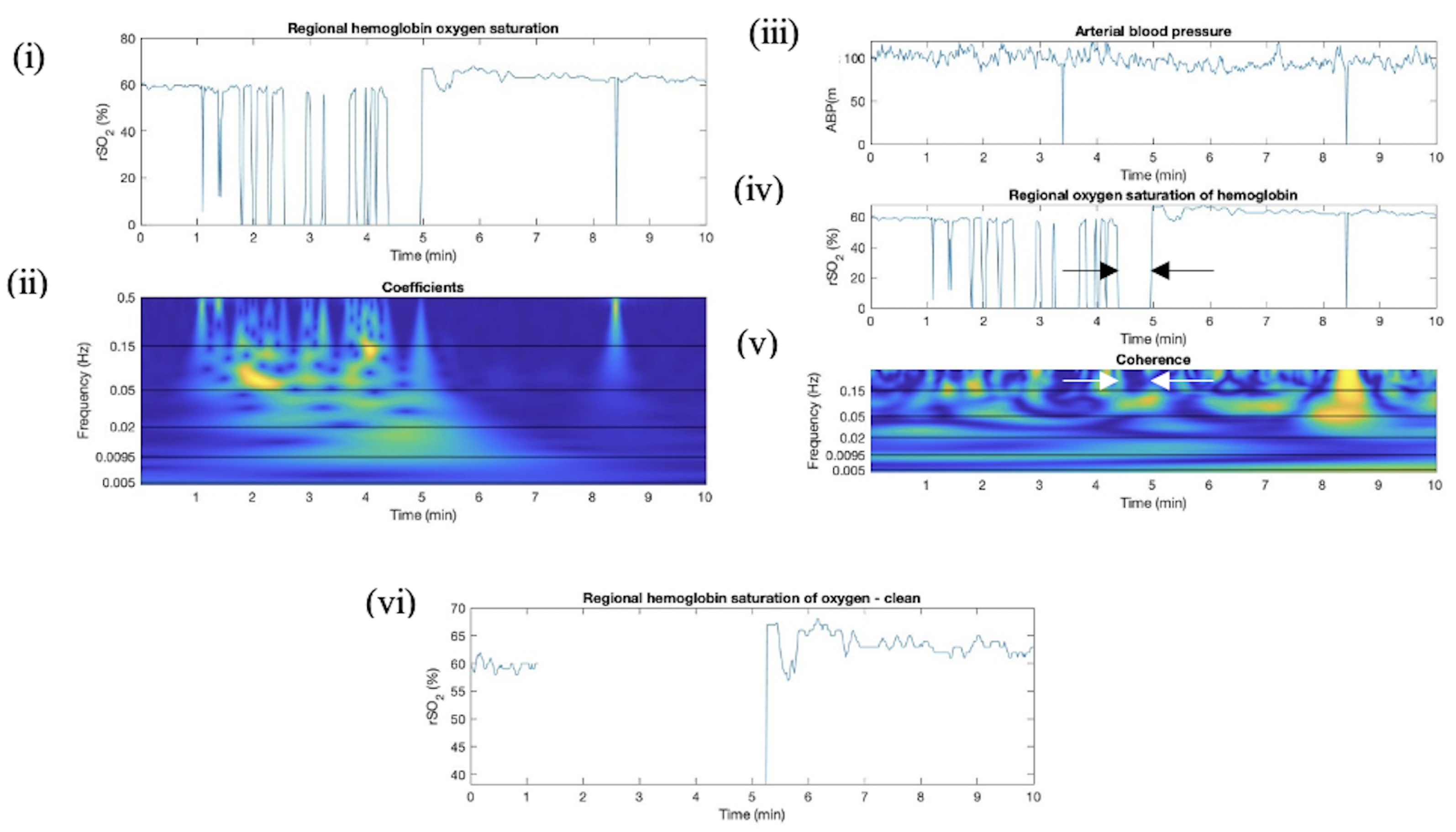

Appendix E. Artifact Detection in SP Additional Plots

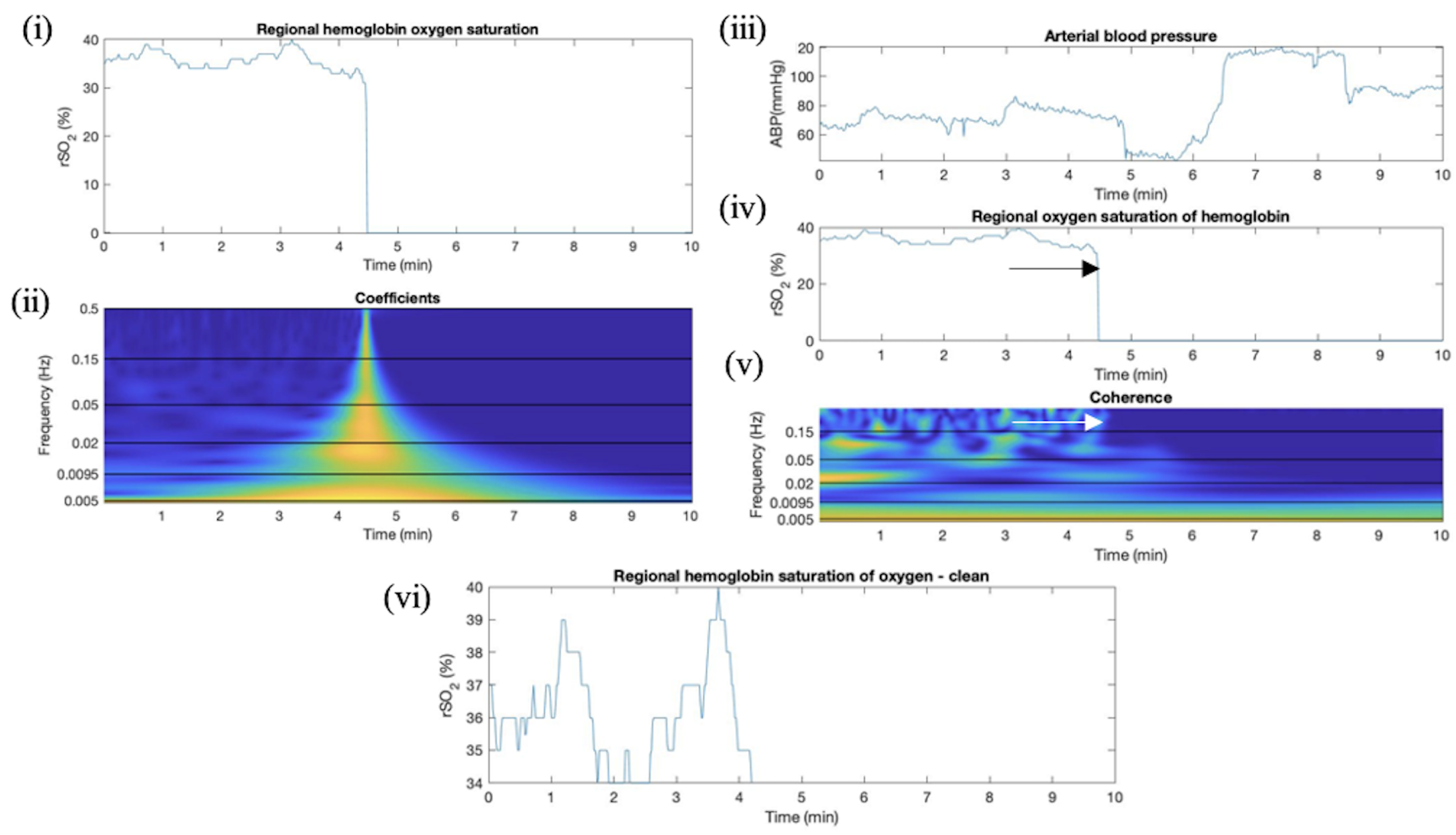
Appendix F. Artifact Detection in TBI Additional Plots


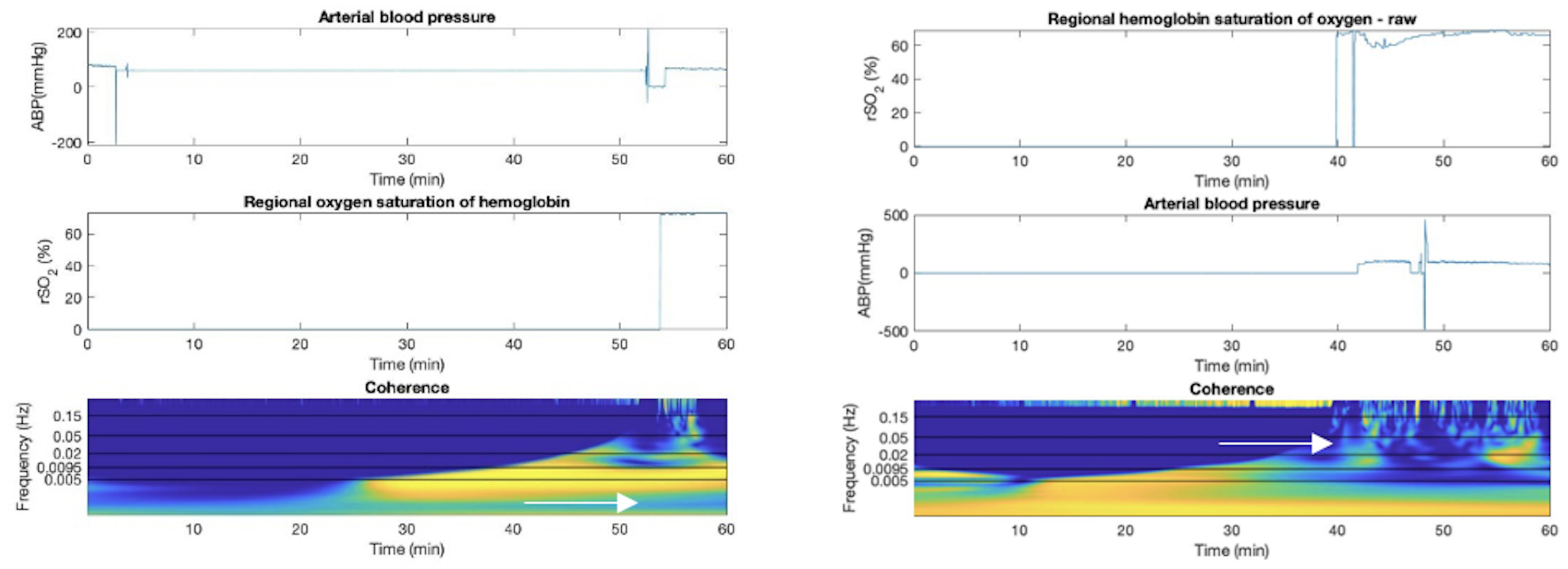
Appendix G. Sub-Group Analyses—Cerebral Autoregulation State
| Data Set | CA Health | Data Points Analyzed | Artifact Points Identified (rSO2 < 1) | Removed Using abs Wavelet Coefficient | Removed Using Coherence | Success Rate |
|---|---|---|---|---|---|---|
| HC | Intact | 64,916 | 511 | 511 | 0 | 100% |
| SP | Intact | 558,892 | 32,102 | 6131 | 25,921 | 99.8% |
| SP | Impaired | 208,448 | 5713 | 1470 | 4218 | 99.6% |
| TBI | Intact | 15,556,279 | 247,624 | 147,620 | 100,314 | 100% |
| TBI | Impaired | 9,495,501 | 101,894 | 37,703 | 62,802 | 98.6% |
Appendix H. Pearson Correlation between rSO2 Signals
| Data Set | p > 0.2 (%) | p > 0.5 (%) | Total Windows |
|---|---|---|---|
| TBI data | 57.76 | 45.36 | 43,936 |
| SP data | 82.67 | 63.36 | 4760 |
| TBI cleaned | 85.94 | 67.35 | 26,919 |
| SP cleaned | 87.18 | 64.48 | 3885 |
| HC cleaned | 91.68 | 68.85 | 793 |
| HC (COx_a < 0) | 100 | 97.22 | 36 |
| SP (COx_a > 0.3) | 98.63 | 87.67 | 219 |
| SP (COx_a < 0) | 99.29 | 86.43 | 140 |
| TBI (COx_a > 0.3) | 100 | 97.53 | 481 |
| TBI (COx_a < 0) | 94.47 | 85.43 | 199 |
References
- Zeiler, F.A.; Iturria-Medina, Y.; Thelin, E.P.; Gomez, A.; Shankar, J.J.; Ko, J.H.; Figley, C.R.; Wright, G.E.; Anderson, C.M. Integrative Neuroinformatics for Precision Prognostication and Personalized Therapeutics in Moderate and Severe Traumatic Brain Injury. Front. Neurol. 2021, 12, 729184. