Quantitative Analysis of Core Lipid Production in Methanothermobacter marburgensis at Different Scales
Abstract
:1. Introduction
2. Materials and Methods
2.1. Archaeal Strain and Culture Set-Up
2.2. Cultivation Medium
2.3. Incoulation
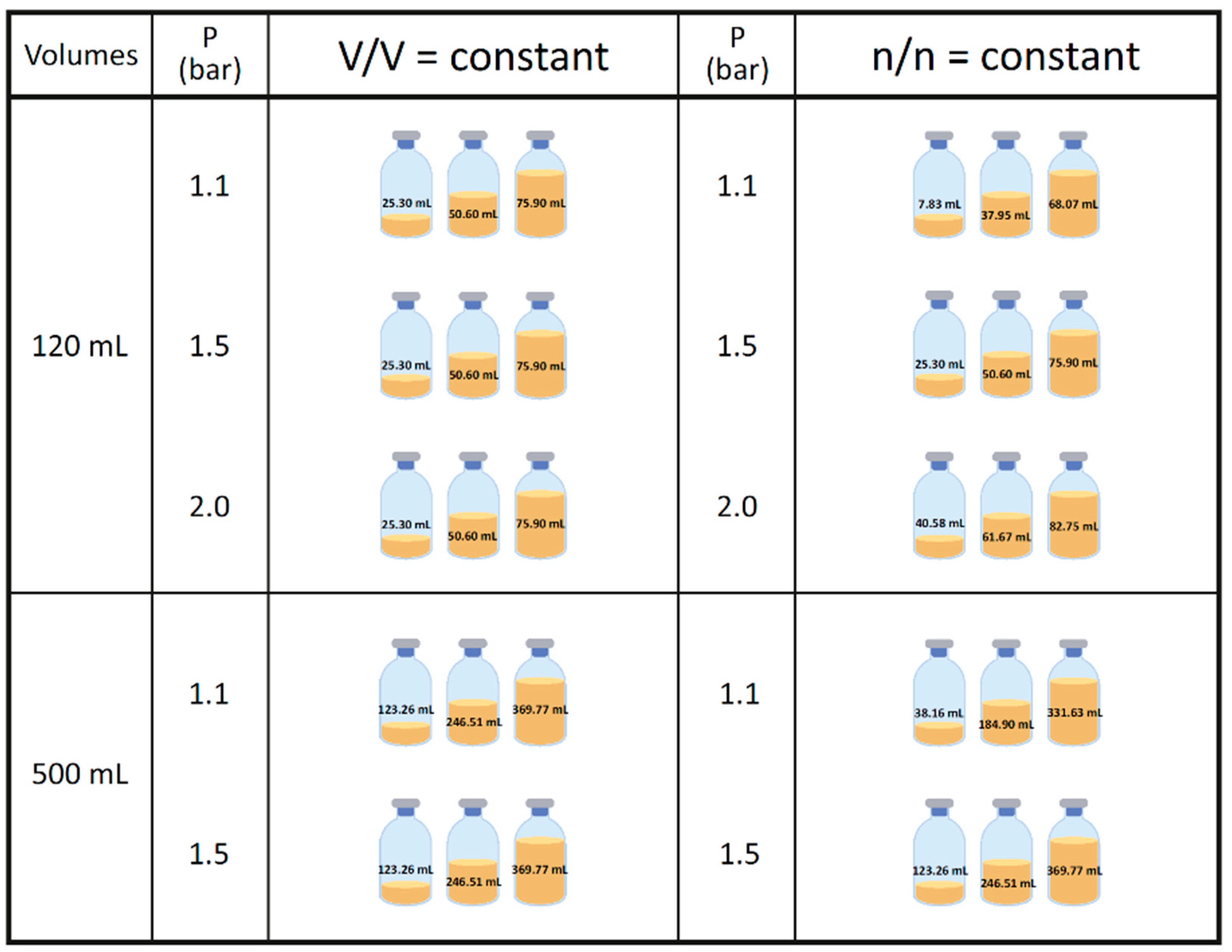
2.4. Pressure Measurement and Gassing
2.5. End Point OD Measurement and Harvesting
2.6. Lipid Extraction
3. Results
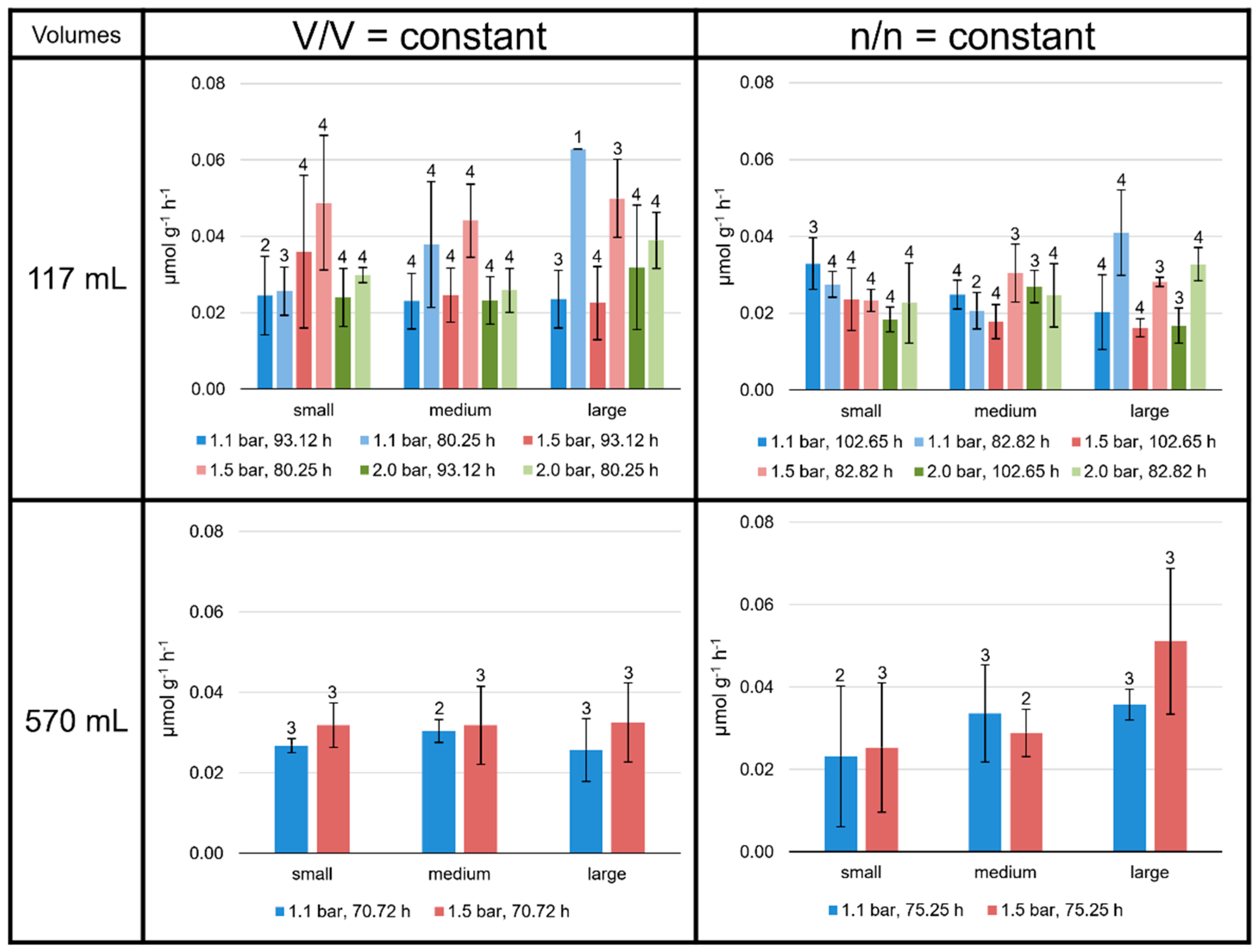
3.1. Specific Total Lipid Production Rates Depend on Culture Conditions
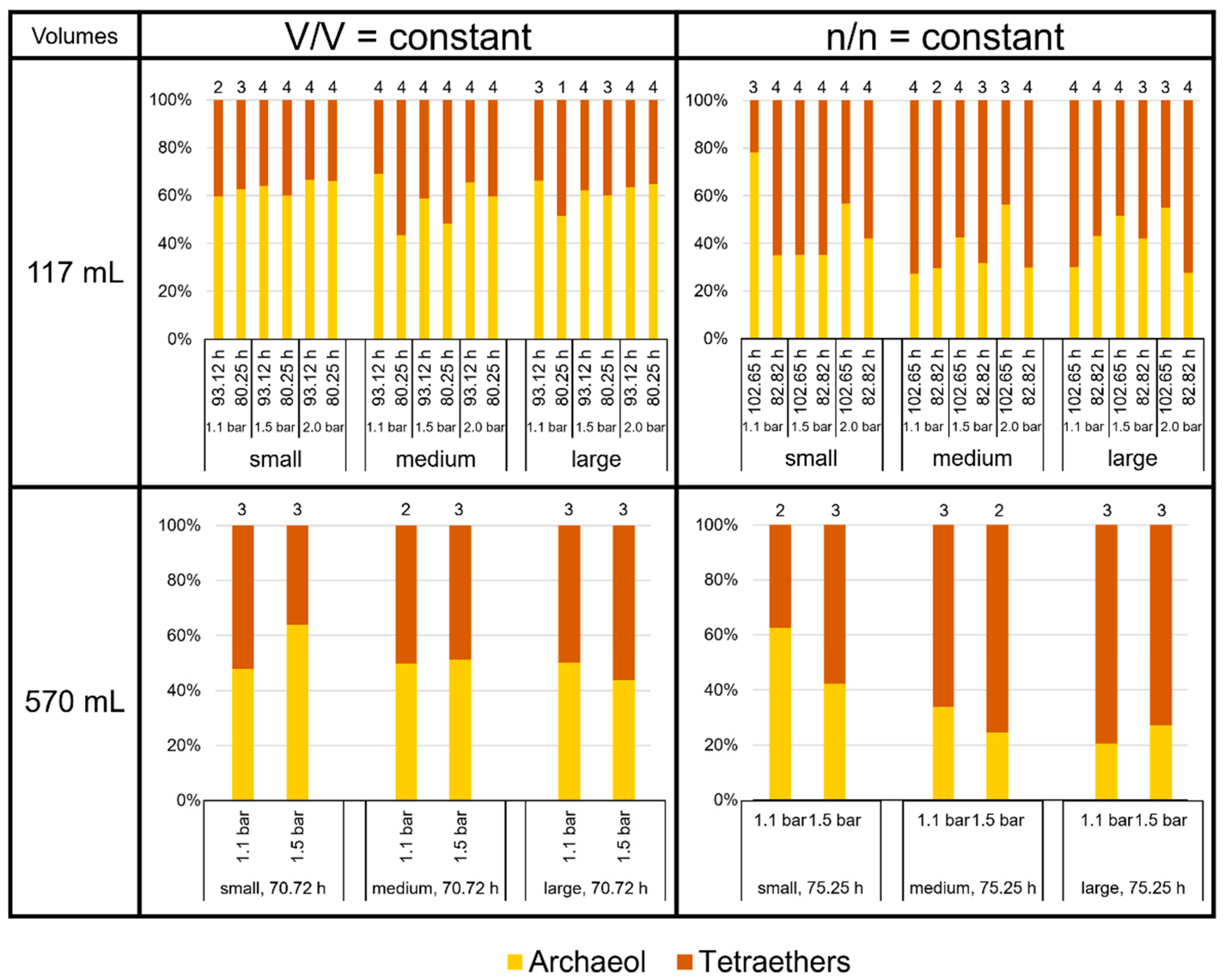
3.2. Product-to-Product Yield Followed the Trends of Specific Lipid Production Rates
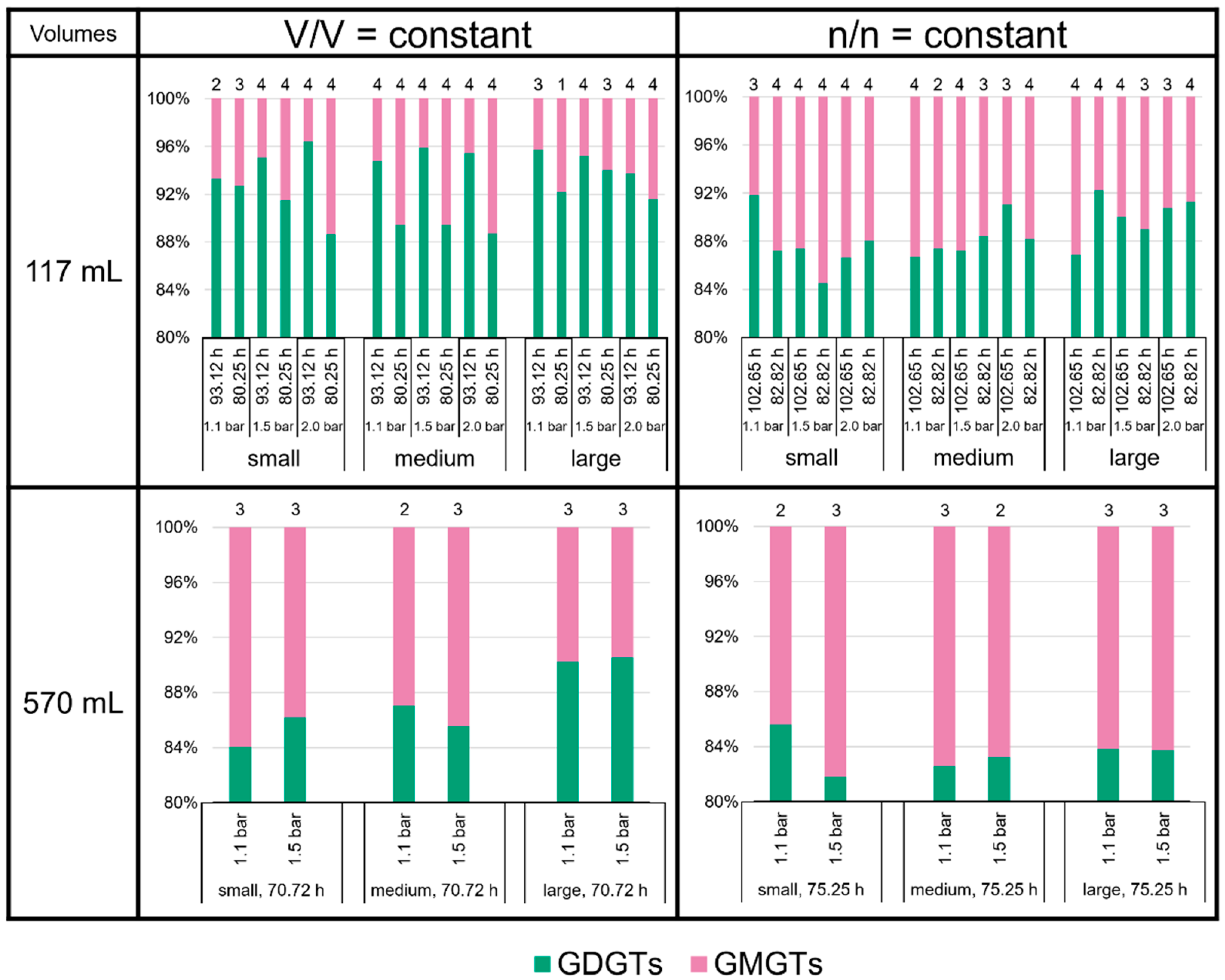
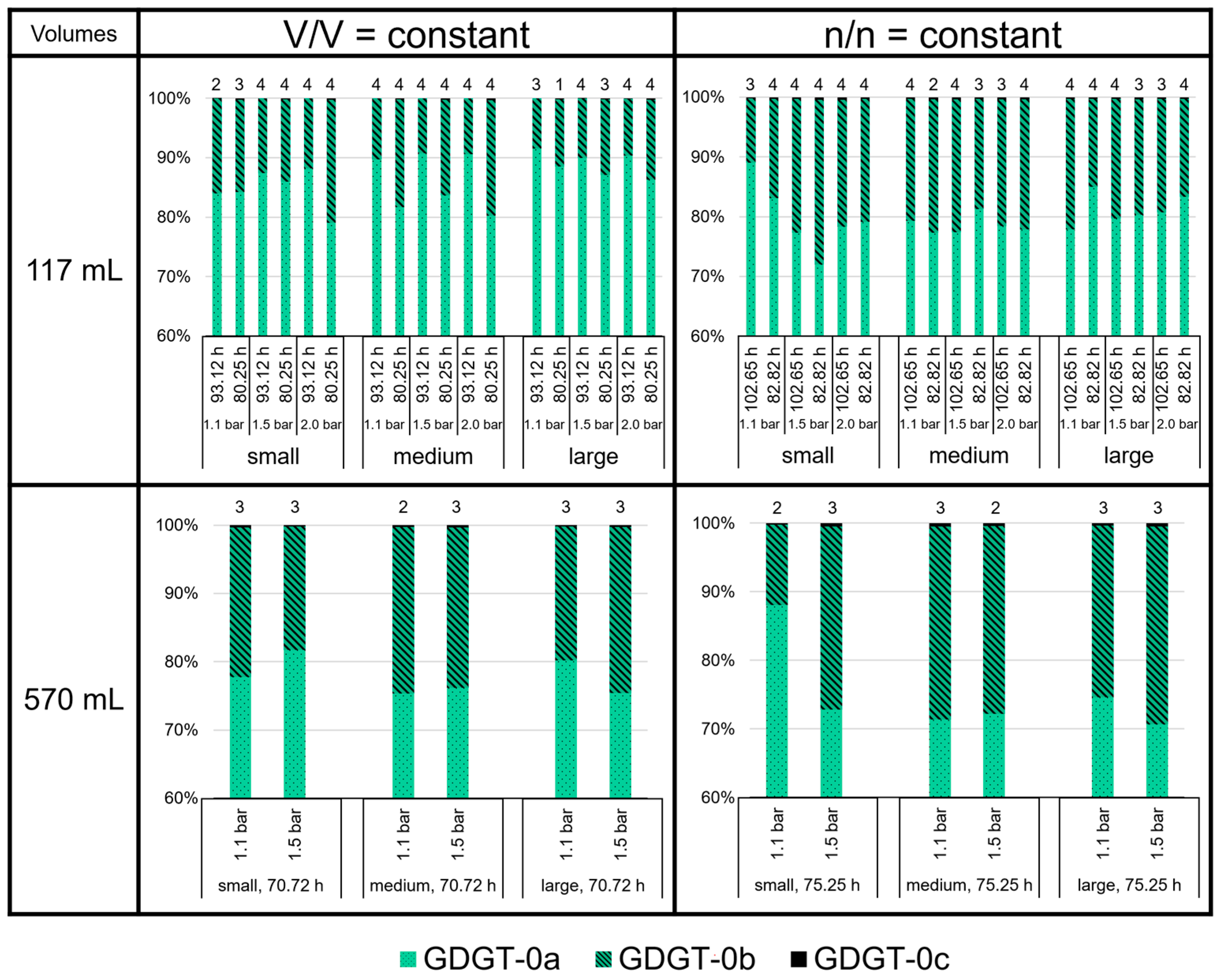
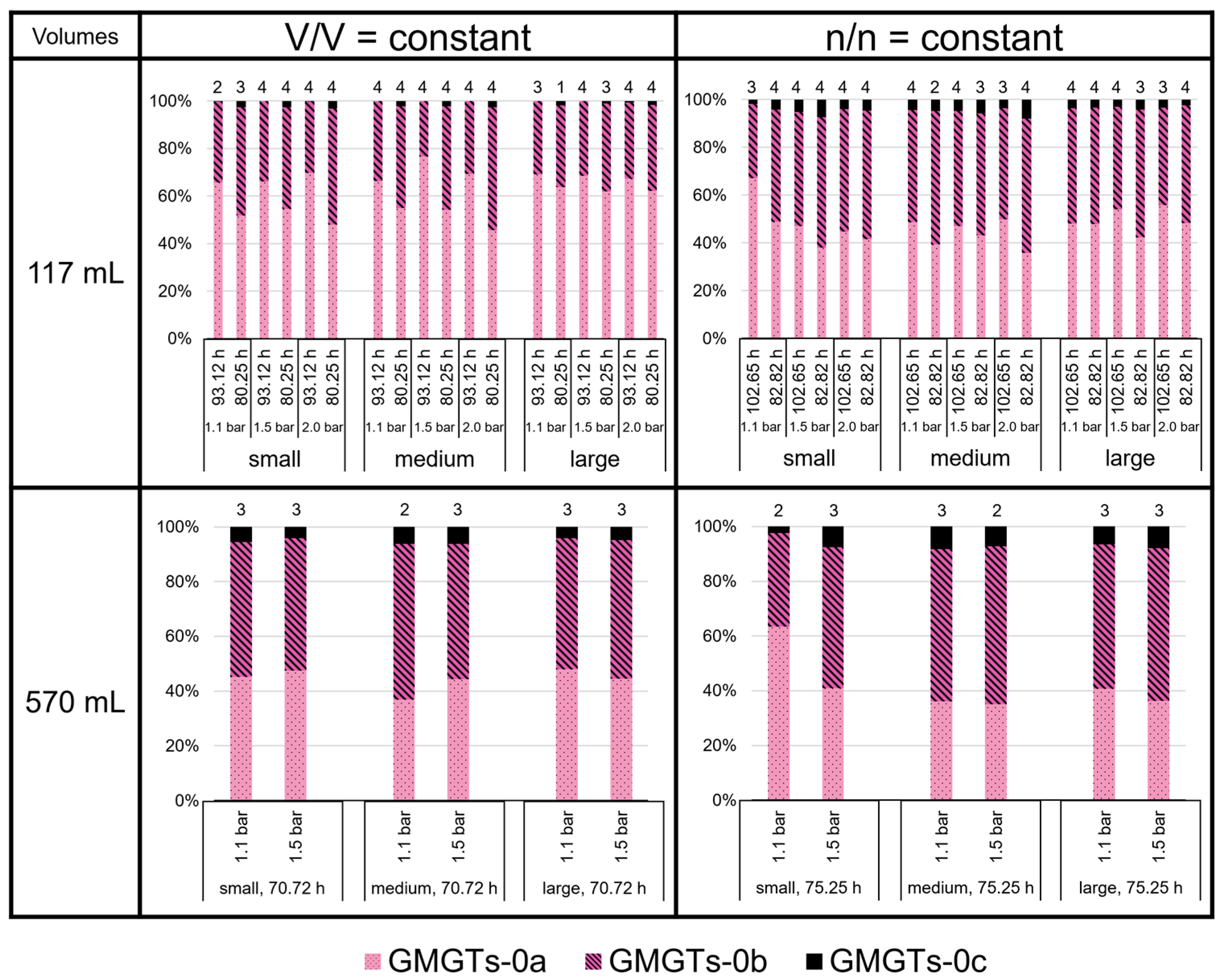
3.3. High Variability of Lipid Ratios Challenges Maintenance of Constant Lipid Quality
3.4. Impact of Interaction-Area-to-Volume Ratio on Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siliakus, M.F.; van der Oost, J.; Kengen, S.W.M. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 2017, 21, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Valentine, D.L. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat. Rev. Microbiol. 2007, 5, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Adam, P.S.; Borrel, G.; Brochier-Armanet, C.; Gribaldo, S. The growing tree of Archaea: New perspectives on their diversity, evolution and ecology. ISME J. 2017, 11, 2407–2425. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Gambacorta, A. The lipids of archaebacteria. Prog. Lipid Res. 1988, 27, 153–175. [Google Scholar] [CrossRef]
