Optic Nerve Head Pulsatile Displacement in Open-Angle Glaucoma after Intraocular Pressure Reduction Measured by Optical Coherence Tomography: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Clinical Examination
2.2. Image Processing
2.3. Statistics
3. Results
3.1. Demographic and Clinical Data
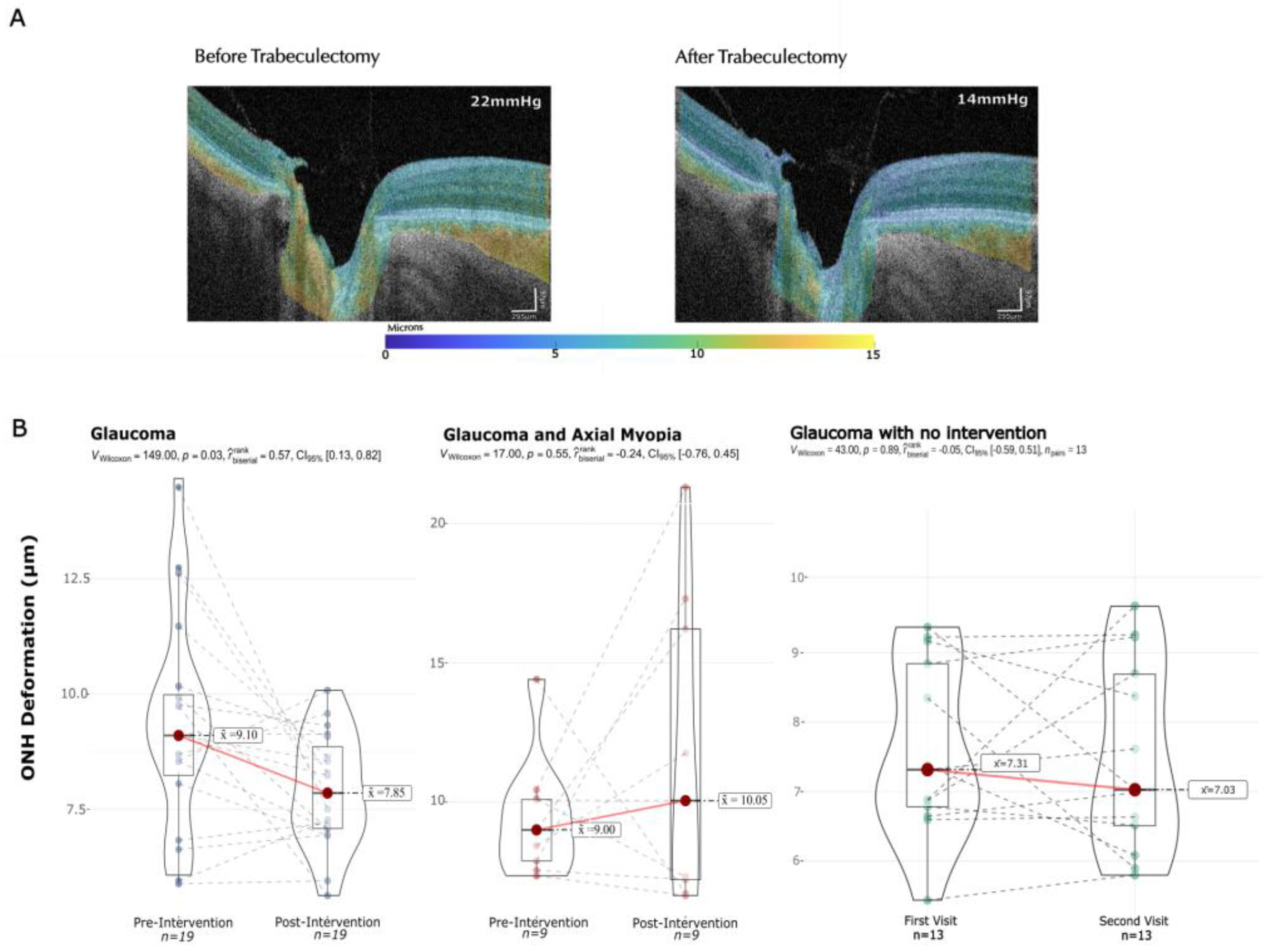
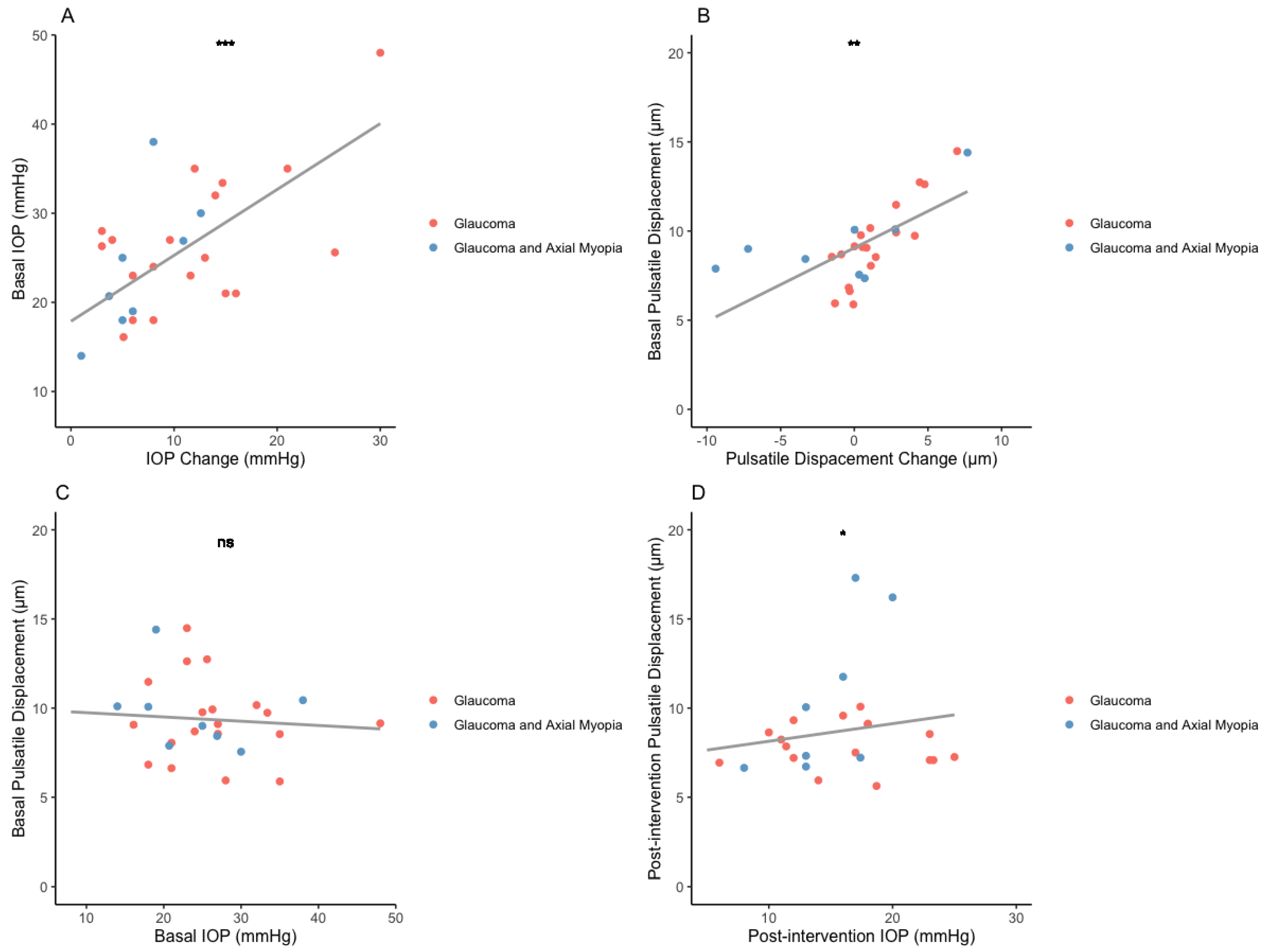
3.2. Pulsatile Displacement Change
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varma, R.; Lee, P.P.; Goldberg, I.; Kotak, S. An Assessment of the Health and Economic Burdens of Glaucoma. Am. J. Ophthalmol. 2011, 152, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.J.A.; Beotra, M.R.; Chin, K.S.; Sandhu, A.; Clemo, M.; Nikita, E.; Kamal, D.S.; Papadopoulos, M.; Mari, J.M.; Aung, T.; et al. In Vivo 3-Dimensional Strain Mapping of the Optic Nerve Head Following Intraocular Pressure Lowering by Trabeculectomy. Ophthalmology 2016, 123, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, R.D.; Weinreb, R.N. Mechanisms of Optic Nerve Damage in Primary Open Angle Glaucoma. Surv. Ophthalmol. 1994, 39, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Flower, R.W.; Addicks, E.M.; McLeod, D.S. The Mechanism of Optic Nerve Damage in Experimental Acute Intraocular Pressure Elevation. Investig. Ophthalmol. Vis. Sci. 1980, 19, 505–517. [Google Scholar]
- Johnson, E.C.; Jia, L.; Cepurna, W.O.; Doser, T.A.; Morrison, J.C. Global Changes in Optic Nerve Head Gene Expression after Exposure to Elevated Intraocular Pressure in a Rat Glaucoma Model. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3161–3177. [Google Scholar] [CrossRef] [PubMed]
- Ravier, M.; Hong, S.; Girot, C.; Ishikawa, H.; Tauber, J.; Wollstein, G.; Schuman, J.; Fishbaugh, J.; Gerig, G. Analysis of Morphological Changes of Lamina Cribrosa Under Acute Intraocular Pressure Change. Med. Image Comput. Comput. Assist. Interv. 2018, 11071, 364–371. [Google Scholar] [PubMed]
- Kirby, M.A.; Pelivanov, I.; Song, S.; Ambrozinski, Ł.; Yoon, S.J.; Gao, L.; Li, D.; Shen, T.T.; Wang, R.K.; O’Donnell, M. Optical Coherence Elastography in Ophthalmology. J. Biomed. Opt. 2017, 22, 121720. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Yoo, L.; Park, J.; Demer, J.L. Finite Element Biomechanics of Optic Nerve Sheath Traction in Adduction. J. Biomech. Eng. 2017, 139, 101010. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.S.; Dharsee, M.; Ackloo, S.; Sivak, J.M.; Flanagan, J.G. Proteomics Analyses of Human Optic Nerve Head Astrocytes Following Biomechanical Strain. Mol. Cell. Proteom. 2012, 11, M111.012302. [Google Scholar] [CrossRef]
