Influence of Incident Wavelength and Detector Material Selection on Fluorescence in the Application of Raman Spectroscopy to a Fungal Fermentation Process
Abstract
:1. Introduction
2. Experimental Section
2.1. Microorganism and Media
2.2. Bioreactor Conditions
2.3. Raman Spectroscopy Devices
2.4. Raman Spectra Preprocessing and Wavelength Selection
2.5. Partial Least Model Generation
2.6. Validation of PLS Model
| n: | calibration samples |
| p: | validation samples |
| : | ith calibration sample |
| : | ith validation sample |
2.7. Raman Spectroscopic Fundamentals
3. Results and Discussion
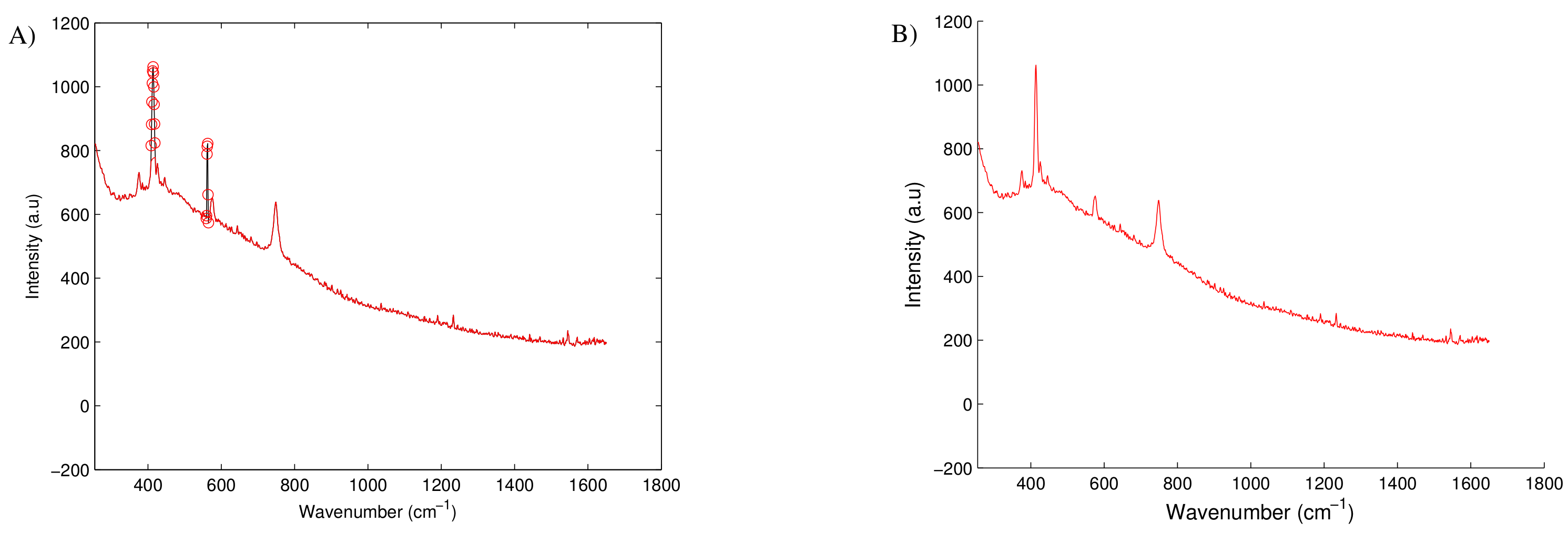
3.1. Fluorescence Observations
3.2. Glucose Predictions of 903 and 993 nm Raman Spectroscopic Devices
3.3. Influence of Raman Spectroscopic Incident Wavelength and Detector on Fluorescence
| : | Intensity of Raman scattered light |
| : | Frequency of light source |
| : | Wavelength of light source |
3.4. API Predictions of 993 nm Raman Spectroscopic Device
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| Inner-relationship matrix for PLS model | |
| CCD | Charged couple device |
| CPP | Critical process parameters |
| CQA | Critical quality attributes |
| Residual matrix in PLS model for X-data | |
| Residual matrix in PLS model for Y-data | |
| FDA | Food and drug administration |
| HPLC | High pressure liquid chromatography |
| InGaAs | Indium gallium arsenide |
| n | Number of calibration points in PLS model |
| NIPLAS | Non-linear iterative partial least squares |
| p | Number of validation points in PLS model |
| Loadings matrix in PLS model | |
| PAT | Process analytic technology |
| PID | Proportional integral derivative |
| PLS | Partial least squares |
| R | Number of latent variables in PLS model |
| RMSEC | Root mean square error of calibration |
| RMSEP | Root mean square error of prediction |
| UV | Ultra-violet |
| Scores matrix in PLS model of Y-data | |
| Loadings matrix in PLS model of Y-data | |
| Scores matrix in PLS model of X-data | |
| X-data (spectral) in PLS model | |
| ith calibration point in PLS model | |
| ith validation point in PLS model | |
| Y-data (glucose) in PLS model | |
| Y-data (API) in PLS model | |
| Regression coefficients for PLS model | |
| Incident wavelength of Raman device | |
| h | Planks constant |
Appendix A. Raman Spectroscopy Operation
- Integration time: relates to the detector exposure time, the longer the integration time the larger the intensity of the Raman spectra. The intensity relates to the total accumulated charge recorded on a single pixel. Large integration times can saturate the detector whereas small integration times can decrease the Raman peaks below detectable levels, therefore a balance is required.
- Number of averages: refers to the number of spectra that were averaged to obtain a single spectrum, used to improve the signal to noise (SNR) ratio.
Appendix Overview of Spectral Preprocessing Methods
- Fluorescence and background Baseline Increase
- Noise
- Cosmic spikes

References
