Investigating the Effect of Processing and Material Parameters of Alginate Dialdehyde-Gelatin (ADA-GEL)-Based Hydrogels on Stiffness by XGB Machine Learning Model
Abstract
:1. Introduction
2. Methodology
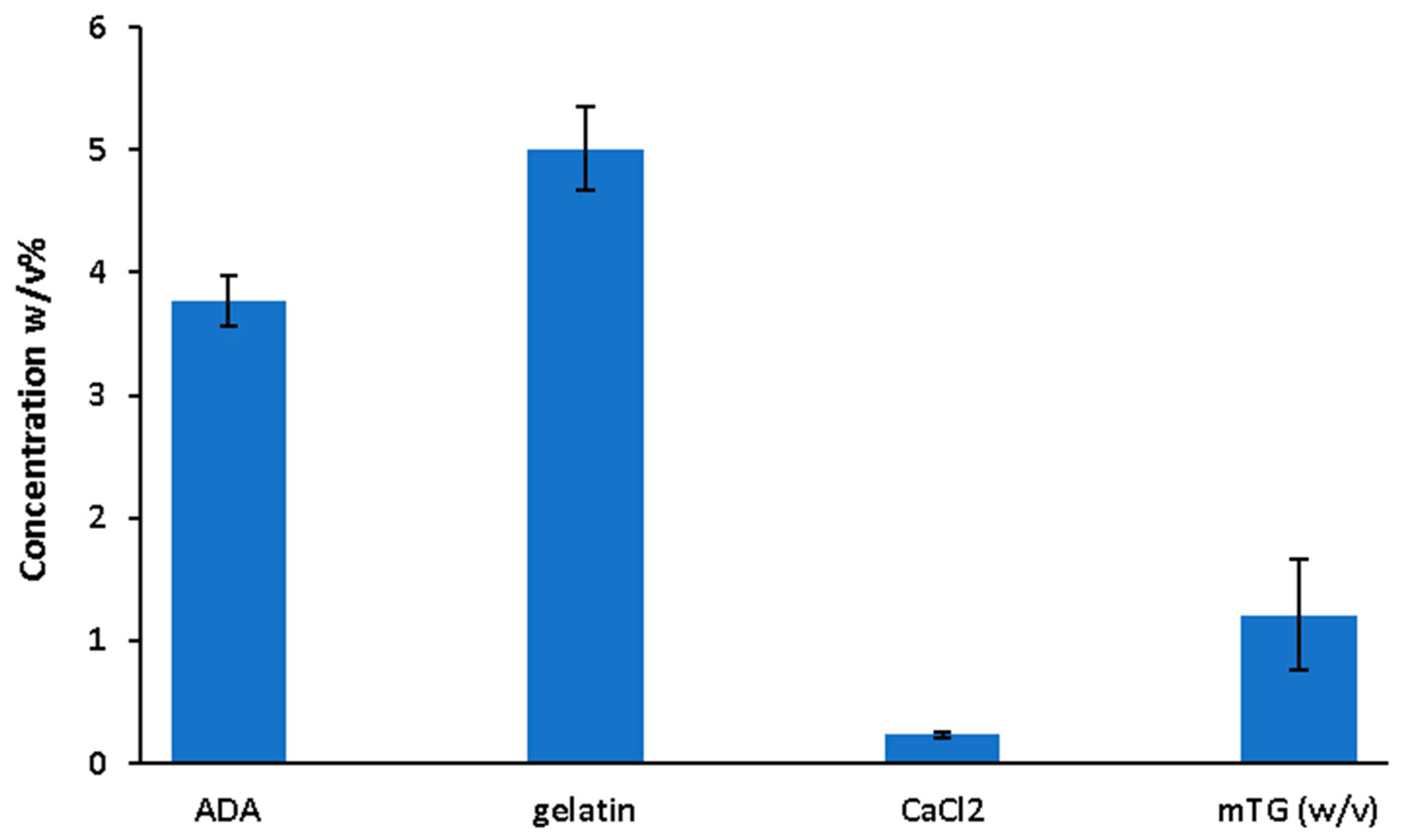
2.1. Data Collection
2.2. Computational Modeling
2.2.1. XGB Regressor
2.2.2. Training, Hyper Tuning and Validation Processes
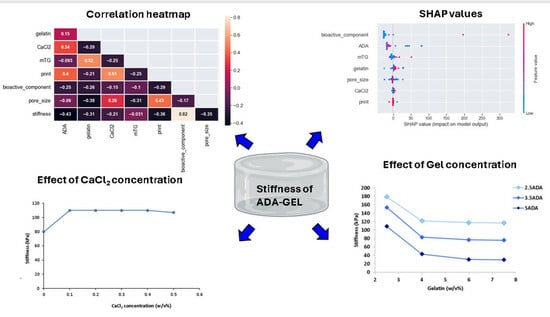
2.2.3. Correlation Heatmap
2.2.4. Feature Importance
2.2.5. Determination of the Model Performance
2.2.6. Shapley Additive Explanation
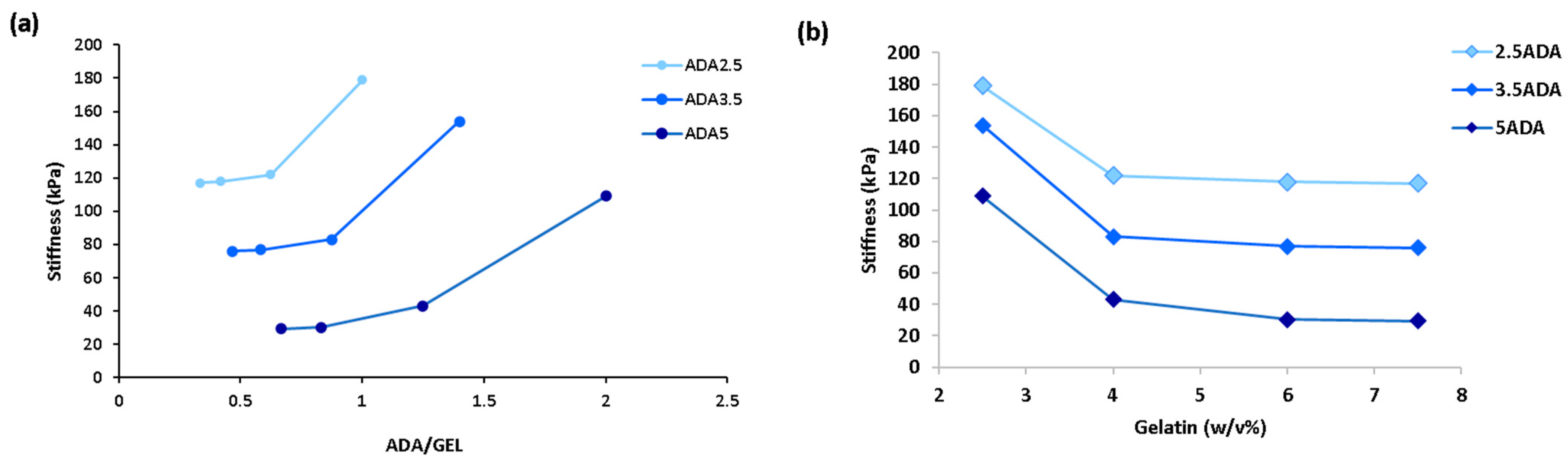
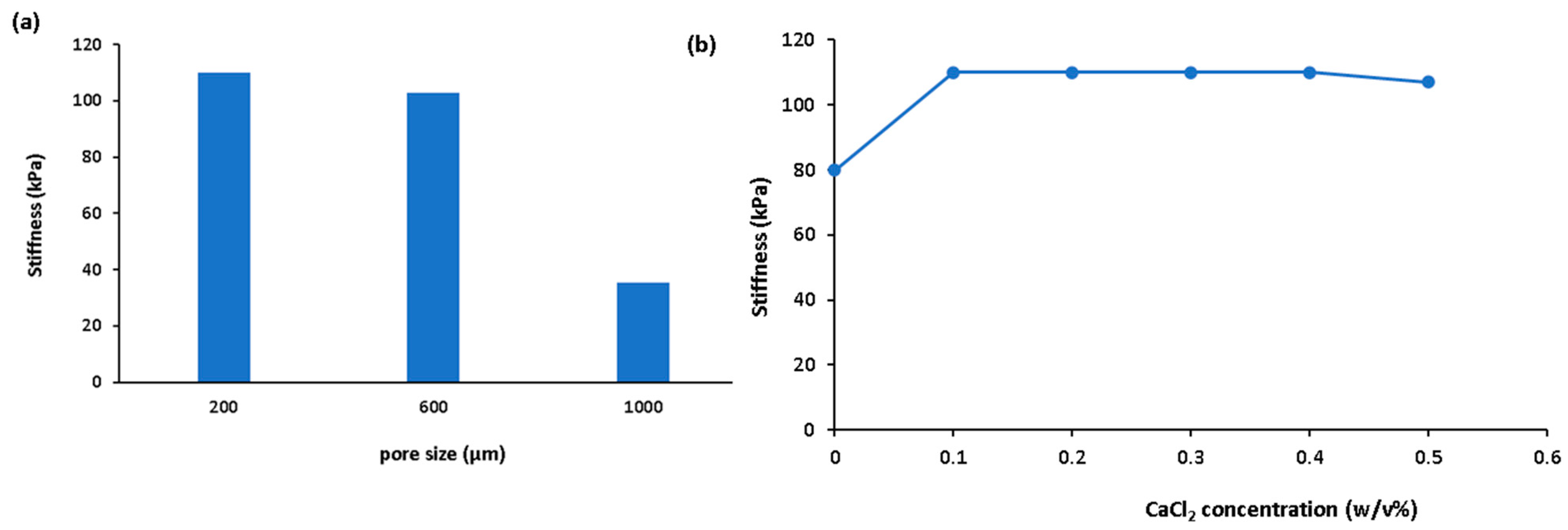
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monavari, M.; Homaeigohar, S.; Medhekar, R.; Nawaz, Q.; Monavari, M.; Zheng, K.; Boccaccini, A.R. A 3D-Printed Wound-Healing Material Composed of Alginate Dialdehyde-Gelatin Incorporating Astaxanthin and Borate Bioactive Glass Microparticles. ACS Appl. Mater. Interfaces 2023, 15, 50626–50637. [Google Scholar] [CrossRef] [PubMed]
- Monavari, M.; Medhekar, R.; Nawaz, Q.; Monavari, M.; Fuentes-Chandía, M.; Homaeigohar, S.; Boccaccini, A.R. A 3D Printed Bone Tissue Engineering Scaffold Composed of Alginate Dialdehyde-Gelatine Reinforced by Lysozyme Loaded Cerium Doped Mesoporous Silica-Calcia Nanoparticles. Macromol. Biosci. 2022, 22, 2200113. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate Composites for Bone Tissue Engineering: A Review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research Progress on Chemical Modification of Alginate: A Review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-Based Composite Materials for Wound Dressing Application:A Mini Review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.I.; Alshaer, W.; Hatmal, M.M.; Lambuk, L.; Ahmed, N.; Mustafa, M.Z.; Low, S.C.; Jaafar, J.; Ferji, K.; Six, J.L.; et al. Applications of Alginate-Based Nanomaterials in Enhancing the Therapeutic Effects of Bee Products. Front. Mol. Biosci. 2022, 9, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kuth, S.; Distler, T.; Gögele, C.; Stölzel, K.; Detsch, R.; Boccaccini, A.R.; Schulze-Tanzil, G. 3D Printing and Characterization of Human Nasoseptal Chondrocytes Laden Dual Crosslinked Oxidized Alginate-Gelatin Hydrogels for Cartilage Repair Approaches. Mater. Sci. Eng. C 2020, 116, 111189. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.F.; Heid, S.; Boccaccini, A.R. Potential of Laponite® Incorporated Oxidized Alginate–Gelatin (ADA-GEL) Composite Hydrogels for Extrusion-Based 3D Printing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Lee, M.; Oh, S.; Seok, J.M.; Kim, J.; Im, S.; Park, S.A.; Lee, J.Y. Three-Dimensional Bioprinting of Mesenchymal Stem Cells Using an Osteoinductive Bioink Containing Alginate and BMP-2-Loaded PLGA Nanoparticles for Bone Tissue Engineering. Biomater. Adv. 2022, 136, 212789. [Google Scholar] [CrossRef]