- Sigal, I.A.; Flanagan, J.G.; Tertinegg, I.; Ethier, C.R. Predicted Extension, Compression and Shearing of Optic Nerve Head Tissues. Exp. Eye Res. 2007, 85, 312–322. [Google Scholar] [CrossRef]
- Wang, Y.X.; Panda-Jonas, S.; Jonas, J.B. Optic Nerve Head Anatomy in Myopia and Glaucoma, Including Parapapillary Zones Alpha, Beta, Gamma and Delta: Histology and Clinical Features. Prog. Retin. Eye Res. 2021, 83, 100933. [Google Scholar] [CrossRef] [PubMed]
- Dastiridou, A.I.; Ginis, H.; Tsilimbaris, M.; Karyotakis, N.; Detorakis, E.; Siganos, C.; Cholevas, P.; Tsironi, E.E.; Pallikaris, I.G. Ocular Rigidity, Ocular Pulse Amplitude, and Pulsatile Ocular Blood Flow: The Effect of Axial Length. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Parrish, R.K.; Khanna, C.L.; Brandt, J.D.; Soltau, J.B.; Johnson, C.A.; Keltner, J.L.; Huecker, J.B.; et al. Assessment of Cumulative Incidence and Severity of Primary Open-Angle Glaucoma Among Participants in the Ocular Hypertension Treatment Study After 20 Years of Follow-Up. JAMA Ophthalmol. 2021, 139, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Grytz, R.; Sigal, I.A.; Ruberti, J.W.; Meschke, G.; Downs, J.C. Lamina Cribrosa Thickening in Early Glaucoma Predicted by a Microstructure Motivated Growth and Remodeling Approach. Mech. Mater. 2012, 44, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Dastiridou, A.I.; Ginis, H.S.; De Brouwere, D.; Tsilimbaris, M.K.; Pallikaris, I.G. Ocular Rigidity, Ocular Pulse Amplitude, and Pulsatile Ocular Blood Flow: The Effect of Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5718–5722. [Google Scholar] [CrossRef] [PubMed]
- Lesk, M.R.; Spaeth, G.L.; Azuara-Blanco, A.; Araujo, S.V.; Katz, L.J.; Terebuh, A.K.; Wilson, R.P.; Moster, M.R.; Schmidt, C.M. Reversal of Optic Disc Cupping after Glaucoma Surgery Analyzed with a Scanning Laser Tomograph. Ophthalmology 1999, 106, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Czerpak, C.A.; Kashaf, M.S.; Zimmerman, B.K.; Quigley, H.A.; Nguyen, T.D. The Strain Response to Intraocular Pressure Decrease in the Lamina Cribrosa of Patients with Glaucoma. Ophthalmol. Glaucoma 2023, 6, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Thornton, I.L.; Dupps, W.J.; Sinha Roy, A.; Krueger, R.R. Biomechanical Effects of Intraocular Pressure Elevation on Optic Nerve/Lamina Cribrosa before and after Peripapillary Scleral Collagen Cross-Linking. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1227–1233. [Google Scholar] [CrossRef]
