Human versus Rat PRF on Collagen Membranes: A Pilot Study of Mineralization in Rat Calvaria Defect Model
Abstract
:1. Introduction
2. Material and Methods
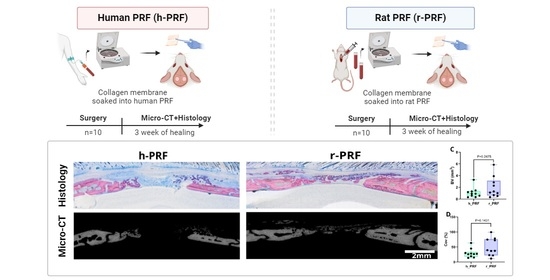
2.1. Study Design
2.2. Preparation of C-PRF
2.3. Surgery
2.4. Micro-CT Analysis
2.5. Histological Analysis
2.6. Statistics
3. Results
3.1. Histological Analysis
3.2. Collagen Membrane Soaked in Human PRF (h-PRF)
3.3. Collagen Membrane Soaked in Rat PRF (r-PRF)
3.4. Micro-CT Assessment of Defects Treated with Membranes Soaked in h-PRF and r-PRF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miron, R.J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Optimization of platelet-rich fibrin. Periodontol. 2000, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Moraschini, V.; Del Fabbro, M.; Piattelli, A.; Fujioka-Kobayashi, M.; Zhang, Y.; Saulacic, N.; Schaller, B.; Kawase, T.; Cosgarea, R.; et al. Use of platelet-rich fibrin for the treatment of gingival recessions: A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 2543–2557. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Moraschini, V.; Fujioka-Kobayashi, M.; Zhang, Y.; Kawase, T.; Cosgarea, R.; Jepsen, S.; Bishara, M.; Canullo, L.; Shirakata, Y.; et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.J.; Stahli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 6–19. [Google Scholar] [CrossRef] [PubMed]
- Valentini, P.; Calciolari, E.; Monlezun, S.; Akcali, A.; Donos, N.; Quirynen, M. APCs in sinus floor augmentation. Periodontol. 2000, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Quirynen, M.; Siawasch, S.; Temmerman, A.; Cortellini, S.; Dhondt, R.; Teughels, W.; Castro, A.B. Do autologous platelet concentrates (APCs) have a role in intra-oral bone regeneration? A critical review of clinical guidelines on decision-making process. Periodontol. 2000 2023, 93, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Chen, C.J.; Barootchi, S.; Kim, D.M. Efficacy of biologics for the treatment of periodontal infrabony defects: An American Academy of Periodontology best evidence systematic review and network meta-analysis. J. Periodontol. 2022, 93, 1803–1826. [Google Scholar] [CrossRef] [PubMed]
- Cano-Duran, J.A.; Pena-Cardelles, J.F.; Ortega-Concepcion, D.; Paredes-Rodriguez, V.M.; Garcia-Riart, M.; Lopez-Quiles, J. The role of Leucocyte-rich and platelet-rich fibrin (L-PRF) in the treatment of the medication-related osteonecrosis of the jaws (MRONJ). J. Clin. Exp. Dent. 2017, 9, e1051–e1059. [Google Scholar] [CrossRef]
- Pinto, N.R.; Ubilla, M.; Zamora, Y.; Del Rio, V.; Dohan Ehrenfest, D.M.; Quirynen, M. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: A prospective cohort study. Platelets 2018, 29, 468–475. [Google Scholar] [CrossRef]
- Bai, M.Y.; Vy, V.P.T.; Tang, S.L.; Hung, T.N.K.; Wang, C.W.; Liang, J.Y.; Wong, C.C.; Chan, W.P. Current Progress of Platelet-Rich Derivatives in Cartilage and Joint Repairs. Int. J. Mol. Sci. 2023, 24, 2608. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Rojas, E. Platelet-Rich Fibrin Membrane for Pterygium Surgery: Literature Review and Feasibility Assessment. Cureus 2021, 13, e17884. [Google Scholar] [CrossRef]