- Tornabene, T.G.; Langworthy, T.A.; Holzer, G.; Oró, J. Squalenes, phytanes and other isoprenoids as major neutral lipids of methanogenic and thermoacidophilic “archaebacteria”. J. Mol. Evol. 1979, 13, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, A.; Barbeau, J.; Lemiègre, L.; Benvegnu, T. Archaeal tetraether bipolar lipids: Structures, functions and applications. Biochimie 2009, 91, 711–717. [Google Scholar] [CrossRef]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.-M.R. Archaea Biotechnology. Biotechnol. Adv. 2021, 47, 107668. [Google Scholar] [CrossRef]
- Patel, G.B.; Sprott, G.D. Archaeobacterial Ether Lipid Liposomes (Archaeosomes) as Novel Vaccine and Drug Delivery Systems. Crit. Rev. Biotechnol. 1999, 19, 317–357. [Google Scholar] [CrossRef]
- Koga, Y.; Nishihara, M.; Morii, H.; Akagawa-Matsushita, M. Ether polar lipids of methanogenic bacteria: Structures, comparative aspects, and biosyntheses. Microbiol. Mol. Biol. Rev. 1993, 57, 164–182. [Google Scholar] [CrossRef]
- Baumann, L.M.F.; Taubner, R.-S.; Bauersachs, T.; Steiner, M.; Schleper, C.; Peckmann, J.; Rittmann, S.K.-M.R.; Birgel, D. Intact polar lipid and core lipid inventory of the hydrothermal vent methanogens Methanocaldococcus villosus and Methanothermococcus okinawensis. Org. Geochem. 2018, 126, 33–42. [Google Scholar] [CrossRef]
- Knappy, C.S.; Nunn, C.E.M.; Morgan, H.W.; Keely, B.J. The major lipid cores of the archaeon Ignisphaera aggregans: Implications for the phylogeny and biosynthesis of glycerol monoalkyl glycerol tetraether isoprenoid lipids. Extremophiles 2011, 15, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Golyshina, O.V.; Lünsdorf, H.; Kublanov, I.V.; Goldenstein, N.I.; Hinrichs, K.