- Bellezza, A.J.; Rintalan, C.J.; Thompson, H.W.; Downs, J.C.; Hart, R.T.; Burgoyne, C.F. Anterior Scleral Canal Geometry in Pressurised (IOP 10) and Non-Pressurised (IOP 0) Normal Monkey Eyes. Br. J. Ophthalmol. 2003, 87, 1284–1290. [Google Scholar] [CrossRef]
- Wei, J.; Hua, Y.; Yang, B.; Wang, B.; Schmitt, S.E.; Wang, B.; Lucy, K.A.; Ishikawa, H.; Schuman, J.S.; Smith, M.A.; et al. Comparing Acute IOP-Induced Lamina Cribrosa Deformations Premortem and Postmortem. Transl. Vis. Sci. Technol. 2022, 11, 1. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Morgan, W.H.; Hazelton, M.L.; House, P.H.; Barry, C.J.; Chan, H.; Cringle, S.J.; Yu, D.-Y. Value of Retinal Vein Pulsation Characteristics in Predicting Increased Optic Disc Excavation. Br. J. Ophthalmol. 2007, 91, 441–444. [Google Scholar] [CrossRef]
- Daneshvar, R.; Nouri-Mahdavi, K. Optical Coherence Tomography Angiography: A New Tool in Glaucoma Diagnostics and Research. J. Ophthalmic Vis. Res. 2017, 12, 325–332. [Google Scholar] [CrossRef]
- Cheng, R.W.; Yusof, F.; Tsui, E.; Jong, M.; Duffin, J.; Flanagan, J.G.; Fisher, J.A.; Hudson, C. Relationship between Retinal Blood Flow and Arterial Oxygen. J. Physiol. 2016, 594, 625–640. [Google Scholar] [CrossRef]
- Schmidt, K.G.; Rückmann, A.V.; Mittag, T.W.; Hessemer, V.; Pillunat, L.E. Reduced Ocular Pulse Amplitude in Low Tension Glaucoma Is Independent of Vasospasm. Eye 1997, 11 Pt 4, 485–488. [Google Scholar] [CrossRef]
- Ch’ng, T.W.; Chua, C.Y.; Ummi Kalsom, M.A.; Azhany, Y.; Gong, V.; Rasool, A.; Liza-Sharmini, A.T. Ocular Perfusion Pressure and Severity of Glaucoma: Is There a Link? J. Curr. Glaucoma Pract. 2021, 15, 78–85. [Google Scholar] [CrossRef]
- Zhang, J.; Murgoitio-Esandi, J.; Qian, X.; Li, R.; Gong, C.; Nankali, A.; Hao, L.; Xu, B.Y.; Kirk Shung, K.; Oberai, A.; et al. High-Frequency Ultrasound Elastography to Assess the Nonlinear Elastic Properties of the Cornea and Ciliary Body. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 2621–2629. [Google Scholar] [CrossRef]
