Embryonal Tumors of the Central Nervous System in Children: The Era of Targeted Therapeutics
Abstract
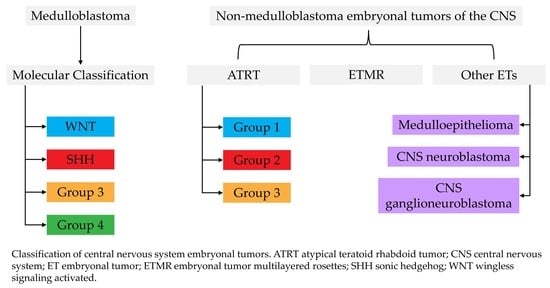
:1. Introduction
2. Medulloblastoma
2.1. Introduction
2.2. Molecular Subgroups
2.3. Standard Therapy
2.4. Molecularly Targeted Therapy
2.5. Conclusion
3. Atypical Teratoid Rhabdoid Tumors (ATRT)
3.1. Introduction
3.2. Clinical Features and Diagnosis
3.3. Molecular Era of ATRT
3.4. Current Treatment
3.5. Newer Therapeutic Insights
3.6. Conclusions
4. Embryonal Tumor with Multilayered Rosettes (ETMR)
4.1. Introduction
4.2. Clinical Features and Diagnosis
4.3. Current Treatment Strategies
4.4. Molecular Characteristics and Therapeutic Insights
4.5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pomeroy, S.L.; Tamayo, P.; Gaasenbeek, M.; Sturla, L.M.; Angelo, M.; McLaughlin, M.E.; Kim, J.Y.; Goumnerova, L.C.; Black, P.M.; Lau, C.; et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 2002, 415, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Sin-Chan, P.; Li, B.K.; Ho, B.; Fonseca, A.; Huang, A. Molecular classification and management of rare pediatric embryonal brain tumors. Curr. Oncol. Rep. 2018, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- McNeil, D.E.; Cote, T.R.; Clegg, L.; Rorke, L.B. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: A seer update. Surveillance epidemiology and end results. Med. Pediatr. Oncol. 2002, 39, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Weil, A.G.; Wang, A.C.; Westwick, H.J.; Ibrahim, G.M.; Ariani, R.T.; Crevier, L.; Perreault, S.; Davidson, T.; Tseng, C.H.; Fallah, A. Survival in pediatric medulloblastoma: A population-based observational study to improve prognostication. J. Neuro-oncol. 2017, 132, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorn, W.R.; Tarbell, N.J. Medulloblastoma: Tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nature Clin. Pract. Oncol. 2007, 4, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.A.; Colaluca, B.; Bailey, L.; Stavinoha, P.L. Impact of attention on social functioning in pediatric medulloblastoma survivors. Pediatr. Hematol. Oncol. 2018, 35, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, V.; Chevignard, M.P.; Dellatolas, G.; Puget, S.; Dhermain, F.; Grill, J.; Valteau-Couanet, D.; Dufour, C. Intellectual, educational, and situation-based social outcome in adult survivors of childhood medulloblastoma. Dev. Neurorehabil. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Uday, S.; Murray, R.D.; Picton, S.; Chumas, P.; Raju, M.; Chandwani, M.; Alvi, S. Endocrine sequelae beyond 10 years in survivors of medulloblastoma. Clin. Endocrinol. 2015, 83, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Bavle, A.; Tewari, S.; Sisson, A.; Chintagumpala, M.; Anderson, M.; Paulino, A.C. Meta-analysis of the incidence and patterns of second neoplasms after photon craniospinal irradiation in children with medulloblastoma. Pediatr. Blood Cancer 2018, 65, e27095. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Koster, J.; Bunt, J.; Hasselt, N.E.; Lakeman, A.; van Sluis, P.; Troost, D.; Meeteren, N.S.; Caron, H.N.; Cloos, J.; et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS ONE 2008, 3, e3088. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Tsherniak, A.; Tamayo, P.; Santagata, S.; Ligon, A.; Greulich, H.; Berhoukim, R.; Amani, V.; Goumnerova, L.; Eberhart, C.G.; et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J. Clin. Oncol. 2011, 29, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Dubuc, A.M.; Pfister, S.; Taylor, M.D. Molecular subgroups of medulloblastoma. Expert Rev. Neurother. 2012, 12, 871–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northcott, P.A.; Jones, D.T.; Kool, M.; Robinson, G.W.; Gilbertson, R.J.; Cho, Y.J.; Pomeroy, S.L.; Korshunov, A.; Lichter, P.; Taylor, M.D.; et al. Medulloblastomics: The end of the beginning. Nature Rev. Cancer 2012, 12, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Grobner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, P.; Tong, Y.; Robinson, G.; Thompson, M.C.; Currle, D.S.; Eden, C.; Kranenburg, T.A.; Hogg, T.; Poppleton, H.; Martin, J.; et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 2010, 468, 1095–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellison, D.W.; Onilude, O.E.; Lindsey, J.C.; Lusher, M.E.; Weston, C.L.; Taylor, R.E.; Pearson, A.D.; Clifford, S.C. Beta-catenin status predicts a favorable outcome in childhood medulloblastoma: The united kingdom children’s cancer study group brain tumour committee. J. Clin. Oncol. 