- Smith, E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- McCreery, R.L. Raman Spectroscopy for Chemical Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kornecki, M.; Strube, J. Process Analytical Technology for Advanced Process Control in Biologics Manufacturing with the Aid of Macroscopic Kinetic Modeling. Bioengineering 2018, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; DHHS: Rockville, MD, USA, 2004. [Google Scholar]
- Lourenço, N.D.; Lopes, J.A.; Almeida, C.F.; Sarraguça, M.C.; Pinheiro, H.M. Bioreactor monitoring with spectroscopy and chemometrics: A review. Anal. Bioanal. Chem. 2012, 404, 1211–1237. [Google Scholar] [CrossRef] [PubMed]
- Bocklitz, T.; Walter, A.; Hartmann, K.; Rösch, P.; Popp, J. How to pre-process Raman spectra for reliable and stable models? Anal. Chim. Acta 2011, 704, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Abu-Absi, N.R.; Kenty, B.M.; Cuellar, M.E.; Borys, M.C.; Sakhamuri, S.; Strachan, D.J.; Hausladen, M.C.; Li, Z.J. Real time monitoring of multiple parameters in mammalian cell culture bioreactors using an in-line Raman spectroscopy probe. Biotechnol. Bioeng. 2011, 108, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, H.; Lauri, D.; Karry, K.M.; Moshgbar, M.; Procopio-Melino, R.; Drapeau, D. Generic R aman-based calibration models enabling real-time monitoring of cell culture bioreactors. Biotechnol. Prog. 2015, 31, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Picard, A.; Daniel, I.; Montagnac, G.; Oger, P. In situ monitoring by quantitative Raman spectroscopy of alcoholic fermentation by Saccharomyces cerevisiae under high pressure. Extremophiles 2007, 11, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Iversen, J.A.; Berg, R.W.; Ahring, B.K. Quantitative monitoring of yeast fermentation using Raman spectroscopy. Anal. Bioanal. Chem. 2014, 406, 4911–4919. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Boccazzi, P.; Gorret, N.; Ram, R.J.; Sinskey, A.J. In situ bioprocess monitoring of Escherichia coli bioreactions using Raman spectroscopy. Vib. Spectrosc. 2004, 35, 131–137. [Google Scholar] [CrossRef]
- André, S.; Saint Cristau, L.; Gaillard, S.; Devos, O.; Calvosa, É.; Duponchel, L. In-line and real-time prediction of recombinant antibody titer by in situ Raman spectroscopy. Anal. Chim. Acta 2015, 892, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Ebel, B.; Paris, C.; Chauchard, F.; Guedon, E.; Marc, A. Real-time monitoring of antibody glycosylation site occupancy by in situ Raman spectroscopy during bioreactor CHO cell cultures. Biotechnol. Prog. 2018, 34, 486–493. [Google Scholar] [CrossRef] [PubMed]
- De Beer, T.; Burggraeve, A.; Fonteyne, M.; Saerens, L.; Remon, J.P.; Vervaet, C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int. J. Pharm. 2011, 417, 32–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, E.; Manoharan, R.; Koo, T.; Shafer, K.; Motz, J.; Fitzmaurice, M.; Kramer, J.; Itzkan, I.; Dasari, R.; Feld, M. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 2000, 45, R1. [Google Scholar] [CrossRef] [PubMed]
- Golcuk, K.; Mandair, G.S.; Callender, A.F.; Sahar, N.; Kohn, D.H.; Morris, M.D. Is photobleaching necessary for Raman imaging of bone tissue using a green laser? Biochim. Biophys. Acta 2006, 1758, 868–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Li, Y.Q. Confocal micro-Raman spectroscopy of single biological cells using optical trapping and shifted excitation difference techniques. J. Appl. Phys. 2003, 93, 2982–2986. [Google Scholar] [CrossRef]
