Multi-Response Optimization of Granaticinic Acid Production by Endophytic Streptomyces thermoviolaceus NT1, Using Response Surface Methodology
Abstract
:1. Introduction
2. Experimental Section
2.1. Microbial Strains, Maintenance, and Chemicals
2.2. Inoculum Preparation for Fermentation
2.3. Production of Granaticinic Acid
2.4. OVAT Optimization for Production of Granaticinic Acid
2.5. Optimization of Media
2.6. Determination of Optimum Glucose Concentration
2.7. Determination of Initial Media pH
2.8. Determination of Incubation Temperature
2.9. Determination of Fermentation Period
2.10. Determination of Most Suitable Inoculum Size
2.11. Optimization of Granaticinic Acid Production Using Box–Behnken Design
2.12. Validation of RSM Predictions
2.13. Statistical Analysis
2.14. Estimation of Granaticinic Acid at Optimization Phases
= (π) (0.25)20.5 [well diameter = 0.5 cm and height = 0.5 cm]
3. Results
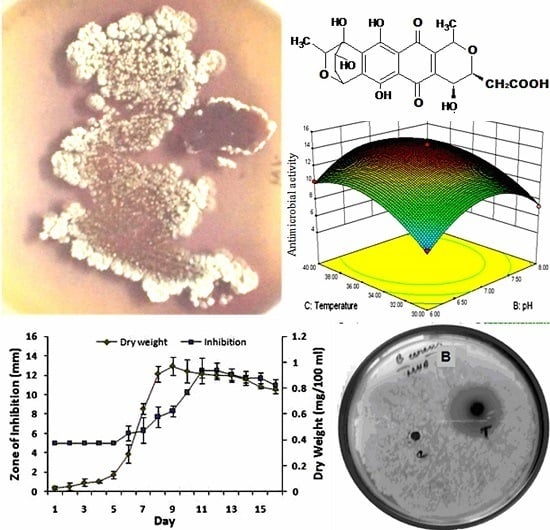
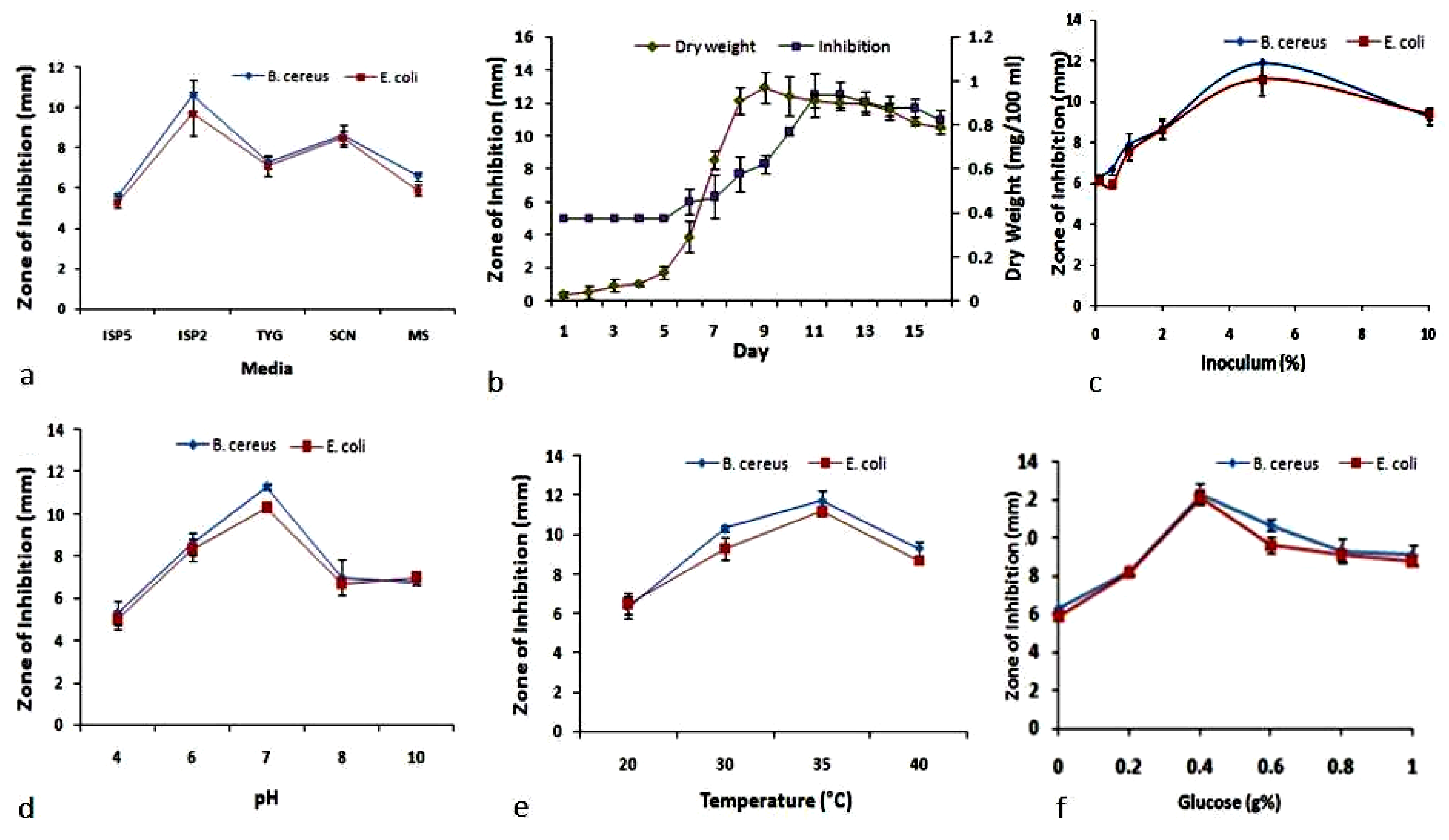
3.1. OVAT Optimization of Granaticinic Acid Production
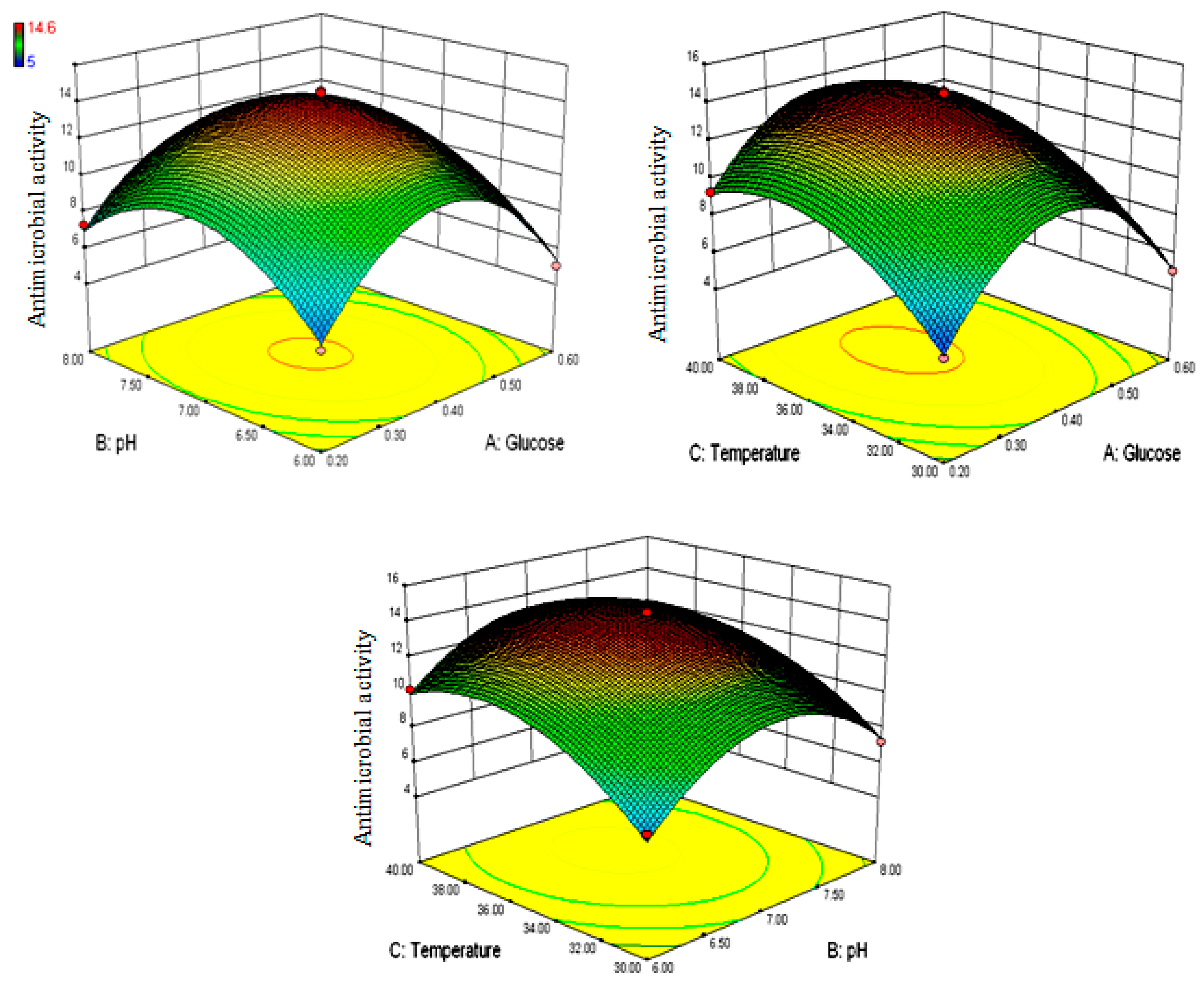
3.2. Box–Behnken Optimization for Granaticinic Acid Production
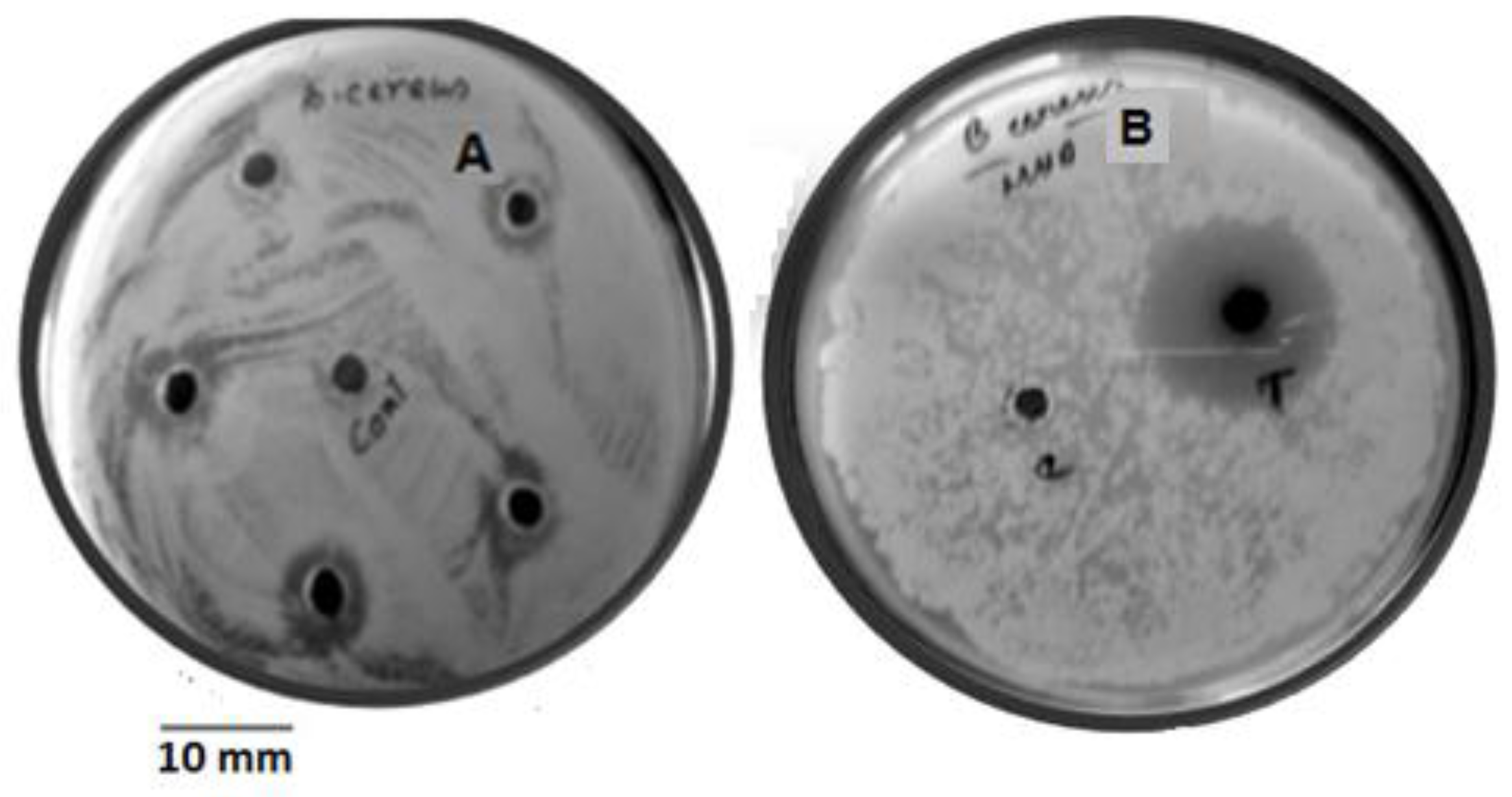
3.3. Validation Study
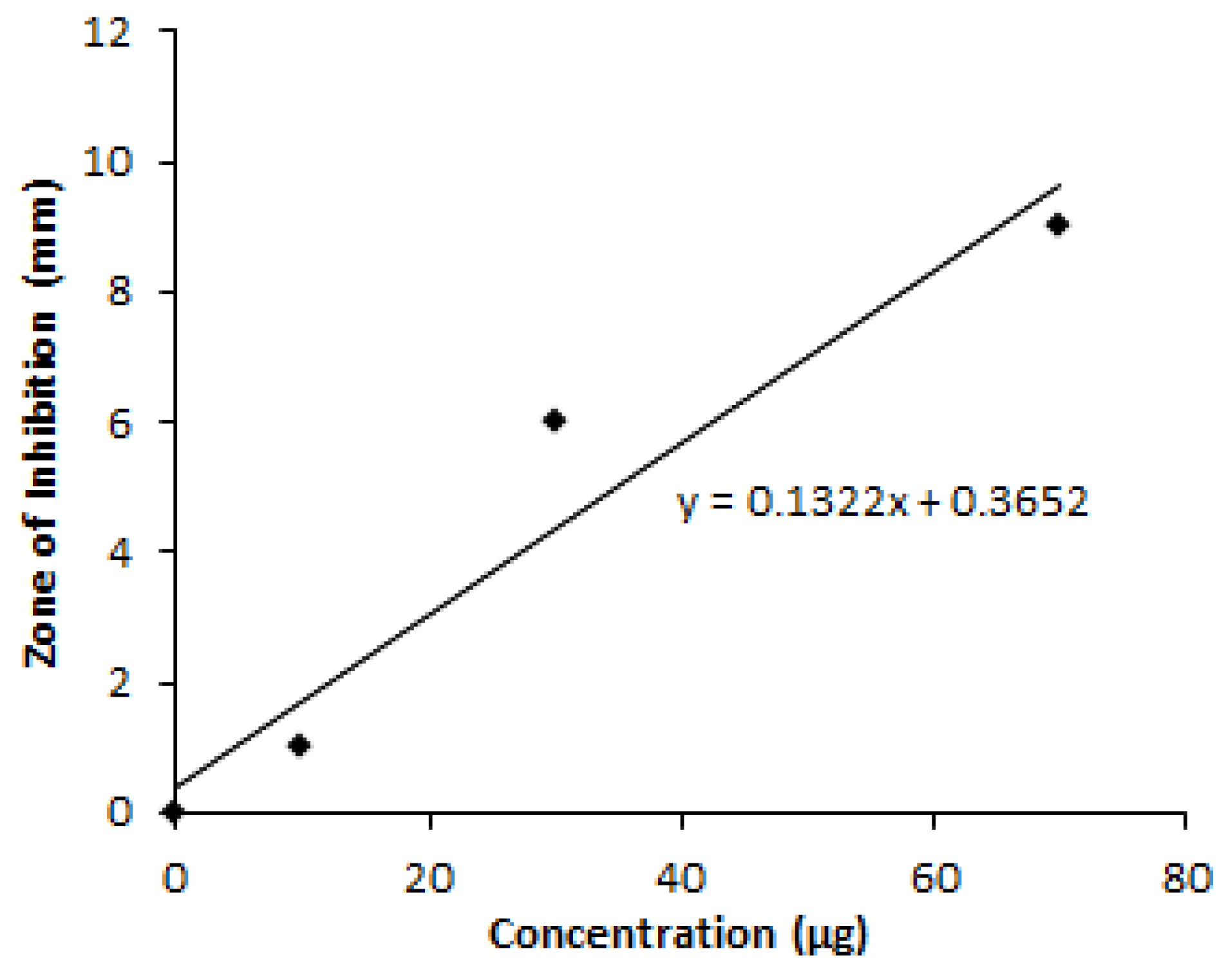
3.4. Yield of Granaticinic Acid