-U.; Golyshin, P.N. The novel extremely acidophilic, cell-wall-deficient archaeon Cuniculiplasma divulgatum gen. nov., sp. nov. represents a new family, Cuniculiplasmataceae fam. nov., of the order Thermoplasmatales. Int. J. Syst. Evol. Microbiol. 2016, 66, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Tourte, M.; Kuentz, V.; Schaeffer, P.; Grossi, V.; Cario, A.; Oger, P.M. Novel Intact Polar and Core Lipid Compositions in the Pyrococcus Model Species, P. furiosus and P. yayanosii, Reveal the Largest Lipid Diversity Amongst Thermococcales. Biomolecules 2020, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Knappy, C.; Barillà, D.; Chong, J.; Hodgson, D.; Morgan, H.; Suleman, M.; Tan, C.; Yao, P.; Keely, B. Mono-, di- and trimethylated homologues of isoprenoid tetraether lipid cores in archaea and environmental samples: Mass spectrometric identification and significance. J. Mass Spectrom. 2015, 50, 1420–1432. [Google Scholar] [CrossRef]
- Borrel, G.; Brugère, J.-F.; Gribaldo, S.; Schmitz, R.A.; Moissl-Eichinger, C. The host-associated archaeome. Nat. Rev. Microbiol. 2020, 18, 622–636. [Google Scholar] [CrossRef]
- Taubner, R.-S.; Schleper, C.; Firneis, M.G.; Rittmann, S.K.-M.R. Assessing the Ecophysiology of Methanogens in the Context of Recent Astrobiological and Planetological Studies. Life 2015, 5, 1652–1686. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef]
- Mauerhofer, L.M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.S.; Paulik, C.; Rittmann, S.K.-M.R. Hyperthermophilic methanogenic archaea act as high-pressure CH4 cell factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef]