- Sowoidnich, K.; Kronfeldt, H.D. Fluorescence rejection by shifted excitation Raman difference spectroscopy at multiple wavelengths for the investigation of biological samples. ISRN Spectrosc. 2012, 2012, 256326. [Google Scholar] [CrossRef]
- Shreve, A.P.; Cherepy, N.J.; Mathies, R.A. Effective rejection of fluorescence interference in Raman spectroscopy using a shifted excitation difference technique. Appl. Spectrosc. 1992, 46, 707–711. [Google Scholar] [CrossRef]
- Everall, N.; Hahn, T.A.S.; Atousek, P.M.; Parker, A.W. Picosecond time-resolved Raman spectroscopy of solids: Capabilities and limitations for fluorescence rejection and the influence of diffuse reflectance. Appl. Spectrosc. 2001, 55, 1701–1708. [Google Scholar] [CrossRef]
- Knorr, F.; Smith, Z.J.; Wachsmann-Hogiu, S. Development of a time-gated system for Raman spectroscopy of biological samples. Opt. Express 2010, 18, 20049–20058. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, S.P.; Keating, C.D. Raman spectroscopy. Anal. Chem. 2000, 72, 145–158. [Google Scholar] [CrossRef]
- Mori, N.; Suzuki, T.; Kakuno, S. Noise of acoustic Doppler velocimeter data in bubbly flow. J. Eng. Mech. 2007, 133, 122–125. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Boelens, H.F.M. Baseline Correction with Asymmetric Least Squares Smoothing. Leiden Univ. Med. Cent. Rep. 2005, 1, 5. [Google Scholar]
- Wold, S.; Geladi, P.; Esbensen, K.; Öhman, J. Multi-way principal components-and PLS-analysis. J. Chemom. 1987, 1, 41–56. [Google Scholar] [CrossRef]
- Gemperline, P. Practical Guide to Chemometrics; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Alexander, R. Advantages of Raman Spectroscopy When Analyzing Materials through Glass or Polymer Containers and in Aqueous Solution; Technical Report, Perkin Elmer Application Report: Waltham, MA, USA, 2008. [Google Scholar]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ferraro, J.R.; Nakamoto, K.; Brown, C.W. Introductory Raman Spectroscopy; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Goldrick, S. Application of Multivariate Data Analysis and First Principle Mathematical Modelling to the Biotechnology Industry. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2015. [Google Scholar]
- Cannizzaro, C.; Rhiel, M.; Marison, I.; von Stockar, U. On-line monitoring of Phaffia rhodozyma fed-batch process with in situ dispersive Raman spectroscopy. Biotechnol. Bioeng. 2003, 83, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Smith, E.A. Determination of glucose and ethanol after enzymatic hydrolysis and fermentation of biomass using Raman spectroscopy. Anal. Chim. Acta 2009, 653, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Craven, S.; Glennon, B. In situ Raman spectroscopy for simultaneous monitoring of multiple process parameters in mammalian cell culture bioreactors. Biotechnol. Prog. 2012, 28, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.J.; Redd, D.C.B.; Gansler, T.S.; McCreery, R.L. Characterization of human breast biopsy specimens with near-IR Raman spectroscopy. Anal. Chem. 1994, 66, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Volodin, B.L.; Dolgy, S.; Lieber, C.; Wu, H.; Yang, W. Quantitative and qualitative analysis of fluorescent substances and binary mixtures by use of shifted excitation Raman difference spectroscopy. SPIE Proc. 2013, 8572, 857211. [Google Scholar] [CrossRef]