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bibb, M.J. Regulation of secondary metabolism in Streptomycetes. Curr. Opin. Microbiol. Microbiol. 2005, 8, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, L.; Kang, Z.; Buchenauer, H.; Gao, X. Optimization of the fermentation process of actinomycetes strain Hhs015T. J. Biomed. Biotechnol. 2010, 141876. [Google Scholar] [CrossRef]
- Jose, P.A.; Santhi, V.S.; Jebakumar, S.R.D. Phylogenetic- affiliation, antimicrobial potential and PKS gene sequence analysis of moderately halophilic Streptomyces sp. inhabiting an Indian saltpan. J. Basic Microbiol. 2011, 51, 348–356. [Google Scholar]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Banerjee, D. Bioactive endophytic actinomycetes of Cinnamomum sp.; isolation, identification, activity guided purification and process optimization of active metabolite. Am. J. Microbiol. 2015, 6, 4–13. [Google Scholar] [CrossRef]
- St. Pyrek, J.S.; Achmatowicz, O.; Zamojski, A. Naphtho- and anthraquinones of Streptomyces thermoviolaceous WR14, structure and model synthesis. Tetrahedron 1977, 33, 673–680. [Google Scholar] [CrossRef]
- Arnone, A.; Camarda, L.; Cardillo, R.; Fronza, G.; Merlini, L.; Mondelli, R.; Nasini, G.; St. Pyrek, J.S. 13-NMR analysis of dihydrogranaticin methyl ester. A case of mixed biogenesis. Helv. Chim. Acta 1979, 62, 30–33. [Google Scholar] [CrossRef]