- Li, R.; Qian, X.; Gong, C.; Zhang, J.; Liu, Y.; Xu, B.; Humayun, M.S.; Zhou, Q. Simultaneous Assessment of the Whole Eye Biomechanics Using Ultrasonic Elastography. IEEE Trans. Biomed. Eng. 2023, 70, 1310–1317. [Google Scholar] [CrossRef]
- Flammer, J.; Konieczka, K.; Flammer, A.J. The Primary Vascular Dysregulation Syndrome: Implications for Eye Diseases. EPMA J. 2013, 4, 14. [Google Scholar] [CrossRef]
- Hidalgo-Aguirre, M.; Costantino, S.; Lesk, M.R. Pilot Study of the Pulsatile Neuro-Peripapillary Retinal Deformation in Glaucoma and Its Relationship with Glaucoma Risk Factors. Curr. Eye Res. 2017, 42, 1620–1627. [Google Scholar] [CrossRef]
- Solano, M.M.; Richer, E.; Cheriet, F.; Lesk, M.R.; Costantino, S. Mapping Pulsatile Optic Nerve Head Deformation Using OCT. Ophthalmol. Sci. 2022, 2, 100205. [Google Scholar] [CrossRef]
- Fortune, B.; Reynaud, J.; Hardin, C.; Wang, L.; Sigal, I.A.; Burgoyne, C.F. Experimental Glaucoma Causes Optic Nerve Head Neural Rim Tissue Compression: A Potentially Important Mechanism of Axon Injury. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4403–4411. [Google Scholar] [CrossRef] [PubMed]
- Stowell, C.; Burgoyne, C.F.; Tamm, E.R.; Ethier, C.R. Lasker/IRRF Initiative on Astrocytes and Glaucomatous Neurodegeneration Participants Biomechanical Aspects of Axonal Damage in Glaucoma: A Brief Review. Exp. Eye Res. 2017, 157, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, C.F.; Downs, J.C.; Bellezza, A.J.; Suh, J.-K.F.; Hart, R.T. The Optic Nerve Head as a Biomechanical Structure: A New Paradigm for Understanding the Role of IOP-Related Stress and Strain in the Pathophysiology of Glaucomatous Optic Nerve Head Damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef] [PubMed]
- Safa, B.N.; Wong, C.A.; Ha, J.; Ethier, C.R. Glaucoma and Biomechanics. Curr. Opin. Ophthalmol. 2022, 33, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Pahlavian, S.H.; Loth, F.; Luciano, M.; Oshinski, J.; Martin, B.A. Neural Tissue Motion Impacts Cerebrospinal Fluid Dynamics at the Cervical Medullary Junction: A Patient-Specific Moving-Boundary Computational Model. Ann. Biomed. Eng. 2015, 43, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Breusegem, C.; Fieuws, S.; Zeyen, T.; Stalmans, I. The Effect of Trabeculectomy on Ocular Pulse Amplitude. Investig. Ophthalmol. Vis. Sci. 2010, 51, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, U.; Garip, R.; Solmaz, B.; Altan, C. Changes in Intra-Ocular Pressure, Ocular Pulse Amplitude and Choroidal Thickness after Trabeculectomy. Clin. Exp. Optom. 2023, 106, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Kiel, J.W.; van Heuven, W.A. Ocular Perfusion Pressure and Choroidal Blood Flow in the Rabbit. Investig. Ophthalmol. Vis. Sci. 1995, 36, 579–585. [Google Scholar]