- Piras, C.C.; Smith, D.K. Multicomponent Polysaccharide Alginate-Based Bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef]
- Datta, S.; Barua, R.; Das, J. Importance of Alginate Bioink for 3D Bioprinting in Tissue Engineering and Regenerative Medicine. In Alginates—Recent Uses of This Natural Polymer; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Devina, N.; Eriwati, Y.K.; Santosa, A.S. The Purity and Viscosity of Sodium Alginate Extracted from Sargassum Brown Seaweed Species as a Basic Ingredient in Dental Alginate Impression Material. J. Phys. Conf. Ser. 2018, 1073, 052012. [Google Scholar] [CrossRef]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering Alginate as Bioink for Bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Distler, T.; McDonald, K.; Heid, S.; Karakaya, E.; Detsch, R.; Boccaccini, A.R. Ionically and Enzymatically Dual Cross-Linked Oxidized Alginate Gelatin Hydrogels with Tunable Stiffness and Degradation Behavior for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 3899–3914. [Google Scholar] [CrossRef]
- Kong, X.; Chen, L.; Li, B.; Quan, C.; Wu, J. Applications of Oxidized Alginate in Regenerative Medicine. J. Mater. Chem. B 2021, 9, 2785–2801. [Google Scholar] [CrossRef]
- Salem, D.M.S.A.; Sallam, M.A.E.; Youssef, T.N.M.A. Synthesis of Compounds Having Antimicrobial Activity from Alginate. Bioorg. Chem. 2019, 87, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Smidsrød, O.; Painter, T. Effect of Periodate Oxidation upon the Stiffness of the Alginate Molecule in Solution. Carbohydr. Res. 1973, 26, 125–132. [Google Scholar] [CrossRef]
- Kim, N.-G.; Kim, S.-C.; Kim, T.-H.; Je, J.-Y.; Lee, B.; Lee, S.G.; Kim, Y.-M.; Kang, H.W.; Qian, Z.-J.; Kim, N.; et al. Ishophloroglucin A-Based Multifunctional Oxidized Alginate/Gelatin Hydrogel for Accelerating Wound Healing. Int. J. Biol. Macromol. 2023, 245, 125484. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Solisito, A.A.; Schneidereit, D.; Friedrich, O.; Detsch, R.; Boccaccini, A.R. 3D Printed Oxidized Alginate-Gelatin Bioink Provides Guidance for C2C12 Muscle Precursor Cell Orientation and Differentiation via Shear Stress during Bioprinting. Biofabrication 2020, 12, 045005. [Google Scholar] [CrossRef]
- Ansari, M.; Darvishi, A.; Sabzevari, A. A Review of Advanced Hydrogels for Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2024, 12. [Google Scholar] [CrossRef]