- Temmerman, A.; Vandessel, J.; Castro, A.; Jacobs, R.; Teughels, W.; Pinto, N.; Quirynen, M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: A split-mouth, randomized, controlled clinical trial. J. Clin. Periodontol. 2016, 43, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.B.; Van Dessel, J.; Temmerman, A.; Jacobs, R.; Quirynen, M. Effect of different platelet-rich fibrin matrices for ridge preservation in multiple tooth extractions: A split-mouth randomized controlled clinical trial. J. Clin. Periodontol. 2021, 48, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, S.; Castro, A.B.; Temmerman, A.; Van Dessel, J.; Pinto, N.; Jacobs, R.; Quirynen, M. Leucocyte- and platelet-rich fibrin block for bone augmentation procedure: A proof-of-concept study. J. Clin. Periodontol. 2018, 45, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Priya, A.; Yadav, H.; Mishra, N.; Kumar, L. Bone augmentation with sticky bone and platelet-rich fibrin by ridge-split technique and nasal floor engagement for immediate loading of dental implant after extracting impacted canine. Natl. J. Maxillofac. Surg. 2019, 10, 98–101. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Fernandes, G.V.; Malta Santos, N.B.; Correia de Sousa, M.F.; Fernandes, J.C.H. Liquid Platelet-Rich Fibrin Coating Implant Surface to Enhance Osseointegration: A Double-Blinded, Randomized Split-Mouth Trial with 1-Year Follow-up. Int. J. Oral Maxillofac. Implant. 2022, 37, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Lollobrigida, M.; Maritato, M.; Bozzuto, G.; Formisano, G.; Molinari, A.; De Biase, A. Biomimetic Implant Surface Functionalization with Liquid L-PRF Products: In Vitro Study. Biomed. Res. Int. 2018, 2018, 9031435. [Google Scholar] [CrossRef] [PubMed]
- Oncu, E.; Alaaddinoglu, E.E. The effect of platelet-rich fibrin on implant stability. Int. J. Oral Maxillofac. Implant. 2015, 30, 578–582. [Google Scholar] [CrossRef]
- Strauss, F.J.; Nasirzade, J.; Kargarpoor, Z.; Stahli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Savio, D.S.; Silva, L.; Reis, G.G.D.; Denardi, R.J.; Costa, N.; Chaves Furlaneto, F.A.; Souza, S.L.S.; Mourao, C.; Miron, R.J.; Okamoto, R.; et al. Effects of platelet-rich fibrin produced by three centrifugation protocols on bone neoformation in defects created in rat calvaria. Platelets 2023, 34, 2228417. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.M.P.; Savio, D.S.F.; de Avila, F.C.; Vicente, R.M.; Reis, G.G.D.; Denardi, R.J.; da Costa, N.M.M.; Silva, P.H.F.; Mourao, C.; Miron, R.J.; et al. Comparison of the effects of platelet concentrates produced by high and low-speed centrifugation protocols on the healing of critical-size defects in rat calvaria: A microtomographic and histomorphometric study. Platelets 2022, 33, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Engler-Pinto, A.; Siessere, S.; Calefi, A.; Oliveira, L.; Ervolino, E.; de Souza, S.; Furlaneto, F.; Messora, M.R. Effects of leukocyte- and platelet-rich fibrin associated or not with bovine bone graft on the healing of bone defects in rats with osteoporosis induced by ovariectomy. Clin. Oral Implant. Res. 2019, 30, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Do Lago, E.S.; Ferreira, S.; Garcia, I.R., Jr.; Okamoto, R.; Mariano, R.C. Improvement of bone repair with l-PRF and bovine bone in calvaria of rats. histometric and immunohistochemical study. Clin. Oral Investig. 