- Cahill, N.D.; Noble, J.A.; Hawkes, D.J. A Demons Algorithm for Image Registration with Locally Adaptive Regularization. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2009, London, UK, 20–24 September 2009; Springer: Berlin/Heidelberg, Germany, 2009; pp. 574–581. [Google Scholar]
- Thirion, J.-P. Image Matching as a Diffusion Process: An Analogy with Maxwell’s Demons. Med. Image Anal. 1998, 2, 243–260. [Google Scholar] [CrossRef]
- Vercauteren, T.; Pennec, X.; Malis, E.; Perchant, A.; Ayache, N. Insight into Efficient Image Registration Techniques and the Demons Algorithm. Inf. Process. Med. Imaging 2007, 20, 495–506. [Google Scholar]
- Vercauteren, T.; Pennec, X.; Perchant, A.; Ayache, N. Non-Parametric Diffeomorphic Image Registration with the Demons Algorithm. Med. Image Comput. Comput. Assist. Interv. 2007, 10, 319–326. [Google Scholar] [PubMed]
- Cachier, P.; Pennec, X.; Ayache, N. Fast Non Rigid Matching by Gradient Descent: Study and Improvements of the “Demons” Algorithm; INRIA: Paris, France, 1999. [Google Scholar]
- Oh, B.-L.; Lee, E.J.; Kim, H.; Girard, M.J.A.; Mari, J.M.; Kim, T.-W. Anterior Lamina Cribrosa Surface Depth in Open-Angle Glaucoma: Relationship with the Position of the Central Retinal Vessel Trunk. PLoS ONE 2016, 11, e0158443. [Google Scholar] [CrossRef] [PubMed]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Shen, M.; Fan, F.; Xue, A.; Wang, J.; Zhou, X.; Lu, F. Biomechanical Properties of the Cornea in High Myopia. Vis. Res. 2008, 48, 2167–2171. [Google Scholar] [CrossRef]
- Han, F.; Li, M.; Wei, P.; Ma, J.; Jhanji, V.; Wang, Y. Effect of Biomechanical Properties on Myopia: A Study of New Corneal Biomechanical Parameters. BMC Ophthalmol. 2020, 20, 459. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Yap, M.K.H. Factors Affecting Ocular Rigidity in the Chinese. Clin. Exp. Optom. 1991, 74, 156–159. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Fenerty, C.H.; Crean, J.; Wordinger, R.J.; Clark, A.F.; O’Brien, C.J. Influence of Cyclical Mechanical Strain on Extracellular Matrix Gene Expression in Human Lamina Cribrosa Cells In Vitro. Mol. Vis. 2005, 11, 798–810. [Google Scholar]