- Khalighi, S.; Saadatmand, M. Bioprinting a Thick and Cell-Laden Partially Oxidized Alginate-Gelatin Scaffold with Embedded Micro-Channels as Future Soft Tissue Platform. Int. J. Biol. Macromol. 2021, 193, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Linh, N.T.; Paul, K.; Kim, B.; Lee, B.-T. Augmenting in Vitro Osteogenesis of a Glycine–Arginine–Glycine–Aspartic-Conjugated Oxidized Alginate–Gelatin–Biphasic Calcium Phosphate Hydrogel Composite and in Vivo Bone Biogenesis through Stem Cell Delivery. J. Biomater. Appl. 2016, 31, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-W.; Kim, S.-C.; Kim, T.-H.; Jung, W.-K. Characterization of an Oxidized Alginate-Gelatin Hydrogel Incorporating a COS-Salicylic Acid Conjugate for Wound Healing. Carbohydr. Polym. 2021, 252, 117145. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, J.; Fraguas, J.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Vázquez, J.A. Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities. Mar. Drugs 2021, 19, 491. [Google Scholar] [CrossRef] [PubMed]
- Hidayati, D.; Sabiyla, G.R.; Prasetyo, E.N.; Sa’adah, N.N.; Kurniawan, F. The Characteristic of Gelatin Extracted from The Skin of Adult and Sub-Adult Striped Catfish (Pangasius Hypophthalmus) Using Acid-Base Pretreatment: PH and FTIR. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012018. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Rubini, K.; Panzavolta, S.; Bigi, A. Chemico-Physical Characterization of Gelatin Films Modified with Oxidized Alginate. Acta Biomater. 2010, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Putri, A.P.; Bose, R.K.; Chalid, M.; Picchioni, F. Rheological and Self-Healing Behavior of Hydrogels Synthesized from l-Lysine-Functionalized Alginate Dialdehyde. Polymers 2023, 15, 1010. [Google Scholar] [CrossRef]
- Distler, T.; Lauria, I.; Detsch, R.; Sauter, C.M.; Bendt, F.; Kapr, J.; Rütten, S.; Boccaccini, A.R.; Fritsche, E. Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels. Biomedicines 2021, 9, 261. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Somasekharan, L.T.; Kasoju, N.; Raju, R.; Bhatt, A. Formulation and Characterization of Alginate Dialdehyde, Gelatin, and Platelet-Rich Plasma-Based Bioink for Bioprinting Applications. Bioengineering 2020, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- GRIFFIN, M.; CASADIO, R.; BERGAMINI, C.M. Transglutaminases: Nature’s Biological Glues. Biochem. J. 2002, 368, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Fusco, A.; Al-Asmar, A.; Mariniello, L. Microbial Transglutaminase as a Tool to Improve the Features of Hydrocolloid-Based Bioplastics. Int. J. Mol. Sci. 2020, 21, 3656. [Google Scholar] [CrossRef]