- Mauerhofer, L.-M.; Reischl, B.; Schmider, T.; Schupp, B.; Nagy, K.; Pappenreiter, P.; Zwirtmayr, S.; Schuster, B.; Bernacchi, S.; Seifert, A.H.; et al. Physiology and methane productivity of Methanobacterium thermaggregans. Appl. Microbiol. Biotechnol. 2018, 102, 7643–7656. [Google Scholar] [CrossRef] [Green Version]
- Abdel Azim, A.; Pruckner, C.; Kolar, P.; Taubner, R.S.; Fino, D.; Saracco, G.; Sousa, F.L.; Rittmann, S.K.M.R. The physiology of trace elements in biological methane production. Bioresour. Technol. 2017, 241, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Rittmann, S.K.-M.R.; Seifert, A.H.; Bernacchi, S. Kinetics, multivariate statistical modelling, and physiology of CO2-based biological methane production. Appl. Energy 2018, 216, 751–760. [Google Scholar] [CrossRef]
- Schönheit, P.; Moll, J.; Thauer, R.K. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch. Microbiol. 1979, 123, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Wasserfallen, A.; Nölling, J.; Pfister, P.; Reeve, J.; De Macario, E.C. Phylogenetic analysis of 18 thermophilic Methanobacterium isolates supports the proposals to create a new genus, Methanothermobacter gen. nov., and to reclassify several isolates in three species, Methanothermobacter thermautotrophicus comb. nov., Methano. Int. J. Syst. Evol. Microbiol. 2000, 50, 43–53. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Wolfe, R.S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J. Bacteriol. 1972, 109, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Seifert, A.H.; Rittmann, S.; Herwig, C. Analysis of process related factors to increase volumetric productivity and quality of biomethane with Methanothermobacter marburgensis. Appl. Energy 2014, 132, 155–162. [Google Scholar] [CrossRef]