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Ercole, A.; Placek, M.M.; Hutchinson, P.J.; Stocchetti, N.; Czosnyka, M.; Smielewski, P.; CENTER-TBI High-Resolution ICU (HR ICU) Sub-Study Participants and Investigators. Association between Physiologic Signal Complexity and Outcomes in Moderate and Severe Traumatic Brain Injury: A CENTER-TBI Exploratory Analysis of Multiscale Entropy. J. Neurotrauma 2021, 38, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Wennervirta, J.M.; Tjerkaski, J.; Luoto, T.M.; Posti, J.P.; Nelson, D.W.; Takala, R.; Bendel, S.; Thelin, E.P.; Luostarinen, T.; et al. Dynamic prediction of mortality after traumatic brain injury using a machine learning algorithm. Digit. Med. 2022, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Siironen, J.; Skrifvars, M.B.; Hernesniemi, J.; Kivisaari, R. Predicting Outcome in Traumatic Brain Injury: Development of a Novel Computerized Tomography Classification System (Helsinki Computerized Tomography Score). Neurosurgery 2014, 75, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Ercole, A.; Cabeleira, M.; Carbonara, M.; Stocchetti, N.; Menon, D.K.; Smielewski, P.; Czosnyka, M.; CENTER-TBI High Resolution (HR ICU) Sub-Study Participants and Investigators. Comparison of Performance of Different Optimal Cerebral Perfusion Pressure Parameters for Outcome Prediction in Adult Traumatic Brain Injury: A Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Study. J. Neurotrauma 2019, 36, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- De Georgia, M.A.; Deogaonkar, A. Multimodal Monitoring in the Neurological Intensive Care Unit. Neurologist 2005, 11, 45–54. [Google Scholar] [CrossRef]
- Tasneem, N.; Samaniego, E.A.; Pieper, C.; Leira, E.C.; Adams, H.P.; Hasan, D.; Ortega-Gutierrez, S. Brain Multimodality Monitoring: A New Tool in Neurocritical Care of Comatose Patients. Crit. Care Res. Pract. 2017, 2017, 6097265. [Google Scholar] [CrossRef]
- Fantini, S.; Hueber, D.; Franceschini, M.A.; Gratton, E.; Rosenfeld, W.; Stubblefield, P.G.; Maulik, D.; Stankovic, M.R. Non-invasive optical monitoring of the newborn piglet brain using continuous-wave and frequency-domain spectroscopy. Phys. Med. Biol. 1999, 44, 1543–1563. [Google Scholar] [CrossRef]