- Beninati, S.; Bergamini, C.M.; Piacentini, M. An Overview of the First 50 Years of Transglutaminase Research. Amino Acids 2009, 36, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Misiewicz, A. Microbial Transglutaminase and Its Application in the Food Industry. A Review. Folia Microbiol. 2014, 59, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kolotylo, V.; Piwowarek, K.; Kieliszek, M. Microbiological Transglutaminase: Biotechnological Application in the Food Industry. Open Life Sci. 2023, 18. [Google Scholar] [CrossRef] [PubMed]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Transglutaminase in Foods and Biotechnology. Int. J. Mol. Sci. 2023, 24, 12402. [Google Scholar] [CrossRef]
- Kara Özenler, A.; Distler, T.; Tihminlioglu, F.; Boccaccini, A.R. Fish Scale Containing Alginate Dialdehyde-Gelatin Bioink for Bone Tissue Engineering. Biofabrication 2023, 15. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically Crosslinked Gelatin Hydrogel Promotes the Proliferation of Adipose Tissue-Derived Stromal Cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef]
- Monavari, M.; Homaeigohar, S.; Fuentes-Chandía, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A.R. 3D Printing of Alginate Dialdehyde-Gelatin (ADA-GEL) Hydrogels Incorporating Phytotherapeutic Icariin Loaded Mesoporous SiO2-CaO Nanoparticles for Bone Tissue Engineering. Mater. Sci. Eng. C 2021, 131, 112470. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Li, W.; Zheng, K.; Detsch, R.; Boccaccini, A.R. Designing Porous Bone Tissue Engineering Scaffolds with Enhanced Mechanical Properties from Composite Hydrogels Composed of Modified Alginate, Gelatin, and Bioactive Glass. ACS Biomater. Sci. Eng. 2016, 2, 2240–2254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, Z.; Xiong, H.; Yan, Y.; Chen, X.; Shao, L. A Bioactive Glass Functional Hydrogel Enhances Bone Augmentation via Synergistic Angiogenesis, Self-Swelling and Osteogenesis. Bioact. Mater. 2023, 22, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Chang, Z.; Wan, Z.; Gan, Y.; Schlangen, E.; Šavija, B. Interpretable Ensemble-Machine-Learning Models for Predicting Creep Behavior of Concrete. Cem. Concr. Compos. 2022, 125, 104295. [Google Scholar] [CrossRef]
- Almufti, S.M. EXtreme Gradient Boosting Algorithm with Machine Learning: A Review. Acad. J. Nawroz Univ. 2023, 12, 320–334. [Google Scholar] [CrossRef]
- Ege, D.; Sertturk, S.; Acarkan, B.; Ademoglu, A. Machine Learning Models to Predict the Relationship between Printing Parameters and Tensile Strength of 3D Poly (Lactic Acid) Scaffolds for Tissue Engineering Applications. Biomed. Phys. Eng. Express 2023, 9, 065014. [Google Scholar] [CrossRef] [PubMed]