- Adar, F.; Atzeni, S.; Gilchrist, R.; Goldstone, L. Detectors: Spectroscopy; Optoelectronics World: Houston, TX, USA, 2002. [Google Scholar]
- Li, Z.; Deen, M.; Kumar, S.; Selvaganapathy, P. Raman Spectroscopy for In-Line Water Quality Monitoring—Instrumentation and Potential. Sensors 2014, 14, 17275–17303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Ray, B.H.; Leister, K.J.; Ryder, A.G. Performance monitoring of a mammalian cell based bioprocess using Raman spectroscopy. Anal. Chim. Acta 2013, 796, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Beier, B.D.; Berger, A.J. Method for automated background subtraction from Raman spectra containing known contaminants. Analyst 2009, 134, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Sivakesava, S.; Irudayaraj, J.; Demirci, A. Monitoring a bioprocess for ethanol production using FT-MIR and FT-Raman spectroscopy. J. Ind. Microbiol. Biotechnol. 2001, 26, 185–190. [Google Scholar] [CrossRef] [PubMed]





| Spectra Reference | Start (h) | End (h) | # of Spec | Integration Time (s) | # of Averages | Issues Encountered |
|---|---|---|---|---|---|---|
| 993 nm Raman Device | ||||||
| Spec | 0 | 260 | 520 | 180 | 9 | Moderate Fluorescence |
| 903 nm Raman Device | ||||||
| Spec | 0 | 90.5 | 181 | 180 | 9 | Moderate Fluorescence |
| Spec | 91.5 | 97 | 10 | 270 | 6 | CCD saturated |
| Spec | 98.5 | 114.5 | 32 | 60 | 27 | High Fluorescence |
| Spec | 115.5 | 146.5 | 62 | 90 | 18 | High Fluorescence |
| Spec | 146.5 | 193 | 93 | 60 | 27 | High Fluorescence |
| Spec | 193.5 | 239 | 91 | 30 | 54 | High Fluorescence |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldrick, S.; Lovett, D.; Montague, G.; Lennox, B. Influence of Incident Wavelength and Detector Material Selection on Fluorescence in the Application of Raman Spectroscopy to a Fungal Fermentation Process. Bioengineering 2018, 5, 79. https://doi.org/10.3390/bioengineering5040079
Goldrick S, Lovett D, Montague G, Lennox B. Influence of Incident Wavelength and Detector Material Selection on Fluorescence in the Application of Raman Spectroscopy to a Fungal Fermentation Process. Bioengineering. 2018; 5(4):79. https://doi.org/10.3390/bioengineering5040079
Chicago/Turabian StyleGoldrick, Stephen, David Lovett, Gary Montague, and Barry Lennox. 2018. "Influence of Incident Wavelength and Detector Material Selection on Fluorescence in the Application of Raman Spectroscopy to a Fungal Fermentation Process" Bioengineering 5, no. 4: 79. https://doi.org/10.3390/bioengineering5040079
APA StyleGoldrick, S., Lovett, D., Montague, G., & Lennox, B. (2018). Influence of Incident Wavelength and Detector Material Selection on Fluorescence in the Application of Raman Spectroscopy to a Fungal Fermentation Process. Bioengineering, 5(4), 79. https://doi.org/10.3390/bioengineering5040079