- Huang, R.; Hong, K.-S.; Yang, D.; Huang, G. Motion artifacts removal and evaluation techniques for functional near-infrared spectroscopy signals: A review. Front. Neurosci. 2022, 16, 878750. [Google Scholar] [CrossRef]
- Rozanek, M.; Skola, J.; Horakova, L.; Trukhan, V. Effect of artifacts upon the pressure reactivity index. Sci. Rep. 2022, 12, 15131. [Google Scholar] [CrossRef]
- Robertson, F.C.; Douglas, T.S.; Meintjes, E.M. Motion Artifact Removal for Functional Near Infrared Spectroscopy: A Comparison of Methods. IEEE Trans. Biomed. Eng. 2010, 57, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Radüntz, T.; Scouten, J.; Hochmuth, O.; Meffert, B. Automated EEG artifact elimination by applying machine learning algorithms to ICA-based features. J. Neural Eng. 2017, 14, 046004. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bian, G.-B.; Tian, Z. Removal of Artifacts from EEG Signals: A Review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, J.; Olivers, C.N.L.; Fahrenfort, J.J. High-pass filtering artifacts in multivariate classification of neural time-series data. J. Neurosci. Methods 2021, 352, 109080. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fariha, M.A.Z.; Ikeura, R.; Hayakawa, S.; Tsutsumi, S. Analysis of Pan-Tompkins Algorithm Performance with Noisy ECG Signals. J. Phys. Conf. Ser. 2020, 1532, 012022. [Google Scholar] [CrossRef]
- Moerman, A.; Wouters, P. Near-infrared spectroscopy (NIRS) monitoring in contemporary anesthesia and critical care. Acta Anaesthesiol. Belg. 2010, 61, 185–194. [Google Scholar]
- Highton, D.; Ghosh, A.; Tachtsidis, I.; Panovska-Griffiths, J.; Elwell, C.E.; Smith, M. Monitoring Cerebral Autoregulation After Brain Injury: Multimodal Assessment of Cerebral Slow-Wave Oscillations Using Near-Infrared Spectroscopy. Anesth. Analg. 2015, 121, 198–205. [Google Scholar] [CrossRef]