- Fleck, W.F.; Strauss, D.G.; Prauser, H. Naphthoquinone antibiotics from Streptomyces lateritius, fermentation, isolation and characterization of granatomycins A, C and D. Z. Allg. Mikrobiol. 1980, 20, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Banerjee, D. Broad spectrum antibacterial activity of granaticinic acid, isolated from Streptomyces thermoviolaceus NT1; an endophyte in Catharanthus roseus (L.) G. Don. J. Appl. Pharm. Sci. 2015, 5, 6–11. [Google Scholar] [CrossRef]
- Bartholomäus, R.; Bachmann, J.; Mang, C.; Haustedt, L.O.; Harms, K.; Koer, U. Synthesis of the AB-Ring pyranolactone substructure of granaticin A. Eur. J. Org. Chem. 2013, 180, 190. [Google Scholar] [CrossRef]
- El-Enshasy, H.A.; Farid, M.A.; El-Sayed, A. Influence of inoculum type and cultivation conditions on natamycin production by Streptomyces natalensis. J. Basic Microbiol. 2000, 40, 333–342. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sheriss, J.C.; Turc, M. Antibiotic susceptibility testing by standardized single disc method. Am. J. Clin. Pathol. 1996, 45, 493–496. [Google Scholar]
- Lagorioa, S.H.; Bianchia, D.A.; Sutichb, E.G.; Kaufman, T.S. Synthesis and antimicrobial activity of pyranobenzoquinones related to the pyranonaphthoquinone antibioiotics. Eur. J. Med. Chem. 2006, 41, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Jana, M.; Mahapatra, S. Production of exopolysaccharide by endophytic Stemphylium sp. Micol. Aplicada. Int. 2009, 21, 57–62. [Google Scholar]
- Feng, Y.L.; Li, W.Q.; Wu, X.Q.; Cheng, J.W.; Ma, S.Y. Statistical optimization of media for mycelia growth and exo-polysaccharide production by Lentinus edodes and a kinetic model study of two growth morphologies. Biochem. Eng. J. 2010, 49, 104–112. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific Yew. Science 1993, 260, 214–216. [Google Scholar]
- Zhang, Y.; Huang, H.; Xu, S.; Wang, B.; Ju, J.; Tan, H.; Li, W. Activation and enhancement of Fredericamycin A production in deep sea-derived Streptomyces somaliensis SCSIO ZH66 by using ribosome engineering and response surface methodology. Microb. Cell Fact. 2015, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Yu, Z.; Yan, Y.; Yang, D.; Huang, T.; Vodanovic-Jankovic, S.; Kron, M.A.; Shen, B. Medium optimization of Streptomyces sp. 17944 for tirandamycin B production and isolation and structural elucidation of tirandamycins H, I and J. J. Antibiot. 2014, 67, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Syed, Q.; Adnan, A.; Qureshi, F.A. Isolation, characterization and selection of avermectin-producing Streptomyces avermitilis strains from soil samples. Jundishapur J. Microbiol. 2014, 7, e10366. [Google Scholar] [CrossRef] [PubMed]