2005, 23, 7951–7957. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Korshunov, A.; Witt, H.; Hielscher, T.; Eberhart, C.G.; Mack, S.; Bouffet, E.; Clifford, S.C.; Hawkins, C.E.; French, P.; et al. Medulloblastoma comprises four distinct molecular variants. J. Clin. Oncol. 2011, 29, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Pfaff, E.; Shih, D.J.; Martin, D.C.; Castelo-Branco, P.; Baskin, B.; Ray, P.N.; Bouffet, E.; et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J. Clin. Oncol. 2013, 31, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Jager, N.; Kool, M.; Zichner, T.; Hutter, B.; Sultan, M.; Cho, Y.J.; Pugh, T.J.; Hovestadt, V.; Stutz, A.M.; et al. Dissecting the genomic complexity underlying medulloblastoma. Nature 2012, 488, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, T.J.; Weeraratne, S.D.; Archer, T.C.; Pomeranz Krummel, D.A.; Auclair, D.; Bochicchio, J.; Carneiro, M.O.; Carter, S.L.; Cibulskis, K.; Erlich, R.L.; et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature 2012, 488, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, G.; Parker, M.; Kranenburg, T.A.; Lu, C.; Chen, X.; Ding, L.; Phoenix, T.N.; Hedlund, E.; Wei, L.; Zhu, X.; et al. Novel mutations target distinct subgroups of medulloblastoma. Nature 2012, 488, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.D.; Liu, L.; Raffel, C.; Hui, C.C.; Mainprize, T.G.; Zhang, X.; Agatep, R.; Chiappa, S.; Gao, L.; Lowrance, A.; et al. Mutations in sufu predispose to medulloblastoma. Nature Genet. 2002, 31, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Waszak, S.M.; Northcott, P.A.; Buchhalter, I.; Robinson, G.W.; Sutter, C.; Groebner, S.; Grund, K.B.; Brugieres, L.; Jones, D.T.W.; Pajtler, K.W.; et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: A retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 2018, 19, 785–798. [Google Scholar] [CrossRef]
- Pei, Y.; Moore, C.E.; Wang, J.; Tewari, A.K.; Eroshkin, A.; Cho, Y.J.; Witt, H.; Korshunov, A.; Read, T.A.; Sun, J.L.; et al. An animal model of MYC-driven medulloblastoma. Cancer cell 2012, 21, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.J.; Northcott, P.A.; Remke, M.; Korshunov, A.; Ramaswamy, V.; Kool, M.; Luu, B.; Yao, Y.; Wang, X.; Dubuc, A.M.; et al. Cytogenetic prognostication within medulloblastoma subgroups. J. Clin. Oncol. 2014, 32, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Hielscher, T.; Bouffet, E.; Remke, M.; Luu, B.; Gururangan, S.; McLendon, R.E.; Bigner, D.D.; Lipp, E.S.; Perreault, S.; et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: A retrospective integrated clinical and molecular analysis. Lancet Oncol 2016, 17, 484–495. [Google Scholar] [CrossRef]
- Wong, K.K.; Ragab, O.; Tran, H.N.; Pham, A.; All, S.; Waxer, J.; Olch, A.J. Acute toxicity of craniospinal irradiation with volumetric-modulated arc therapy in children with solid tumors. Pediatr Blood Cancer 2018, 65, e27050. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Gerber, N.U.; von Hoff, K.; Gnekow, A.; Bode, U.; Graf, N.; Berthold, F.; Henze, G.; Wolff, J.E.; Warmuth-Metz, M.; et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro-Oncology 2009, 11, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.A.; Jones, R.M.; MacDonald, S.M.; Pulsifer, M.B.; Lavally, B.; et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016, 17, 287–298. [Google Scholar] [CrossRef]
- Ashley, D.M.; Merchant, T.E.; Strother, D.; Zhou, T.; Duffner, P.; Burger, P.C.; Miller, D.C.; Lyon, N.; Bonner, M.J.; Msall, M.; et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s oncology group study P9934. J. Clin. Oncol. 2012, 30, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Janss, A.; Vezina, G.; Gajjar, A.; Pollack, I.; Merchant, T.E.; FitzGerald, T.J.; Booth, T.; Tarbell, N.J.; Li, Y.; et al. Results of cog acns0331: A phase III trial of involved-field radiotherapy (IFRT) and low dose craniospinal irradiation (Ld-Csi) with chemotherapy in average-risk medulloblastoma: A report from the children’s oncology group. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 937–938. [Google Scholar] [CrossRef]