- Liu, X.; Hu, X.; Brady, K.M.; Koehler, R.; Smielewski, P.; Czosnyka, M.; Donnelly, J.; Lee, J.K. Comparison of wavelet and correlation indices of cerebral autoregulation in a pediatric swine model of cardiac arrest. Sci. Rep. 2020, 10, 5926. [Google Scholar] [CrossRef]
- Noriyuki, T.; Ohdan, H.; Yoshioka, S.; Miyata, Y.; Asahara, T.; Dohi, K. Near-infrared spectroscopic method for assessing the tissue oxygenation state of living lung. Am. J. Respir. Crit. Care Med. 1997, 156, 1656–1661. [Google Scholar] [CrossRef]
- Miller, S. NIRS-based cerebrovascular regulation assessment: Exercise and cerebrovascular reactivity. Neurophoton 2017, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Kurazumi, T.; Kato, T.; Takko, C.; Ogawa, Y.; Iwasaki, K.I. Changes in cerebral oxygen saturation and cerebral blood flow velocity under mild +Gz hypergravity. J. Appl. Physiol. 2019, 127, 190–197. [Google Scholar] [CrossRef]
- Polinder-Bos, H.A.; Elting, J.W.J.; Aries, M.J.; García, D.V.; Willemsen, A.T.; van Laar, P.J.; Kuipers, J.; Krijnen, W.P.; Slart, R.H.; Luurtsema, G.; et al. Changes in cerebral oxygenation and cerebral blood flow during hemodialysis—A simultaneous near-infrared spectroscopy and positron emission tomography study. J. Cereb. Blood Flow Metab. 2020, 40, 328–340. [Google Scholar] [CrossRef]
- Tas, J.; Eleveld, N.; Borg, M.; Bos, K.D.; Langermans, A.P.; van Kuijk, S.M.; van der Horst, I.C.; Elting, J.W.J.; Aries, M.J. Cerebral Autoregulation Assessment Using the Near Infrared Spectroscopy ‘NIRS-Only’ High Frequency Methodology in Critically Ill Patients: A Prospective Cross-Sectional Study. Cells 2022, 11, 2254. [Google Scholar] [CrossRef] [PubMed]