- Kreller, T.; Distler, T.; Heid, S.; Gerth, S.; Detsch, R.; Boccaccini, A.R. Physico-Chemical Modification of Gelatine for the Improvement of 3D Printability of Oxidized Alginate-Gelatine Hydrogels towards Cartilage Tissue Engineering. Mater. Des. 2021, 208, 109877. [Google Scholar] [CrossRef]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H.; et al. Electrically Conductive and 3D-Printable Oxidized Alginate-Gelatin Polypyrrole:PSS Hydrogels for Tissue Engineering. Adv. Healthc. Mater. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Kim, M.; Monavari, M.; Ghalandari, B.; Boccaccini, A.R. Mussel-Inspired Polydopamine Decorated Alginate Dialdehyde-Gelatin 3D Printed Scaffolds for Bone Tissue Engineering Application. Front. Bioeng. Biotechnol. 2022, 10, 1–16. [Google Scholar] [CrossRef]
- Chayanun, S.; Soufivand, A.A.; Faber, J.; Budday, S.; Lohwongwatana, B.; Boccaccini, A.R. Reinforcing Tissue-Engineered Cartilage: Nanofibrillated Cellulose Enhances Mechanical Properties of Alginate Dialdehyde–Gelatin Hydrogel. Adv. Eng. Mater. 2023, 26, 2300641. [Google Scholar] [CrossRef]
- Hao, J.; Ho, T.K. Machine Learning Made Easy: A Review of Scikit-Learn Package in Python Programming Language. J. Educ. Behav. Stat. 2019, 44, 348–361. [Google Scholar] [CrossRef]
- Johansson, R. Numerical Python: Scientific Computing and Data Science Applications with Numpy, SciPy and Matplotlib; Apress: Berkeley, CA, USA, 2019. [Google Scholar] [CrossRef]
- Rickert, C.A.; Hayta, E.N.; Selle, D.M.; Kouroudis, I.; Harth, M.; Gagliardi, A.; Lieleg, O. Machine Learning Approach to Analyze the Surface Properties of Biological Materials. ACS Biomater. Sci. Eng. 2021, 7, 4614–4625. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar] [CrossRef]
- De-Prado-gil, J.; Palencia, C.; Jagadesh, P.; Martínez-García, R. A Comparison of Machine Learning Tools That Model the Splitting Tensile Strength of Self-Compacting Recycled Aggregate Concrete. Materials 2022, 15, 4164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, J.; Yu, T.; Santomauro, A.; Gordon, A.; Gou, J.; Wu, D. Predicting Flexural Strength of Additively Manufactured Continuous Carbon Fiber- Reinforced Polymer Composites Using Machine Learning. J. Comput. Inf. Sci. Eng. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Guo, L.; Li, Z.; Tian, Q.; Guo, L.; Wang, Q. Prediction of CSG Splitting Tensile Strength Based on XGBoost-RF Model. Mater. Today Commun. 2023, 34, 105350. [Google Scholar] [CrossRef]