2020, 24, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Feher, B.; Apaza Alccayhuaman, K.A.; Strauss, F.J.; Lee, J.S.; Tangl, S.; Kuchler, U.; Gruber, R. Osteoconductive properties of upside-down bilayer collagen membranes in rat calvarial defects. Int. J. Implant. Dent. 2021, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Nasirzade, J.; Alccayhuaman, K.A.A.; Kargarpour, Z.; Kuchler, U.; Strauss, F.J.; Panahipour, L.; Kampleitner, C.; Heimel, P.; Schwarz, F.; Gruber, R. Acid Dentin Lysate Failed to Modulate Bone Formation in Rat Calvaria Defects. Biology 2021, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.J.; Kuchler, U.; Kobatake, R.; Heimel, P.; Tangl, S.; Gruber, R. Acid bone lysates reduce bone regeneration in rat calvaria defects. J. Biomed. Mater. Res. A 2021, 109, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, U.; Rybaczek, T.; Dobask, T.; Heimel, P.; Tangl, S.; Klehm, J.; Menzel, M.; Gruber, R. Bone-conditioned medium modulates the osteoconductive properties of collagen membranes in a rat calvaria defect model. Clin. Oral Implant. Res. 2018, 29, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Apaza Alccayhuaman, K.A.; Heimel, P.; Tangl, S.; Lettner, S.; Kampleitner, C.; Panahipour, L.; Kuchler, U.; Gruber, R. Active and Passive Mineralization of Bio-Gide((R)) Membranes in Rat Calvaria Defects. J. Funct. Biomater. 2024, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Al-Sharabi, N.; Kampleitner, C.; Mohamed-Ahmed, S.; Kristoffersen, E.K.; Tangl, S.; Mustafa, K.; Gruber, R.; Sanz, M. The use of mesenchymal stromal cell secretome to enhance guided bone regeneration in comparison with leukocyte and platelet-rich fibrin. Clin. Oral Implant. Res. 2024, 35, 141–154. [Google Scholar] [CrossRef]
- Shanbhag, S.; Kampleitner, C.; Al-Sharabi, N.; Mohamed-Ahmed, S.; Apaza Alccayhuaman, K.A.; Heimel, P.; Tangl, S.; Beinlich, A.; Rana, N.; Sanz, M.; et al. Functionalizing Collagen Membranes with MSC-Conditioned Media Promotes Guided Bone Regeneration in Rat Calvarial Defects. Cells 2023, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, R.; Zayed, N.M.A.; Pascu, E.I.; Cavanagh, B.; Hobbs, C.; Santarella, F.; Simpson, C.R.; Murphy, C.M.; Sridharan, R.; Gonzalez-Vazquez, A.; et al. Functionalising Collagen-Based Scaffolds With Platelet-Rich Plasma for Enhanced Skin Wound Healing Potential. Front. Bioeng. Biotechnol. 2019, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Fahimipour, F.; Bastami, F.; Khoshzaban, A.; Jahangir, S.; Eslaminejad, M.B.; Khayyatan, F.; Safiaghdam, H.; Sadooghi, Y.; Safa, M.; Jafarzadeh Kashi, T.S.; et al. Critical-sized bone defects regeneration using a bone-inspired 3D bilayer collagen membrane in combination with leukocyte and platelet-rich fibrin membrane (L-PRF): An in vivo study. Tissue Cell 2020, 63, 101326. [Google Scholar] [CrossRef] [PubMed]
- Horimizu, M.; Kubota, T.; Kawase, T.; Nagata, M.; Kobayashi, M.; Okuda, K.; Nakata, K.; Yoshie, H. Synergistic effects of the combined use of human-cultured periosteal sheets and platelet-rich fibrin on bone regeneration: An animal study. Clin. Exp. Dent. Res. 2017, 3, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Meira, R.O.; Braga, D.N.M.; Pinheiro, L.S.G.; Amorim, I.F.G.; Vasconcellos, L.S.; Alberti, L.R. Effects of homologous and heterologous rich platelets plasma, compared to poor platelets plasma, on cutaneous healing of rabbits. Acta Cir. Bras. 2020, 35, e202001006. [Google Scholar] [CrossRef]