- Büchner, K.; Meixensberger, J.; Dings, J.; Roosen, K. Near-infrared spectroscopy—Not useful to monitor cerebral oxygenation after severe brain injury. Zentralbl. Neurochir. 2000, 61, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Shaaban-Ali, M.; Momeni, M.; Denault, A. Clinical and Technical Limitations of Cerebral and Somatic Near-Infrared Spectroscopy as an Oxygenation Monitor. J. Cardiothorac. Vasc. Anesth. 2021, 35, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zhang, M.; Li, Z.; Xin, Q.; Lu, L.; Zhou, W.; Han, Q.; Gao, Y. Wavelet coherence analysis of spontaneous oscillations in cerebral tissue oxyhemoglobin concentrations and arterial blood pressure in elderly subjects. Microvasc. Res. 2014, 93, 14–20. [Google Scholar] [CrossRef]
- Tian, F.; Tarumi, T.; Liu, H.; Zhang, R.; Chalak, L. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-Ischemic encephalopathy. NeuroImage Clin. 2016, 11, 124–132. [Google Scholar] [CrossRef]
- Shiogai, Y.; Stefanovska, A.; McClintock, P.V.E. Nonlinear dynamics of cardiovascular ageing. Phys. Rep. 2010, 488, 51–110. [Google Scholar] [CrossRef]
- Shu, L.; Xie, J.; Yang, M.; Li, Z.; Li, Z.; Liao, D.; Xu, X.; Yang, X. A Review of Emotion Recognition Using Physiological Signals. Sensors 2018, 18, 2074. [Google Scholar] [CrossRef]
- Karim, S.A.A.; Kamarudin, M.H.; Karim, B.A.; Hasan, M.K.; Sulaiman, J. Wavelet Transform and Fast Fourier Transform for signal compression: A comparative study. In Proceedings of the 2011 International Conference on Electronic Devices, Systems and Applications (ICEDSA), Kuala Lumpur, Malaysia, 25–27 April 2011. [Google Scholar]
- Arts, L.P.A.; Van Den Broek, E.L. The fast continuous wavelet transformation (fCWT) for real-time, high-quality, noise-resistant time-frequency analysis. Nat. Comput. Sci. 2022, 2, 47–58. [Google Scholar] [CrossRef]