- Song, K.; Yan, F.; Ding, T.; Gao, L.; Lu, S. A Steel Property Optimization Model Based on the XGBoost Algorithm and Improved PSO. Comput. Mater. Sci. 2020, 174, 109472. [Google Scholar] [CrossRef]
- Sri Chandrahas, N.; Choudhary, B.S.; Vishnu Teja, M.; Venkataramayya, M.S.; Krishna Prasad, N.S.R. XG Boost Algorithm to Simultaneous Prediction of Rock Fragmentation and Induced Ground Vibration Using Unique Blast Data. Appl. Sci. 2022, 12, 5269. [Google Scholar] [CrossRef]
- Wang, Q.; Hussain, A.; Farooqi, M.U.; Deifalla, A.F. Artificial Intelligence-Based Estimation of Ultra-High-Strength Concrete’s Flexural Property. Case Stud. Constr. Mater. 2022, 17, e01243. [Google Scholar] [CrossRef]
- Pervaiz, M.; Shorfuzzaman, M.; Alsufyani, A.; Jalal, A.; Alsuhibany, S.A.; Park, J. Tracking and Analysis of Pedestrian’s Behavior in Public Places. Comput. Mater. Contin. 2023, 74, 841–853. [Google Scholar] [CrossRef]
- Han, T.; Stone-Weiss, N.; Huang, J.; Goel, A.; Kumar, A. Machine Learning as a Tool to Design Glasses with Controlled Dissolution for Healthcare Applications. Acta Biomater. 2020, 107, 286–298. [Google Scholar] [CrossRef]
- Khokhar, S.A.; Ahmed, T.; Khushnood, R.A.; Ali, S.M.; Shahnawaz. A Predictive Mimicker of Fracture Behavior in Fiber Reinforced Concrete Using Machine Learning. Materials 2021, 14, 7669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jin, L.; Li, W.; Han, S.; Yan, J. The Prediction of Wear Depth Based on Machine Learning Algorithms. Lubricants 2024, 12, 34. [Google Scholar] [CrossRef]
- De-Prado-Gil, J.; Palencia, C.; Silva-Monteiro, N.; Martínez-García, R. To Predict the Compressive Strength of Self Compacting Concrete with Recycled Aggregates Utilizing Ensemble Machine Learning Models. Case Stud. Constr. Mater. 2022, 16, e01046. [Google Scholar] [CrossRef]
- Salminen, J.; Corporan, J.; Marttila, R.; Salenius, T.; Jansen, B.J. Using Machine Learning to Predict Ranking of Webpages in the Gift Industry. In Proceedings of the 9th International Conference on Information Systems and Technologies, Cairo, Egypt, 24–26 March 2019; ACM: New York, NY, USA, 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Conev, A.; Litsa, E.E.; Perez, M.R.; Diba, M.; Mikos, A.G.; Kavraki, L.E. Machine Learning-Guided Three-Dimensional Printing of Tissue Engineering Scaffolds. Tissue Eng. Part A 2020, 26, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, K.; Wen, W.; Peng, Y.; Baniassadi, M.; Ahzi, S. Application of Machine Learning Methods on Dynamic Strength Analysis for Additive Manufactured Polypropylene-Based Composites. Polym. Test. 2022, 110, 107580. [Google Scholar] [CrossRef]