- Mourão, C.F.; Lowenstein, A.; Mello-Machado, R.C.; Ghanaati, S.; Pinto, N.; Kawase, T.; Alves, G.G.; Messora, M.R. Standardization of Animal Models and Techniques for Platelet-Rich Fibrin Production: A Narrative Review and Guideline. Bioengineering 2023, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, G.; Davidzohn, A.; Gursoy, M.; Gursoy, U.K.; Shapira, L.; Wilensky, A. Membrane barriers for guided bone regeneration: An overview of available biomaterials. Periodontol. 2000 2023, 93, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, R.J. Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin. Oral Investig. 2020, 24, 4373–4383. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Zhang, P.; Li, Y.; Wang, Y.; Mourao, C.; Sculean, A.; Fujioka Kobayashi, M.; Zhang, Y. A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin. Oral Investig. 2020, 24, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Miron, R.J.; Gruber, R. Liquid Platelet-Rich Fibrin and Heat-Coagulated Albumin Gel: Bioassays for TGF-beta Activity. Materials 2020, 13, 3466. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Tamai, N.; Naka, N.; Yoshikawa, H.; Myoui, A. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J. Artif. Organs 2013, 16, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, D.V.; Laposy, C.B.; Abegao, K.G.; Nogueira, R.M.; Nai, G.A.; Bracale, B.N.; Delfim, I.G. Comparison of experimentally-induced wounds in rabbits treated with different sources of platelet-rich plasma. Lab. Anim. 2015, 49, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Noguchi, K.; Morishima, Y.; Yamaguchi, K. Generation and characterization of tissue-type plasminogen activator transgenic rats. J. Thromb. Thrombolysis 2018, 45, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manzano, A.; Gonzalez-Llaven, J.; Lemini, C.; Rubio-Poo, C. Standardization of rat blood clotting tests with reagents used for humans. Proc. West. Pharmacol. Soc. 2001, 44, 153–155. [Google Scholar] [PubMed]
- Yuasa, M.; Mignemi, N.A.; Nyman, J.S.; Duvall, C.L.; Schwartz, H.S.; Okawa, A.; Yoshii, T.; Bhattacharjee, G.; Zhao, C.; Bible, J.E.; et al. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification. J. Clin. Investig. 2015, 125, 3117–3131. [Google Scholar] [CrossRef] [PubMed]
- Andreev, D.; Kachler, K.; Schett, G.; Bozec, A. Rheumatoid arthritis and osteoimmunology: The adverse impact of a deregulated immune system on bone metabolism. Bone 2022, 162, 116468. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apaza Alccayhuaman, K.A.; Heimel, P.; Tangl, S.; Lettner, S.; Kampleitner, C.; Panahipour, L.; Kuchler, U.; Gruber, R. Human versus Rat PRF on Collagen Membranes: A Pilot Study of Mineralization in Rat Calvaria Defect Model. Bioengineering 2024, 11, 414. https://doi.org/10.3390/bioengineering11050414
Apaza Alccayhuaman KA, Heimel P, Tangl S, Lettner S, Kampleitner C, Panahipour L, Kuchler U, Gruber R. Human versus Rat PRF on Collagen Membranes: A Pilot Study of Mineralization in Rat Calvaria Defect Model. Bioengineering. 2024; 11(5):414. https://doi.org/10.3390/bioengineering11050414
Chicago/Turabian StyleApaza Alccayhuaman, Karol Ali, Patrick Heimel, Stefan Tangl, Stefan Lettner, Carina Kampleitner, Layla Panahipour, Ulrike Kuchler, and Reinhard Gruber. 2024. "Human versus Rat PRF on Collagen Membranes: A Pilot Study of Mineralization in Rat Calvaria Defect Model" Bioengineering 11, no. 5: 414. https://doi.org/10.3390/bioengineering11050414