- Johnson, S.B.; Hung, J.; Kapadia, N.; Oh, K.S.; Kim, M.; Hamstra, D.A. Spinal growth patterns following craniospinal irradiation in children with medulloblastoma. Pract. Radiat. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.H.; Geyer, J.R.; Miller, D.C.; Curran, J.G.; Zhou, T.; Holmes, E.; Ingles, S.A.; Dunkel, I.J.; Hilden, J.; Packer, R.J.; et al. Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report from the children’s oncology group. Pediat. Neurol. 2015, 53, 31–46. [Google Scholar] [CrossRef] [PubMed]
- St. Jude Children’s Research Hospital; Genentech, Inc.; National Cancer Institute. A Clinical and Molecular Risk-Directed Therapy for Newly Diagnosed Medulloblastoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01878617 (accessed on 21 September 2018).
- Children’s Oncology Group; National Cancer Institute. Reduced Craniospinal Radiation Therapy and Chemotherapy in Treating Younger Patients with Newly Diagnosed Wnt-Driven Medulloblastoma. Available online: https://clinicaltrials.gov/ct2/show/NCT02724579 (accessed on 21 September 2018).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. Study Assessing the Feasibility of a Surgery and Chemotherapy-Only in Children with Wnt Positive Medulloblastoma. Available online: https://clinicaltrials.gov/ct2/show/NCT02212574 (accessed on 21 September 2018).
- LoRusso, P.M.; Rudin, C.M.; Reddy, J.C.; Tibes, R.; Weiss, G.J.; Borad, M.J.; Hann, C.L.; Brahmer, J.R.; Chang, I.; Darbonne, W.C.; et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin. Cancer Res. 2011, 17, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Romer, J.T.; Kimura, H.; Magdaleno, S.; Sasai, K.; Fuller, C.; Baines, H.; Connelly, M.; Stewart, C.F.; Gould, S.; Rubin, L.L.; et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer cell 2004, 6, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Rohner, A.; Spilker, M.E.; Lam, J.L.; Pascual, B.; Bartkowski, D.; Li, Q.J.; Yang, A.H.; Stevens, G.; Xu, M.; Wells, P.A.; et al. Effective targeting of hedgehog signaling in a medulloblastoma model with PF-5274857, a potent and selective smoothened antagonist that penetrates the blood-brain barrier. Mol. Cancer Ther. 2012, 11, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.W.; Orr, B.A.; Wu, G.; Gururangan, S.; Lin, T.; Qaddoumi, I.; Packer, R.J.; Goldman, S.; Prados, M.D.; Desjardins, A.; et al. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: Results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J. Clin. Oncol. 2015, 33, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Kieran, M.W.; Chisholm, J.; Casanova, M.; Brandes, A.A.; Aerts, I.; Bouffet, E.; Bailey, S.; Leary, S.; MacDonald, T.J.; Mechinaud, F.; et al. Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro-Oncology 2017, 19, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Buonamici, S.; Williams, J.; Morrissey, M.; Wang, A.; Guo, R.; Vattay, A.; Hsiao, K.; Yuan, J.; Green, J.; Ospina, B.; et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2010, 2, 51ra70. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Hann, C.L.; Laterra, J.; Yauch, R.L.; Callahan, C.A.; Fu, L.; Holcomb, T.; Stinson, J.; Gould, S.E.; Coleman, B.; et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. New Engl. J. Med. 2009, 361, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Matheson, C.J.; Venkataraman, S.; Amani, V.; Harris, P.S.; Backos, D.S.; Donson, A.M.; Wempe, M.F.; Foreman, N.K.; Vibhakar, R.; Reigan, P. A WEE1 inhibitor analog of AZD1775 maintains synergy with cisplatin and demonstrates reduced single-agent cytotoxicity in medulloblastoma cells. ACS Chem. Biol. 2016, 11, 2066–2067. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Fouladi, M.; Olson, J.; Daryani, V.M.; Stewart, C.F.; Wetmore, C.; Kocak, M.; Onar-Thomas, A.; Wagner, L.; Gururangan, S.; et al. Phase I trial of weekly MK-0752 in children with refractory central nervous system malignancies: A pediatric brain tumor consortium study. Child’s nerv. Syst. 2015, 31, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.J.; Aguilera, D.; Castellino, R.C. The rationale for targeted therapies in medulloblastoma. Neuro-Oncology 2014, 16, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.J.; Wagner, J.P.; Hofmann, N.E.; Eide, C.A.; Cho, Y.J.; Druker, B.J.; Davare, M.A. Functional validation of the oncogenic cooperativity and targeting potential of tuberous sclerosis mutation in medullblastoma using a MYC-amplified model cell line. Pediat. Blood Cancer 2017, 64, e26553. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. PI3K/mTOR Inhibitor LY3023414 in Treating Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphoma, or Histiocytic Disorders with TSC or PI3K/mTOR Mutations (a Pediatric Match Treatment Trial). Available online: https://clinicaltrials.gov/ct2/show/NCT03213678 (accessed on 21 September 2018).
- Pei, Y.; Liu, K.W.; Wang, J.; Garancher, A.; Tao, R.; Esparza, L.A.; Maier, D.L.; Udaka, Y.T.; Murad, N.; Morrissy, S.; et al. HDAC and PI3K antagonists cooperate to inhibit growth of MYC-driven medulloblastoma. Cancer cell 2016, 29, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhayay, P.; Bergthold, G.; Nguyen, B.; Schubert, S.; Gholamin, S.; Tang, Y.; Bolin, S.; Schumacher, S.E.; Zeid, R.; Masoud, S.; et al. Bet bromodomain inhibition of myc-amplified medulloblastoma. Clin. Cancer Res. 2014, 20, 912–925. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Vorinostat Combined with Isotretinoin and Chemotherapy in Treating Younger Patients with Embryonal Tumors of the Central Nervous System. Available online: https://clinicaltrials.gov/ct2/show/NCT00867178 (accessed on 21 September 2018).
- Li, M.; Lockwood, W.; Zielenska, M.; Northcott, P.; Ra, Y.S.; Bouffet, E.; Yoshimoto, M.; Rutka, J.T.; Yan, H.; Taylor, M.D.; et al. Multiple CDK/CYCLIND genes are amplified in medulloblastoma and supratentorial primitive neuroectodermal brain tumor. Cancer Genet. 2012, 205, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Pediatric Brain Tumor Consortium; National Cancer Institute. Palbociclib Isethionate in Treating Younger Patients with Recurrent, Progressive, or Refractory Central Nervous System Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT02255461 (accessed on 21 September 2018).
- Fouladi, M.; Stewart, C.F.; Blaney, S.M.; Onar-Thomas, A.; Schaiquevich, P.; Packer, R.J.; Goldman, S.; Geyer, J.R.; Gajjar, A.; Kun, L.E.; et al. A molecular biology and phase II trial of lapatinib in children with refractory CNS malignancies: A pediatric brain tumor consortium study. J. Neuro-oncol. 2013, 114, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jakacki, R.I.; Hamilton, M.; Gilbertson, R.J.; Blaney, S.M.; Tersak, J.; Krailo, M.D.; Ingle, A.M.; Voss, S.D.; Dancey, J.E.; Adamson, P.C. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: A children’s oncology group phase I consortium study. J. Clin. Oncol. 2008, 26, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Kieran, M.W.; Chi, S.; Goldman, S.; Onar-Thomas, A.; Poussaint, T.Y.; Vajapeyam, S.; Fahey, F.; Wu, S.; Turner, D.C.; Stewart, C.F.; et al. A phase I trial and pk study of cediranib (AZD2171), an orally bioavailable pan-VEGFR inhibitor, in children with recurrent or refractory primary CNS tumors. Child’s Nerv. Syst. 2015, 31, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Shin, S.J.; Vats, T.; Guha-Thakurta, N.; Aaron, J.; Rytting, M.; Kleinerman, E.; Kurzrock, R. Pediatric patients with refractory central nervous system tumors: Experiences of a clinical trial combining bevacizumab and temsirolimus. Anticancer Res. 2014, 34, 1939–1945. [Google Scholar] [PubMed]
- St. Jude Children’s Research Hospital; Novartis Pharmaceuticals. SJDAWN: St. Jude Children’s Research Hospital Phase 1 Study Evaluating Molecularly-Driven Doublet Therapies for Children and Young Adults with Recurrent Brain Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT03434262 (accessed on 21 September 2018).