- Jin, Y.; Wang, X.; Zhang, L.; Jonas, J.B.; Aung, T.; Schmetterer, L.; Girard, M.J.A. Modeling the Origin of the Ocular Pulse and Its Impact on the Optic Nerve Head. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3997–4010. [Google Scholar] [CrossRef]
- Singh, K.; Dion, C.; Godin, A.G.; Lorghaba, F.; Descovich, D.; Wajszilber, M.; Ozaki, T.; Costantino, S.; Lesk, M.R. Pulsatile Movement of the Optic Nerve Head and the Peripapillary Retina in Normal Subjects and in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7819–7824. [Google Scholar] [CrossRef]
- Riva, C.E.; Titze, P.; Hero, M.; Petrig, B.L. Effect of Acute Decreases of Perfusion Pressure on Choroidal Blood Flow in Humans. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1752–1760. [Google Scholar]
- Sayah, D.N.; Lesk, M.R. Ocular Rigidity and Glaucoma. In Ocular Rigidity, Biomechanics and Hydrodynamics of the Eye; Pallikaris, I., Tsilimbaris, M.K., Dastiridou, A.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 267–290. ISBN 978-3-03-064422-2. [Google Scholar]
- Wang, J.; Freeman, E.E.; Descovich, D.; Harasymowycz, P.J.; Kamdeu Fansi, A.; Li, G.; Lesk, M.R. Estimation of Ocular Rigidity in Glaucoma Using Ocular Pulse Amplitude and Pulsatile Choroidal Blood Flow. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tun, T.A.; Baskaran, M.; Atalay, E.; Thakku, S.G.; Liang, Z.; Milea, D.; Strouthidis, N.G.; Aung, T.; Girard, M.J. Effect of Acute Intraocular Pressure Elevation on the Minimum Rim Width in Normal, Ocular Hypertensive and Glaucoma Eyes. Br. J. Ophthalmol. 2018, 102, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, A.; Izadi, S.; Jeffery, G. Age-Related Changes in the Thickness of the Human Lamina Cribrosa. Br. J. Ophthalmol. 2006, 90, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, G.; Zhou, X.; Xu, R.; Wang, S.; Guan, Z.; Lu, J.; Srinivasalu, N.; Shen, M.; Jin, Z.; et al. Changes in Choroidal Thickness and Choroidal Blood Perfusion in Guinea Pig Myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Sayah, D.N. Ocular Rigidity: A Previously Unexplored Risk Factor in the Pathophysiology of Open-Angle Glaucoma: Assessment Using a Novel OCT-Based Measurement Method. 2020. Available online: https://papyrus.bib.umontreal.ca/xmlui/handle/1866/24253 (accessed on 4 March 2024).