- Kaster, A.-K.; Moll, J.; Parey, K.; Thauer, R.K. Coupling of ferredoxin and heterodisulfide reduction via electron bifurcation in hydrogenotrophic methanogenic archaea. Proc. Natl. Acad. Sci. USA 2011, 108, 2981–2986. [Google Scholar] [CrossRef] [Green Version]
- Heiko, L.; Anne-Kristin, K.; Arnim, W.; Meike, G.; Antje, W.; Henning, S.; Gerhard, G.; Rudolf, K.T. Complete Genome Sequence of Methanothermobacter marburgensis, a Methanoarchaeon Model Organism. J. Bacteriol. 2010, 192, 5850–5851. [Google Scholar]
- Taubner, R.-S.; Rittmann, S.K.-M.R. Method for Indirect Quantification of CH4 Production via H2O Production Using Hydrogenotrophic Methanogens. Front. Microbiol. 2016, 7, 532. [Google Scholar] [CrossRef] [Green Version]
- Bernacchi, S.; Rittmann, S.; Seifert, H.A.; Krajete, A.; Herwig, C. Experimental methods for screening parameters influencing the growth to product yield (Y(x/CH4)) of a biological methane production (BMP) process performed with Methanothermobacter marburgensis. AIMS Bioeng. 2014, 1, 72–86. [Google Scholar] [CrossRef]
- Rittmann, S.; Seifert, A.; Herwig, C. Quantitative analysis of media dilution rate effects on Methanothermobacter marburgensis grown in continuous culture on H2 and CO2. Biomass Bioenergy 2012, 36, 293–301. [Google Scholar] [CrossRef]
- Rittmann, S.K.-M.R.; Pfeifer, K.; Palabikyan, H.; Ergal, İ.; Schuster, B. Archaea in der Biotechnologie. BIOspektrum 2021, 27, 96–98. [Google Scholar] [CrossRef]
- Gräther, O.W. Zur Struktur und Biosynthese der Tetraetherlipide der Archea; ETH Zürich: Zürich, Switzerland, 1994. [Google Scholar]
- Paltauf, F. Ether lipids in biomembranes. Chem. Phys. Lipids 1994, 74, 101–139. [Google Scholar] [CrossRef]