- Martin, A.M.; Nirschl, C.J.; Polanczyk, M.J.; Bell, W.R.; Nirschle, T.R.; Harris-Bookman, S.; Phallen, J.; Hicks, J.; Martinez, D.; Ogurtsova, A.; et al. PD-L1 expression in medulloblastoma: An evaluation by subgroup. Oncotarget 2018, 9, 19177–19191. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.D.; Mitchell, D.A. Know your neighbors: Different tumor microenvironments have implications in immunotherapeutic targeting strategies across MB subgroups. Oncoimmunology 2016, 5, e1144002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, C.D.; Flores, C.; Yang, C.; Pinheiro, E.M.; Yearley, J.H.; Sayour, E.J.; Pei, Y.; Moore, C.; McLendon, R.E.; Huang, J.; et al. Differential immune microenvironements and response to immune checkpoint blockade among molecular subtypes of murine medulloblastoma. Clin. Cancer Res. 2016, 22, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.; Lode, H. MYCN-targeting vaccines and immunotherapeutics. Hum. Vaccines Immunother. 2016, 12, 2257–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, S.; Carrera, D.; Phillips, J.J.; Weiss, W.A.; Raffel, C. An oncolytic measles virus-sensitive group 3 medulloblastoma model in immune-competent mice. Neuro-Oncology 2018. [Google Scholar] [CrossRef] [PubMed]
- Rorke, L.B.; Packer, R.J.; Biegel, J.A. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: Definition of an entity. J. Neurosurg. 1996, 85, 56–65. [Google Scholar] [CrossRef] [PubMed]
- McGovern, S.L.; Grosshans, D.; Mahajan, A. Embryonal brain tumors. Cancer J. 2014, 20, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, C.; Boyett, J.; Li, S.; Lin, T.; Bendel, A.; Gajjar, A.; Orr, B.A. Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro-Oncology 2015, 17, 882–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruhwald, M.C.; Biegel, J.A.; Bourdeaut, F.; Roberts, C.W.; Chi, S.N. Atypical teratoid/rhabdoid tumors-current concepts, advances in biology, and potential future therapies. Neuro-Oncology 2016, 18, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Feng, X.Y. MRI features of atypical teratoid/rhabdoid tumors in children. Pediatr. Radiol. 2013, 43, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Warmuth-Metz, M.; Bison, B.; Dannemann-Stern, E.; Kortmann, R.; Rutkowski, S.; Pietsch, T. Ct and mr imaging in atypical teratoid/rhabdoid tumors of the central nervous system. Neuroradiology 2008, 50, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Nemes, K.; Hohm, A.; Vandergrift, L.A.; Hasselblatt, M.; Johann, P.D.; Kool, M.; Fruhwald, M.C.; Warmuth-Metz, M. Magnetic resonance imaging surrogates of molecular subgroups in atypical teratoid/rhabdoid tumor (ATRT). Neuro-Oncology 2018. [Google Scholar] [CrossRef] [PubMed]
- Bikowska, B.; Grajkowska, W.; Jozwiak, J. Atypical teratoid/rhabdoid tumor: Short clinical description and insight into possible mechanism of the disease. Eur. J. Neurol. 2011, 18, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Biegel, J.A. Molecular genetics of atypical teratoid/rhabdoid tumor. Neurosurg. Focus 2006, 20, 1–7. [Google Scholar] [CrossRef]
- Haberler, C.; Laggner, U.; Slavc, I.; Czech, T.; Ambros, I.M.; Ambros, P.F.; Budka, H.; Hainfellner, J.A. Immunohistochemical analysis of INI1 protein in malignant pediatric CNS tumors: Lack of INI1 in atypical teratoid/rhabdoid tumors and in a fraction of primitive neuroectodermal tumors without rhabdoid phenotype. Am. J. Surg Pathol. 2006, 30, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Eaton, K.W.; Tooke, L.S.; Wainwright, L.M.; Judkins, A.R.; Biegel, J.A. Spectrum of smarcb1/ini1 mutations in familial and sporadic rhabdoid tumors. Pediatr. Blood Cancer 2011, 56, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Biegel, J.A.; Zhou, J.Y.; Rorke, L.B.; Stenstrom, C.; Wainwright, L.M.; Fogelgren, B. Germ-line and acquired mutations of ini1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999, 59, 74–79. [Google Scholar] [PubMed]