- Tanito, M.; Sugihara, K.; Tsutsui, A.; Hara, K.; Manabe, K.; Matsuoka, Y. Effects of Preoperative Intraocular Pressure Level on Surgical Results of Microhook Ab Interno Trabeculotomy. J. Clin. Med. Res. 2021, 10, 3327. [Google Scholar] [CrossRef] [PubMed]
- Pillunat, K.R.; Spoerl, E.; Elfes, G.; Pillunat, L.E. Preoperative Intraocular Pressure as a Predictor of Selective Laser Trabeculoplasty Efficacy. Acta Ophthalmol. 2016, 94, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.I.; Chansangpetch, S.; Nguyen, A.; Feinstein, M.; Mora, M.; Badr, M.; Masis, M.; Porco, T.; Lin, S.C. How to Predict Intraocular Pressure Reduction after Cataract Surgery? A Prospective Study. Curr. Eye Res. 2019, 44, 623–631. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, J. Measurements of Ocular Properties in Response to Intraocular Pressure Changes Using an Ultrasonic System. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 5076–5079. [Google Scholar]
- Roberts, M.D.; Sigal, I.A.; Liang, Y.; Burgoyne, C.F.; Downs, J.C. Changes in the Biomechanical Response of the Optic Nerve Head in Early Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5675–5684. [Google Scholar] [CrossRef] [PubMed]
- Strouthidis, N.G.; Girard, M.J.A. Altering the Way the Optic Nerve Head Responds to Intraocular Pressure—A Potential Approach to Glaucoma Therapy. Curr. Opin. Pharmacol. 2013, 13, 83–89. [Google Scholar] [CrossRef]
- Raghu, N.; Pandav, S.S.; Kaushik, S.; Ichhpujani, P.; Gupta, A. Effect of Trabeculectomy on RNFL Thickness and Optic Disc Parameters Using Optical Coherence Tomography. Eye 2012, 26, 1131–1137. [Google Scholar] [CrossRef]
- Aydin, A.; Wollstein, G.; Price, L.L.; Fujimoto, J.G.; Schuman, J.S. Optical Coherence Tomography Assessment of Retinal Nerve Fiber Layer Thickness Changes after Glaucoma Surgery. Ophthalmology 2003, 110, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Ivers, K.M.; Sredar, N.; Patel, N.B.; Rajagopalan, L.; Queener, H.M.; Twa, M.D.; Harwerth, R.S.; Porter, J. In Vivo Changes in Lamina Cribrosa Microarchitecture and Optic Nerve Head Structure in Early Experimental Glaucoma. PLoS ONE 2015, 10, e0134223. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, H.; Gardiner, S.K.; Williams, G.; Hardin, C.; Strouthidis, N.G.; Fortune, B.; Burgoyne, C.F. Longitudinal Detection of Optic Nerve Head Changes by Spectral Domain Optical Coherence Tomography in Early Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Gusek, G.C.; Naumann, G.O. Optic Disc, Cup and Neuroretinal Rim Size, Configuration and Correlations in Normal Eyes. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1151–1158. [Google Scholar]
- Park, H.-Y.L.; Yi, R.; Jung, Y.; Park, C.K. Effect of Glaucoma Surgery on the Progression Rate and Pattern in Glaucoma Patients with Myopia. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4170–4179. [Google Scholar] [CrossRef]