- Jin, S.; Goh, G. Bayesian Selection of Best Subsets via Hybrid Search. Comput. Stat. 2021, 36, 1991–2007. [Google Scholar] [CrossRef]
- Anjum, M.; Khan, K.; Ahmad, W.; Ahmad, A.; Amin, M.N.; Nafees, A. New SHapley Additive ExPlanations (SHAP) Approach to Evaluate the Raw Materials Interactions of Steel-Fiber-Reinforced Concrete. Materials 2022, 15, 6261. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, J.; Liang, S. Interpretable Machine Learning Models for Punching Shear Strength Estimation of FRP Reinforced Concrete Slabs. Crystals 2022, 12, 259. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. Adv. Neural Inf. Process. Syst. 2017, 2017, 4766–4775. [Google Scholar]
- Meghanathan, N. Assortativity Analysis of Real-World Network Graphs Based on Centrality Metrics. Comput. Inf. Sci. 2016, 9, 7. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, J.E.; Jeong, S.H.; Choi, Y.J.; Ryu, J.J. Printing Accuracy, Mechanical Properties, Surface Characteristics, and Microbial Adhesion of 3D-Printed Resins with Various Printing Orientations. J. Prosthet. Dent. 2020, 124, 468–475. [Google Scholar] [CrossRef]
- Vatcheva, K.P.; Lee, M. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology 2016, 06, 1–20. [Google Scholar] [CrossRef]
- Kalnins, A. Multicollinearity: How Common Factors Cause Type 1 Errors in Multivariate Regression. Strateg. Manag. J. 2018, 39, 2362–2385. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Wang, S.; Ma, Y.; Wei, Q.; Li, X.; Cong, R.; Ren, T. Methods for Estimating Leaf Nitrogen Concentration of Winter Oilseed Rape (Brassica Napus L.) Using in Situ Leaf Spectroscopy. Ind. Crops Prod. 2016, 91, 194–204. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.J.; Jurman, G. The Coefficient of Determination R-Squared Is More Informative than SMAPE, MAE, MAPE, MSE and RMSE in Regression Analysis Evaluation. PeerJ Comput. Sci. 2021, 7, e623. [Google Scholar] [CrossRef]
- Nohara, Y.; Matsumoto, K.; Soejima, H.; Nakashima, N. Explanation of Machine Learning Models Using Shapley Additive Explanation and Application for Real Data in Hospital. Comput. Methods Programs Biomed. 2022, 214, 106584. [Google Scholar] [CrossRef]
- Zhang, Z.; Ghasemi, A.; Koutná, N.; Xu, Z.; Grünstäudl, T.; Song, K.; Holec, D.; He, Y.; Mayrhofer, P.H.; Bartosik, M. Correlating Point Defects with Mechanical Properties in Nanocrystalline TiN Thin Films. Mater. Des. 2021, 207, 109844. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, J.; Long, X.; Zhao, L.; Chen, C.; Liu, C. Statistical Effects of Pore Features on Mechanical Properties and Fracture Behaviors of Heterogeneous Random Porous Materials by Phase-Field Modeling. Int. J. Solids Struct. 