- Torchia, J.; Picard, D.; Lafay-Cousin, L.; Hawkins, C.E.; Kim, S.K.; Letourneau, L.; Ra, Y.S.; Ho, K.C.; Chan, T.S.; Sin-Chan, P.; et al. Molecular subgroups of atypical teratoid rhabdoid tumours in children: An integrated genomic and clinicopathological analysis. Lancet Oncol. 2015, 16, 569–582. [Google Scholar] [CrossRef]
- Hasselblatt, M.; Gesk, S.; Oyen, F.; Rossi, S.; Viscardi, E.; Giangaspero, F.; Giannini, C.; Judkins, A.R.; Fruhwald, M.C.; Obser, T.; et al. Nonsense mutation and inactivation of smarca4 (brg1) in an atypical teratoid/rhabdoid tumor showing retained smarcb1 (ini1) expression. Am. J. Surg Pathol 2011, 35, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Birks, D.K.; Donson, A.M.; Patel, P.R.; Dunham, C.; Muscat, A.; Algar, E.M.; Ashley, D.M.; Kleinschmidt-Demasters, B.K.; Vibhakar, R.; Handler, M.H.; et al. High expression of bmp pathway genes distinguishes a subset of atypical teratoid/rhabdoid tumors associated with shorter survival. Neuro-Oncology 2011, 13, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Johann, P.D.; Erkek, S.; Zapatka, M.; Kerl, K.; Buchhalter, I.; Hovestadt, V.; Jones, D.T.W.; Sturm, D.; Hermann, C.; Segura Wang, M.; et al. Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell. 2016, 29, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; Richer, W.; Freneaux, P.; Chauvin, C.; Lucchesi, C.; Guillemot, D.; Grison, C.; Lequin, D.; Pierron, G.; Masliah-Planchon, J.; et al. The occurrence of intracranial rhabdoid tumours in mice depends on temporal control of smarcb1 inactivation. Nat. Commun. 2016, 7, 10421. [Google Scholar] [CrossRef] [PubMed]
- Ginn, K.F.; Gajjar, A. Atypical teratoid rhabdoid tumor: Current therapy and future directions. Front. Oncol. 2012, 2, 114. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.N.; Zimmerman, M.A.; Yao, X.; Cohen, K.J.; Burger, P.; Biegel, J.A.; Rorke-Adams, L.B.; Fisher, M.J.; Janss, A.; Mazewski, C.; et al. Intensive multimodality treatment for children with newly diagnosed cns atypical teratoid rhabdoid tumor. J. Clin. Oncol. 2009, 27, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Tekautz, T.M.; Fuller, C.E.; Blaney, S.; Fouladi, M.; Broniscer, A.; Merchant, T.E.; Krasin, M.; Dalton, J.; Hale, G.; Kun, L.E.; et al. Atypical teratoid/rhabdoid tumors (ATRT): Improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J. Clin. Oncol. 2005, 23, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Fangusaro, J.; Finlay, J.; Sposto, R.; Ji, L.; Saly, M.; Zacharoulis, S.; Asgharzadeh, S.; Abromowitch, M.; Olshefski, R.; Halpern, S.; et al. Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): Report of the head start I and II experience. Pediatr. Blood Cancer 2008, 50, 312–318. [Google Scholar] [PubMed]
- Lafay-Cousin, L.; Hawkins, C.; Carret, A.S.; Johnston, D.; Zelcer, S.; Wilson, B.; Jabado, N.; Scheinemann, K.; Eisenstat, D.; Fryer, C.; et al. Central nervous system atypical teratoid rhabdoid tumours: The canadian paediatric brain tumour consortium experience. Eur J. Cancer 2012, 48, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hilden, J.M.; Meerbaum, S.; Burger, P.; Finlay, J.; Janss, A.; Scheithauer, B.W.; Walter, A.W.; Rorke, L.B.; Biegel, J.A. Central nervous system atypical teratoid/rhabdoid tumor: Results of therapy in children enrolled in a registry. J. Clin. Oncol. 2004, 22, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Athale, U.H.; Duckworth, J.; Odame, I.; Barr, R. Childhood atypical teratoid rhabdoid tumor of the central nervous system: A meta-analysis of observational studies. J. Pediatr. Hematol. Oncol. 2009, 31, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Buscariollo, D.L.; Park, H.S.; Roberts, K.B.; Yu, J.B. Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a surveillance, epidemiology, and end results analysis. Cancer 2012, 118, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Kurmasheva, R.T.; Sammons, M.; Favours, E.; Wu, J.; Kurmashev, D.; Cosmopoulos, K.; Keilhack, H.; Klaus, C.R.; Houghton, P.J.; Smith, M.A. Initial testing (stage 1) of tazemetostat (EPZ-6438), a novel EZH2 inhibitor, by the pediatric preclinical testing program. Pediatr. Blood Cancer 2017, 64, e26218. [Google Scholar] [CrossRef] [PubMed]
- Unland, R.; Borchardt, C.; Clemens, D.; Kool, M.; Dirksen, U.; Fruhwald, M.C. Analysis of the antiproliferative effects of 3-deazaneoplanocin a in combination with standard anticancer agents in rhabdoid tumor cell lines. Anticancer Drugs 2015, 26, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ribrag, V.; Soria, J.C.; Reyderman, L.; Chen, R.; Salazar, P.; Kumar, N.; Kuznetsov, G.; Keilhack, H.; Ottesen, L.H.; Italiano, A. O7.2phase 1 first-in-human study of the enhancer of zeste-homolog 2 (EZH2) histone methyl transferase inhibitor E7438. Annals of Oncology 2015, 26. [Google Scholar] [CrossRef]
- Kerl, K.; Holsten, T.; Fruhwald, M.C. Rhabdoid tumors: Clinical approaches and molecular targets for innovative therapy. Pediatr. Hematol. Oncol. 2013, 30, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gholamin, S.; Schubert, S.; Willardson, M.I.; Lee, A.; Bandopadhayay, P.; Bergthold, G.; Masoud, S.; Nguyen, B.; Vue, N.; et al. Epigenetic targeting of hedgehog pathway transcriptional output through bet bromodomain inhibition. Nat. Med. 2014, 20, 732–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knipstein, J.A.; Birks, D.K.; Donson, A.M.; Alimova, I.; Foreman, N.K.; Vibhakar, R. Histone deacetylase inhibition decreases proliferation and potentiates the effect of ionizing radiation in atypical teratoid/rhabdoid tumor cells. Neuro-Oncology 2012, 14, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tsikitis, M.; Zhang, Z.; Edelman, W.; Zagzag, D.; Kalpana, G.V. Genetic ablation of cyclin D1 abrogates genesis of rhabdoid tumors resulting from Ini1 loss. Proc. Natl. Acad. Sci. USA 2005, 102, 12129–12134. [Google Scholar] [CrossRef] [PubMed]
- Venneti, S.; Le, P.; Martinez, D.; Eaton, K.W.; Shyam, N.; Jordan-Sciutto, K.L.; Pawel, B.; Biegel, J.A.; Judkins, A.R. P16INK4a and p14ARF tumor suppressor pathways are deregulated in malignant rhabdoid tumors. J. Neuropathol. Exp. Neurol. 2011, 70, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, R.; Zhang, A.; Mueller, S.; Prados, M.D.; Lulla, R.R.; Goldman, S.; Saratsis, A.M.; Mazar, A.P.; Stegh, A.H.; Cheng, S.Y.; et al. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro-Oncology 2016, 18, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Geoerger, B.; Bourdeaut, F.; DuBois, S.G.; Fischer, M.; Geller, J.I.; Gottardo, N.G.; Marabelle, A.; Pearson, A.D.J.; Modak, S.; Cash, T.; et al. A phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin. Cancer Res. 2017, 23, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.; Remke, M.; Castoldi, M.; Bai, A.H.; Muckenthaler, M.U.; Kulozik, A.; von Deimling, A.; Pscherer, A.; Lichter, P.; Korshunov, A. Novel genomic amplification targeting the microrna cluster at 19q13.42 in a pediatric embryonal tumor with abundant neuropil and true rosettes. Acta Neuropathol. 2009, 117, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Sturm, D.; Ryzhova, M.; Hovestadt, V.; Gessi, M.; Jones, D.T.; Remke, M.; Northcott, P.; Perry, A.; Picard, D.; et al. Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol. 2014, 128, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Spence, T.; Perotti, C.; Sin-Chan, P.; Picard, D.; Wu, W.; Singh, A.; Anderson, C.; Blough, M.D.; Cairncross, J.G.; Lafay-Cousin, L.; et al. A novel C19MC amplified cell line links Lin28/let-7 to mtor signaling in embryonal tumor with multilayered rosettes. Neuro-Oncology 2014, 16, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.; Dufour, C.; Leblond, P.; Bourdeaut, F.; Faure-Conter, C.; Bertozzi, A.I.; Delisle, M.B.; Palenzuela, G.; Jouvet, A.; Scavarda, D.; et al. Embryonal tumors with multilayered rosettes in children: The sfce experience. Childs Nerv Syst 2016, 32, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Gogia, B.; Fuller, G.N.; Ketonen, L.M. Embryonal tumor with multilayered rosettes, c19mc-altered: Clinical, pathological, and neuroimaging findings. J. Neuroimaging 2018, 28, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ceccom, J.; Bourdeaut, F.; Loukh, N.; Rigau, V.; Milin, S.; Takin, R.; Richer, W.; Uro-Coste, E.; Couturier, J.; Bertozzi, A.I.; et al. Embryonal tumor with multilayered rosettes: Diagnostic tools update and review of the literature. Clin. Neuropathol. 2014, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Miller, S.; Hawkins, C.E.; Bouffet, E.; Rogers, H.A.; Chan, T.S.; Kim, S.K.; Ra, Y.S.; Fangusaro, J.; Korshunov, A.; et al. Markers of survival and metastatic potential in childhood CNS primitive neuro-ectodermal brain tumours: An integrative genomic analysis. Lancet Oncol. 2012, 13, 838–848. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Stefanaki, K.; Vartholomatos, G.; Sfakianos, G.; Prodromou, N.; Moschovi, M. Embryonal tumor with abundant neuropil and true rosettes: A systematic literature review and report of 2 new cases. J. Child Neurol. 2013, 28, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, C.L.; Gerges, N.; Papillon-Cavanagh, S.; Sin-Chan, P.; Pramatarova, A.; Quang, D.A.; Adoue, V.; Busche, S.; Caron, M.; Djambazian, H.; et al. Fusion of TTYH1 with the C19MC microRNA cluster drives expression of a brain-specific DNMT3B isoform in the embryonal brain tumor ETMR. Nat. Genet. 2014, 46, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Braasch, I.; Bobe, J.; Guiguen, Y.; Postlethwait, J.H. Reply to: ’subfunctionalization versus neofunctionalization after whole-genome duplication'. Nat. Genet. 2018, 50, 910–911. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.E.; Wefers, A.K.; Lambo, S.; Bianchi, E.; Bockstaller, M.; Dorostkar, M.M.; Meister, V.; Schindler, P.; Korshunov, A.; von Hoff, K.; et al. A mouse model for embryonal tumors with multilayered rosettes uncovers the therapeutic potential of sonic-hedgehog inhibitors. Nat. Med. 2017, 23, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Schubert, N.A.; Brabetz, S.; Mack, N.; Schwalm, B.; Chan, J.A.; Selt, F.; Herold-Mende, C.; Witt, O.; Milde, T.; et al. Preclinical drug screen reveals topotecan, actinomycin D, and volasertib as potential new therapeutic candidates for ETMR brain tumor patients. Neuro-Oncology 2017, 19, 1607–1617. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kram, D.E.; Henderson, J.J.; Baig, M.; Chakraborty, D.; Gardner, M.A.; Biswas, S.; Khatua, S. Embryonal Tumors of the Central Nervous System in Children: The Era of Targeted Therapeutics. Bioengineering 2018, 5, 78. https://doi.org/10.3390/bioengineering5040078
Kram DE, Henderson JJ, Baig M, Chakraborty D, Gardner MA, Biswas S, Khatua S. Embryonal Tumors of the Central Nervous System in Children: The Era of Targeted Therapeutics. Bioengineering. 2018; 5(4):78. https://doi.org/10.3390/bioengineering5040078
Chicago/Turabian StyleKram, David E., Jacob J. Henderson, Muhammad Baig, Diya Chakraborty, Morgan A. Gardner, Subhasree Biswas, and Soumen Khatua. 2018. "Embryonal Tumors of the Central Nervous System in Children: The Era of Targeted Therapeutics" Bioengineering 5, no. 4: 78. https://doi.org/10.3390/bioengineering5040078