| Parameter | Group (n = 28) | ||||||
|---|---|---|---|---|---|---|---|
| Glaucoma (n = 19) | Glaucoma and Myopia (n = 9) | ||||||
| Pre-Intervention | Post-Intervention | p-Value * | Pre-Intervention | Post-Intervention | p-Value * | Baseline Comparison (p-Value) | |
| Age (years) | 67.7 ± 11 | - | 64.4 ± 14 | - | 0.4 + | ||
| Sex (female %) | 14 (73%) | - | 4 (44%) | - | 0.1 ο | ||
| Intervention (surgical %) ** | 8 (50%) | - | 4 (44%) | - | - | ||
| AL (mm) | 23.19 ± 0.7 | - | 25.89 ± 0.5 | - | <0.005 + | ||
| IOP (mmHg) | 25.65 ± 8 | 16.5 ± 7 | <0.005 | 22.2 ± 6 | 16.0 ± 9 | 0.01 | 0.2 + |
| IOP change (mmHg) | 9.1 ± 7 | - | 6.2 ± 4 | - | 0.01 + | ||
| Glaucoma severity *** | |||||||
| Early (n (%)) | 7 (37%) | - | 4 (44%) | - | - | ||
| Moderate (n (%)) | 6 (31.5%) | - | 2 (28%) | - | - | ||
| Severe (n (%)) | 6 (31.5%) | - | 3 (28%) | - | - | ||
| Anatomical and Functional Assessment | |||||||
| Visual Field MD (dB) | −3.67 ± 6 | −3.68 ± 6 | 0.4 | −5.51 ± 3 | −5.40 ± 4 | 0.8 | 0.5 + |
| BMO area (μm2) | 1.89 ± 0.4 | 1.88 ± 0.4 | 0.7 | 2.26 ± 0.5 | 2.25 ± 0.5 | 0.1 | 0.4 + |
| GCC volume (mm3) | 0.91 ± 0.1 | 0.88 ± 0.2 | 0.02 | 0.86 ± 0.1 | 0.87 ± 0.1 | 0.7 | 0.6 + |
| Peripapillary CT area (mm2) | 974 ± 353 | 1140 ± 380 | 0.1 | 499 ± 369 | 661 ± 352 | 0.7 | <0.005 + |
| Anterior PLT depth (μm) | 1033.02 ± 600 | 973.39 ± 559 | 0.05 | 753.46 ± 439 | 740.12 ± 452 | 0.5 | 0.3 + |
| RNFL Thickness (μm) | |||||||
| Superior | 96 ± 26 | 94 ± 24 | 0.08 | 96 ± 16 | 92 ± 17 | 0.01 | 0.5 + |
| Inferior | 94 ± 33 | 87 ± 35 | 0.05 | 101 ± 36 | 89 ± 32 | 0.01 | 0.8 + |
| Temporal | 57 ± 15 | 55 ± 13 | 0.7 | 63 ± 11 | 64 ± 13 | 0.3 | 0.4 + |
| Nasal | 59 ± 16 | 61 ± 16 | 0.1 | 56 ± 16 | 61 ± 17 | 0.5 | 0.2 + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masís Solano, M.; Richer, E.; Costantino, S.; Lesk, M.R. Optic Nerve Head Pulsatile Displacement in Open-Angle Glaucoma after Intraocular Pressure Reduction Measured by Optical Coherence Tomography: A Pilot Study. Bioengineering 2024, 11, 411. https://doi.org/10.3390/bioengineering11050411
Masís Solano M, Richer E, Costantino S, Lesk MR. Optic Nerve Head Pulsatile Displacement in Open-Angle Glaucoma after Intraocular Pressure Reduction Measured by Optical Coherence Tomography: A Pilot Study. Bioengineering. 2024; 11(5):411. https://doi.org/10.3390/bioengineering11050411
Chicago/Turabian StyleMasís Solano, Marissé, Emmanuelle Richer, Santiago Costantino, and Mark R. Lesk. 2024. "Optic Nerve Head Pulsatile Displacement in Open-Angle Glaucoma after Intraocular Pressure Reduction Measured by Optical Coherence Tomography: A Pilot Study" Bioengineering 11, no. 5: 411. https://doi.org/10.3390/bioengineering11050411