2023, 264, 112098. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, Z.; Wang, Y.; Zheng, B.; Zhou, H. Voids Formation and Their Effects on Mechanical Properties in Thermoformed Carbon Fiber Fabric-reinforced Composites. Polym. Compos. 2019, 40, E1094–E1102. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Benzvi, C. Microbial Transglutaminase Is a Very Frequently Used Food Additive and Is a Potential Inducer of Autoimmune/ Neurodegenerative Diseases. Toxics 2021, 9, 233. [Google Scholar] [CrossRef]
- Tian, X.Y.; Chen, X.B. Effects of Cell Density on Mechanical Properties of Alginate Hydrogel Tissue Scaffolds. J. Biomim. Biomater. Tissue Eng. 2014, 19, 77–85. [Google Scholar] [CrossRef]
- Yang, L.; Fan, L.; Lin, X.; Yu, Y.; Zhao, Y. Pearl Powder Hybrid Bioactive Scaffolds from Microfluidic 3D Printing for Bone Regeneration. Adv. Sci. 2023, 10, 2304190. [Google Scholar] [CrossRef]
- Yang, P.; Ju, Y.; Hu, Y.; Xie, X.; Fang, B.; Lei, L. Emerging 3D Bioprinting Applications in Plastic Surgery. Biomater. Res. 2023, 27, 1. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]



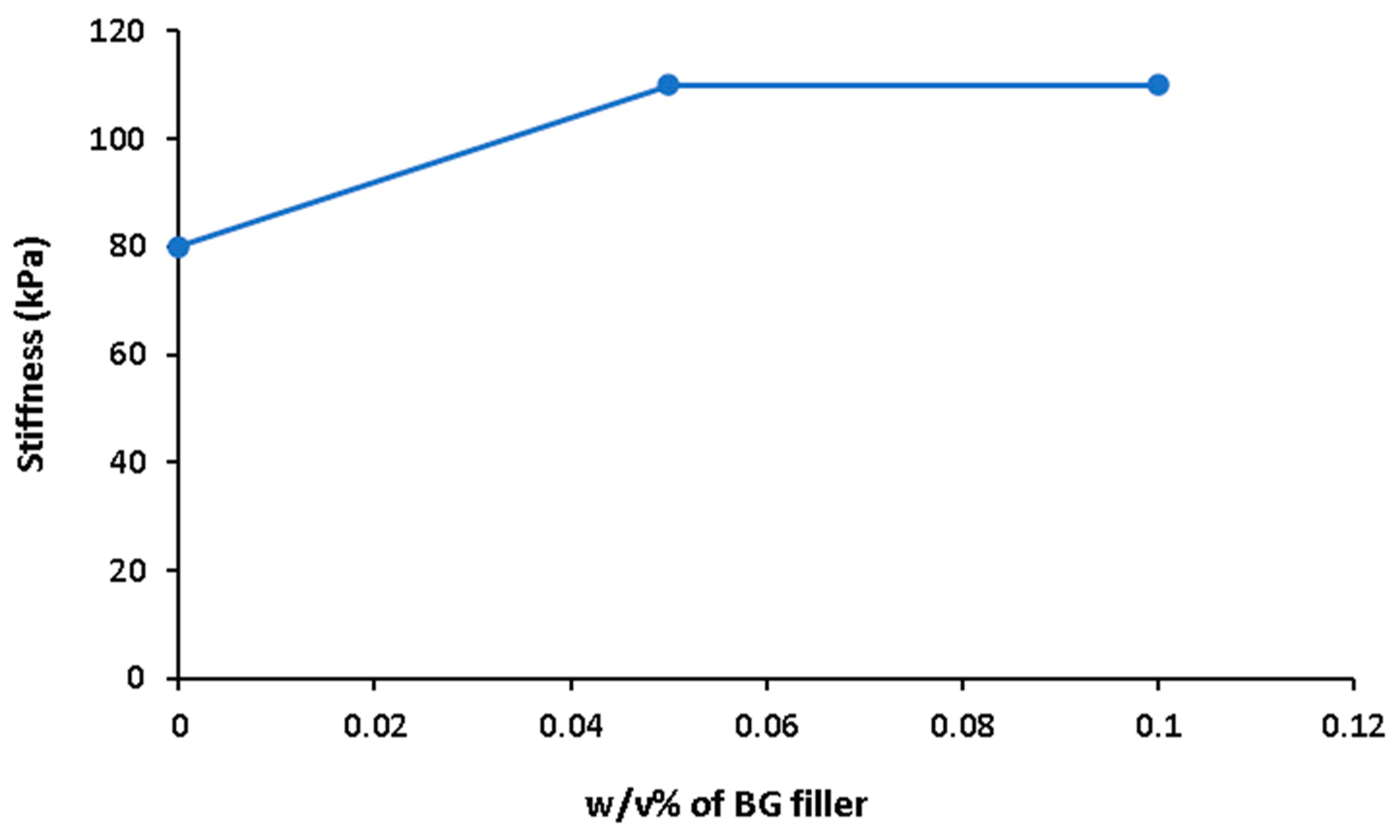
| Printing Temperature (°C) | Printing Speed (mm/s) | Pressure (kPa) | Ref. |
|---|---|---|---|
| 30 | N/A | N/A | [42] |
| 30 | 2 | 160 | [48] |
| 30 | 5 | 165 | [2] |
| 30 | N/A | 8 | [1] |
| 30 | 10 | 250 | [49] |
| 30 | N/A | N/A | [50] |
| 30 | 14 | 35 | [8] |
| 30 | 10 | 100 | [40] |
| Hyperparameters | Subsample Ratio of Columns | Number of Estimators | Maximum Depth | Learning Rate |
|---|---|---|---|---|
| Parameters | 0.5, 0.6, 0.8, 0.9 | 1000, 2000 | 4, 6, 10 | 0.01, 0.05, 0.1, 0.3 |
| Best parameters | 0.8 | 1000 | 4 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ege, D.; Boccaccini, A.R. Investigating the Effect of Processing and Material Parameters of Alginate Dialdehyde-Gelatin (ADA-GEL)-Based Hydrogels on Stiffness by XGB Machine Learning Model. Bioengineering 2024, 11, 415. https://doi.org/10.3390/bioengineering11050415
Ege D, Boccaccini AR. Investigating the Effect of Processing and Material Parameters of Alginate Dialdehyde-Gelatin (ADA-GEL)-Based Hydrogels on Stiffness by XGB Machine Learning Model. Bioengineering. 2024; 11(5):415. https://doi.org/10.3390/bioengineering11050415
Chicago/Turabian StyleEge, Duygu, and Aldo R. Boccaccini. 2024. "Investigating the Effect of Processing and Material Parameters of Alginate Dialdehyde-Gelatin (ADA-GEL)-Based Hydrogels on Stiffness by XGB Machine Learning Model" Bioengineering 11, no. 5: 415. https://doi.org/10.3390/bioengineering11050415