- James, P.D.A.; Edwards, C. The effects of temperature on growth and production of the antibiotic granaticin by a thermotolerant Streptomycete. J. Gen. Microbiol. 1989, 135, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Vu-Trong, K.; Gray, P.P. Influence of ammonia on the biosynthesis of the macrolide antibiotic tylosin. Enzym. Microb. Technol. 1987, 9, 590–593. [Google Scholar] [CrossRef]
- Bommarius, B.; Jenssen, H.; Elliott, M.; Kindrachuk, J.; Pasupuleti, M.; Gieren, H.; Jaeger, K.E.; Hancock, R.E.W.; Kalman, D. Cost-effective expression and purification of antimicrobial and host defense peptides in Escherichia coli. Peptides 2010, 31, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Banerjee, D. Optimization of a bioactive exopolysaccharide production from endophytic Fusariumsolani SD5. Carbohydr. Polym. 2013, 97, 627–634. [Google Scholar] [CrossRef] [PubMed]
| Run | Independent Variables | Response (Growth Inhibition, mm) | |||
|---|---|---|---|---|---|
| A: Glucose (g%) | B: pH | C: Temperature (°C) | Observed | Predicted | |
| 1 | 0.6 | 7.0 | 30 | 5 | 5.0625 |
| 2 | 0.2 | 8.0 | 35 | 7.3 | 6.9625 |
| 3 | 0.2 | 7.0 | 30 | 5.3 | 5.4125 |
| 4 | 0.4 | 8.0 | 30 | 7.2 | 7.425 |
| 5 | 0.6 | 6.0 | 35 | 5 | 5.3375 |
| 6 | 0.2 | 7.0 | 40 | 9.3 | 9.2375 |
| 7 | 0.4 | 7.0 | 35 | 14.2 | 14.24 |
| 8 | 0.4 | 8.0 | 40 | 9.2 | 9.6 |
| 9 | 0.4 | 7.0 | 35 | 13.9 | 14.24 |
| 10 | 0.4 | 7.0 | 35 | 14.6 | 14.24 |
| 11 | 0.4 | 6.0 | 40 | 10.2 | 9.975 |
| 12 | 0.2 | 6.0 | 35 | 5.3 | 5.5875 |
| 13 | 0.6 | 8.0 | 35 | 5 | 4.7125 |
| 14 | 0.4 | 7.0 | 35 | 14.5 | 14.24 |
| 15 | 0.4 | 6.0 | 30 | 6.7 | 6.3 |
| 16 | 0.4 | 7.0 | 35 | 14 | 14.24 |
| 17 | 0.6 | 7.0 | 40 | 7.2 | 7.0875 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob>F |
|---|---|---|---|---|---|
| Model | 228.0028529 | 9 | 25.33365033 | 145.416607 | <0.0001 |
| A | 3.125 | 1 | 3.125 | 17.93767938 | 0.0039 |
| B | 0.28125 | 1 | 0.28125 | 1.614391144 | 0.2445 |
| C | 17.11125 | 1 | 17.11125 | 98.2195572 | <0.0001 |
| AB | 1 | 1 | 1 | 5.740057401 | 0.0478 |
| AC | 0.81 | 1 | 0.81 | 4.649446494 | 0.0680 |
| BC | 0.5625 | 1 | 0.5625 | 3.228782288 | 0.1154 |
| A2 | 109.8381316 | 1 | 109.8381316 | 630.47718 | <0.0001 |
| B2 | 51.06444737 | 1 | 51.06444737 | 293.112859 | <0.0001 |
| C2 | 24.91392105 | 1 | 24.91392105 | 143.0073369 | <0.0001 |
| Residual | 1.2195 | 7 | 0.174214286 | ||
| Lack of fit | 0.8475 | 3 | 0.2825 | 3.037634409 | 0.1556 |