- Kaur, G.; Garg, T.; Rath, G.; Goyal, A.K. Archaeosomes: An excellent carrier for drug and cell delivery. Drug Deliv. 2016, 23, 2497–2512. [Google Scholar] [CrossRef]
- Gambacorta, A.; Gliozzi, A.; De Rosa, M. Archaeal lipids and their biotechnological applications. World J. Microbiol. Biotechnol. 1995, 11, 115–131. [Google Scholar] [CrossRef]
- Liefeith, K.; Frant, M.; Müller, U.; Stenstad, P.; Johnsen, H.; Schmid, R. Archaeal tetraether lipid coatings—A strategy for the development of membrane analog spacer systems for the site-specific functionalization of medical surfaces. Biointerphases 2018, 13, 011004. [Google Scholar] [CrossRef]
- Choquet, C.G.; Patel, G.B.; Sprott, G.D.; Beveridge, T.J. Stability of pressure-extruded liposomes made from archaeobacterial ether lipids. Appl. Microbiol. Biotechnol. 1994, 42, 375–384. [Google Scholar] [CrossRef]
- Choquet, C.G.; Patel, G.B.; Sprott, G.D. Heat sterilization of archaeal liposomes. Can. J. Microbiol. 1996, 42, 183–186. [Google Scholar] [CrossRef]
- Sprott, G.D.; Dicaire, C.J.; Fleming, L.P.; Patel, G.B. Stability of liposomes prepared from archaeobacterial lipids and phosphatidylcholine mixtures. Cells Mater. 1996, 6, 143–155. [Google Scholar]
- Metcalf, W.W.; Zhang, J.K.; Apolinario, E.; Sowers, K.R.; Wolfe, R.S. A genetic system for Archaea of the genus Methanosarcina: Liposome-mediated transformation and construction of shuttle vectors. Proc. Natl. Acad. Sci. USA 1997, 94, 2626–2631. [Google Scholar] [CrossRef] [Green Version]