- Komorowski, D.; Pietraszek, S. The Use of Continuous Wavelet Transform Based on the Fast Fourier Transform in the Analysis of Multi-channel Electrogastrography Recordings. J. Med. Syst. 2016, 40, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, P. Comparisons between fast algorithms for the continuous wavelet transform and applications in cosmology: The 1D case. RAS Tech. Instruments 2023, 2, 307–323. [Google Scholar] [CrossRef]
- Fekete, T.; Rubin, D.; Carlson, J.M.; Mujica-Parodi, L.R. The NIRS Analysis Package: Noise Reduction and Statistical Inference. PLoS ONE 2011, 6, e24322. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Dian, J.; Froese, L.; Zeiler, F.A. Near-Infrared Cerebrovascular Reactivity for Monitoring Cerebral Autoregulation and Predicting Outcomes in Moderate to Severe Traumatic Brain Injury: Proposal for a Pilot Observational Study. JMIR Res. Protoc. 2020, 9, e18740. [Google Scholar] [CrossRef]
- Froese, L.; Dian, J.; Gomez, A.; Batson, C.; Sainbhi, A.S.; Zeiler, F.A. Association between Processed Electroencephalogram-Based Objectively Measured Depth of Sedation and Cerebrovascular Response: A Systematic Scoping Overview of the Human and Animal Literature. Front. Neurol. 2021, 12, 1409. [Google Scholar] [CrossRef]
- Froese, L.; Dian, J.; Batson, C.; Gomez, A.; Unger, B.; Zeiler, F.A. Cerebrovascular Response to Propofol, Fentanyl, and Midazolam in Moderate/Severe Traumatic Brain Injury: A Scoping Systematic Review of the Human and Animal Literature. Neurotrauma Rep. 2020, 12, 100–112. [Google Scholar] [CrossRef]
- Gomez, A.; Sainbhi, A.S.; Froese, L.; Batson, C.; Slack, T.; Stein, K.Y.; Cordingley, D.M.; Mathieu, F.; Zeiler, F.A. The Quantitative Associations between Near Infrared Spectroscopic Cerebrovascular Metrics and Cerebral Blood Flow: A Scoping Review of the Human and Animal Literature. Front. Netw. Physiol. 2022, 13, 934731. [Google Scholar] [CrossRef]
- cwt Continuous 1-D Wavelet Transformation. Available online: https://www.mathworks.com/help/wavelet/ref/cwt.html (accessed on 25 May 2023).