| Pure error | 0.372 | 4 | 0.093 | ||
| Cor total | 229.2223529 | 16 | |||
| R-Squared | 0.994679838 | ||||
| Adj R-Squared | 0.98783963 | ||||
| Pred R-Squared | 0.938307718 | ||||
| Adeq Precision | 29.76196049 |
| Sample Tested | Zone of Inhibition (mm) | Amount of Granaticinic Acid (mg/L) | Fold Increased |
|---|---|---|---|
| Crude | 8.83 | 18.64 | 0 |
| OVAT* | 11.73 | 40.58 | 2.17 |
| RSM | 14.5 | 61.53 | 3.30 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Halder, S.K.; Banerjee, D. Multi-Response Optimization of Granaticinic Acid Production by Endophytic Streptomyces thermoviolaceus NT1, Using Response Surface Methodology. Bioengineering 2016, 3, 19. https://doi.org/10.3390/bioengineering3030019
Roy S, Halder SK, Banerjee D. Multi-Response Optimization of Granaticinic Acid Production by Endophytic Streptomyces thermoviolaceus NT1, Using Response Surface Methodology. Bioengineering. 2016; 3(3):19. https://doi.org/10.3390/bioengineering3030019
Chicago/Turabian StyleRoy, Sudipta, Suman Kumar Halder, and Debdulal Banerjee. 2016. "Multi-Response Optimization of Granaticinic Acid Production by Endophytic Streptomyces thermoviolaceus NT1, Using Response Surface Methodology" Bioengineering 3, no. 3: 19. https://doi.org/10.3390/bioengineering3030019