- Benvegnu, T.; Lemiegre, L.; Cammas-Marion, S. New Generation of Liposomes Called Archaeosomes Based on Natural or Synthetic Archaeal Lipids as Innovative Formulations for Drug Delivery. Recent Pat. Drug Deliv. Formul. 2009, 3, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Leriche, G.; Cifelli, J.L.; Sibucao, K.C.; Patterson, J.P.; Koyanagi, T.; Gianneschi, N.C.; Yang, J. Characterization of drug encapsulation and retention in archaea-inspired tetraether liposomes. Org. Biomol. Chem. 2017, 15, 2157–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beveridge, T.J.; Choquet, C.G.; Patel, G.B.; Sprott, G.D. Freeze-fracture planes of methanogen membranes correlate with the content of tetraether lipids. J. Bacteriol. 1993, 175, 1191–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elferink, M.G.L.; de Wit, J.G.; Driessen, A.J.M.; Konings, W.N. Stability and proton-permeability of liposomes composed of archaeal tetraether lipids. Biochim. Biophys. Acta Biomembr. 1994, 1193, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, L.; Dennis Sprott, G. Archaeosomes as Self-adjuvanting Delivery Systems for Cancer Vaccines*. J. Drug Target. 2003, 11, 515–524. [Google Scholar] [CrossRef]
- Krishnan, L.; Sad, S.; Patel, G.B.; Sprott, G.D. Archaeosomes Induce Long-Term CD8 + Cytotoxic T Cell Response to Entrapped Soluble Protein by the Exogenous Cytosolic Pathway, in the Absence of CD4 + T Cell Help. J. Immunol. 2000, 165, 5177–5185. [Google Scholar] [CrossRef] [Green Version]
- Stark, F.C.; Agbayani, G.; Sandhu, J.K.; Akache, B.; McPherson, C.; Deschatelets, L.; Dudani, R.; Hewitt, M.; Jia, Y.; Krishnan, L.; et al. Simplified Admix Archaeal Glycolipid Adjuvanted Vaccine and Checkpoint Inhibitor Therapy Combination Enhances Protection from Murine Melanoma. Biomedicine 2019, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Huguet, C.; Hopmans, E.C.; Febo-Ayala, W.; Thompson, D.H.; Sinninghe Damsté, J.S.; Schouten, S. An improved method to determine the absolute abundance of glycerol dibiphytanyl glycerol tetraether lipids. Org. Geochem. 2006, 37, 1036–1041. [Google Scholar] [CrossRef] [Green Version]