- Lin, A.; Shelden, C.; Panagiotacopulos, N.; Ross, B. Definition of the Neurochemical Patterns of Human Head Injury in 1 H MRS Using Wavelet Analysis. Proc. Intl. Soc. Mag. Res. Med. 2001, 9, 822. [Google Scholar]
- Bernjak, A.; Stefanovska, A.; McClintock, P.V.E.; Owen-Lynch, P.J.; Clarkson, P.B.M. Coherence between fluctuations in blood flow and oxygen saturation. Fluct. Noise Lett. 2012, 11, 1240013. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Cooper, G.R.J.; Cowan, D.R. Comparing time series using wavelet-based semblance analysis. Comput. Geosci. 2008, 34, 95–102. [Google Scholar] [CrossRef]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlin. Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Stefanovska, A. Coupled Oscillators: Complex But Not Complicated Cardiovascular and Brain Interactions. IEEE Eng. Med. Biol. Mag. 2007, 26, 25–29. [Google Scholar] [CrossRef]
- Kvandal, P.; Sheppard, L.; Landsverk, S.A.; Stefanovska, A.; Kirkeboen, K.A. Impaired cerebrovascular reactivity after acute traumatic brain injury can be detected by wavelet phase coherence analysis of the intracranial and arterial blood pressure signals. J. Clin. Monit. Comput. 2013, 27, 375–383. [Google Scholar] [CrossRef]
- Kvandal, P.; Landsverk, S.A.; Bernjak, A.; Stefanovska, A.; Kvernmo, H.D.; Kirkebøen, K.A. Low-frequency oscillations of the laser Doppler perfusion signal in human skin. Microvasc. Res. 2006, 72, 120–127. [Google Scholar] [CrossRef]
- Brady, K.M.; Lee, J.K.; Kibler, K.K.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Koehler, R.C.; Shaffner, D.H. Continuous Time-Domain Analysis of Cerebrovascular Autoregulation Using Near-Infrared Spectroscopy. Stroke 2007, 38, 2818–2825. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Aries, M.; Czosnyka, M.; Smielewski, P. Cerebral Autoregulation Monitoring in Traumatic Brain Injury: An Overview of Recent Advances in Personalized Medicine. J. Neurotrauma 2022, 39, 1477–1494. [Google Scholar] [CrossRef]
- Froese, L.; Gomez, A.; Sainbhi, A.S.; Vakitbilir, N.; Marquez, I.; Amenta, F.; Park, K.; Stein, K.Y.; Thelin, E.P.; Zeiler, F.A. Cerebrovascular Reactivity Is Not Associated With Therapeutic Intensity in Adult Traumatic Brain Injury: A Validation Study. Neurotrauma Rep. 2023, 4, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Diedler, J.; Kasprowicz, M.; Budohoski, K.P.; Haubrich, C.; Smielewski, P.; Outtrim, J.G.; Manktelow, A.; Hutchinson, P.J.; Pickard, J.D.; et al. Critical Thresholds for Cerebrovascular Reactivity After Traumatic Brain Injury. Neurocrit. Care 2012, 16, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Donnelly, J.; Smielewski, P.; Menon, D.K.; Hutchinson, P.J.; Czosnyka, M. Critical Thresholds of Intracranial Pressure-Derived Continuous Cerebrovascular Reactivity Indices for Outcome Prediction in Noncraniectomized Patients with Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Laurikkala, J.; Aneman, A.; Peng, A.; Reinikainen, M.; Pham, P.; Jakkula, P.; Hästbacka, J.; Wilkman, E.; Loisa, P.; Toppila, J.; et al. Association of deranged cerebrovascular reactivity with brain injury following cardiac arrest: A post-hoc analysis of the COMACARE trial. Crit. Care 2021, 25, 350. [Google Scholar] [CrossRef] [PubMed]
- Weigl, W.; Milej, D.; Janusek, D.; Wojtkiewicz, S.; Sawosz, P.; Kacprzak, M.; Gerega, A.; Maniewski, R.; Liebert, A. Application of optical methods in the monitoring of traumatic brain injury: A review. J. Cereb. Blood Flow Metab. 2016, 36, 1825–1843. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Malik, A.A.; Adil, M.M.; Defillo, A.; Sherr, G.T.; Suri, M.F.K. Hematoma Enlargement Among Patients with Traumatic Brain Injury: Analysis of a Prospective Multicenter Clinical Trial. J. Vasc. Interv. Neurol. 2015, 8, 42–49. [Google Scholar]