- Schouten, S.; Hopmans, E.C.; Sinninghe Damsté, J.S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review. Org. Geochem. 2013, 54, 19–61. [Google Scholar] [CrossRef]
- Taubner, R.-S.; Baumann, L.M.F.; Bauersachs, T.; Clifford, E.L.; Mähnert, B.; Reischl, B.; Seifert, R.; Peckmann, J.; Rittmann, S.K.-M.R.; Birgel, D. Membrane Lipid Composition and Amino Acid Excretion Patterns of Methanothermococcus okinawensis Grown in the Presence of Inhibitors Detected in the Enceladian Plume. Life 2019, 9, 85. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumann, L.M.F.; Taubner, R.-S.; Oláh, K.; Rohrweber, A.-C.; Schuster, B.; Birgel, D.; Rittmann, S.K.-M.R. Quantitative Analysis of Core Lipid Production in Methanothermobacter marburgensis at Different Scales. Bioengineering 2022, 9, 169. https://doi.org/10.3390/bioengineering9040169
Baumann LMF, Taubner R-S, Oláh K, Rohrweber A-C, Schuster B, Birgel D, Rittmann SK-MR. Quantitative Analysis of Core Lipid Production in Methanothermobacter marburgensis at Different Scales. Bioengineering. 2022; 9(4):169. https://doi.org/10.3390/bioengineering9040169
Chicago/Turabian StyleBaumann, Lydia M. F., Ruth-Sophie Taubner, Kinga Oláh, Ann-Cathrin Rohrweber, Bernhard Schuster, Daniel Birgel, and Simon K.-M. R. Rittmann. 2022. "Quantitative Analysis of Core Lipid Production in Methanothermobacter marburgensis at Different Scales" Bioengineering 9, no. 4: 169. https://doi.org/10.3390/bioengineering9040169