- Jacob, M.; Kale, M.N.; Hasnain, S. Correlation between cerebral co-oximetry (rSO2) and outcomes in traumatic brain injury cases: A prospective, observational study. Med. J. Armed. Forces India 2019, 75, 190–196. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]

| Demographics | Median (Interquartile Range) or Number of Patients |
|---|---|
| Age | 42 (27.5–58.6) |
| Sex (% Male) | 68 (81.9%) |
| Best Admission GCS—Total | 6 (4–8) |
| Best Admission GCS—Motor | 4 (2–5) |
| Number with Hypoxia episode | 26 (31.3%) |
| Number with Hypotension episode | 9 (10.8%) |
| Number with Traumatic SAH | 80 (96.4%) |
| Pupils | |
| Bilateral Unreactive | 11 (13.3%) |
| Unilateral Unreactive | 18 (21.7%) |
| Bilateral Reactive | 52 (62.7%) |
| Admission Marshall CT | |
| V | 41 (48.4%) |
| IV | 15 (18.1%) |
| III | 24 (28.9%) |
| II | 3 (3.6%) |
| Mean ICP (mmHg) | 7.9 (4.2–10.7) |
| % Time ICP > 20 mmHg | 0.3 (0.0–2.0) |
| % Time ICP > 22 mmHg | 0.1 (0.0–1.2) |
| Mean CPP (mmHg) | 76 (71.3–83.5) |
| % Time CPP > 70 mmHg | 74 (52–84) |
| % Time CPP < 60 mmHg | 4 (0.4–6.3) |
| Mean rSO (au) | 68.5 (61.3–76.5) |
| Mean CO_a | 0.06 (0.01–0.13) |
| % Time COx_a > 0 | 54 (49–61) |
| % time COx_a > 0.25 | 22 (16–27) |
| Data Set | Data Points Analyzed | Artifact Points Identified (rSO2 < 1) | Removed Using abs Wavelet Coefficient | Removed Using Coherence | Success Rate | Erroneously Removed Points | Error Rate |
|---|---|---|---|---|---|---|---|
| HC (Right) | 154,961 | 522 | 517 | 5 | 100% | 3433 | 2.22% |
| HC (Left) | 154,961 | 522 | 521 | 1 | 100% | 3566 | 2.30% |
| SP (Right) | 279,446 | 15,927 | 3143 | 12,756 | 99.8% | 4645 | 1.66% |
| SP (Left) | 279,446 | 16,175 | 2988 | 13,165 | 97.9% | 5045 | 1.81% |
| TBI (Right) | 25,557,085 | 4,719,300 | 190,505 | 4,513,148 | 99.9% | 311,656 | 1.22% |
| TBI (Left) | 25,557,085 | 5,084,529 | 129,516 | 4,945,166 | 99.8% | 219,791 | 0.86% |
| Data Set | Hematoma/Contusion | Data Points Analyzed | Artifact Points Identified (rSO2 < 1) | Removed Using abs Wavelet Coefficient | Removed Using Coherence | Success Rate | Percent Time Recorded Signal Was Artifact |
|---|---|---|---|---|---|---|---|
| TBI (Right) | Present | 5,683,219 | 1,939,540 | 61,056 | 1,878,141 | 100% | 34.1% |
| TBI (Right) | Absent | 19,873,866 | 2,779,760 | 129,449 | 2,635,007 | 99.4% | 14.0% |
| TBI (Left) | Present | 3,982,816 | 2,496,637 | 6122 | 2,490,401 | 100% | 62.7% |
| TBI (Left) | Absent | 21,574,268 | 2,587,892 | 123,034 | 2,454,765 | 99.6% | 12.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergmann, T.; Froese, L.; Gomez, A.; Sainbhi, A.S.; Vakitbilir, N.; Islam, A.; Stein, K.; Marquez, I.; Amenta, F.; Park, K.; et al. Evaluation of Morlet Wavelet Analysis for Artifact Detection in Low-Frequency Commercial Near-Infrared Spectroscopy Systems. Bioengineering 2024, 11, 33. https://doi.org/10.3390/bioengineering11010033
Bergmann T, Froese L, Gomez A, Sainbhi AS, Vakitbilir N, Islam A, Stein K, Marquez I, Amenta F, Park K, et al. Evaluation of Morlet Wavelet Analysis for Artifact Detection in Low-Frequency Commercial Near-Infrared Spectroscopy Systems. Bioengineering. 2024; 11(1):33. https://doi.org/10.3390/bioengineering11010033
Chicago/Turabian StyleBergmann, Tobias, Logan Froese, Alwyn Gomez, Amanjyot Singh Sainbhi, Nuray Vakitbilir, Abrar Islam, Kevin Stein, Izzy Marquez, Fiorella Amenta, Kevin Park, and et al. 2024. "Evaluation of Morlet Wavelet Analysis for Artifact Detection in Low-Frequency Commercial Near-Infrared Spectroscopy Systems" Bioengineering 11, no. 1: 33. https://doi.org/10.3390/bioengineering11010033






