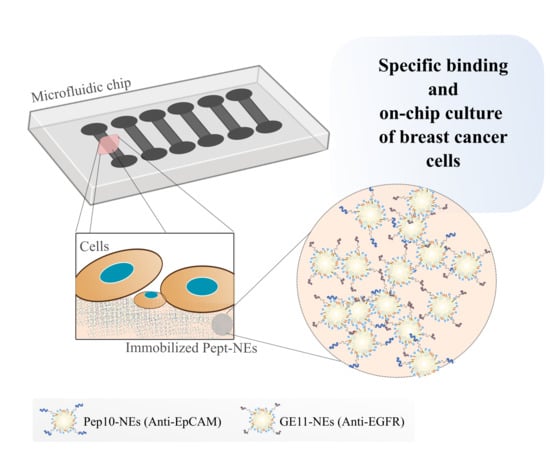
Peptide-Functionalized Nanoemulsions as a Promising Tool for Isolation and Ex Vivo Culture of Circulating Tumor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents and Solvents
2.1.2. Cell Cultures
2.2. Methods
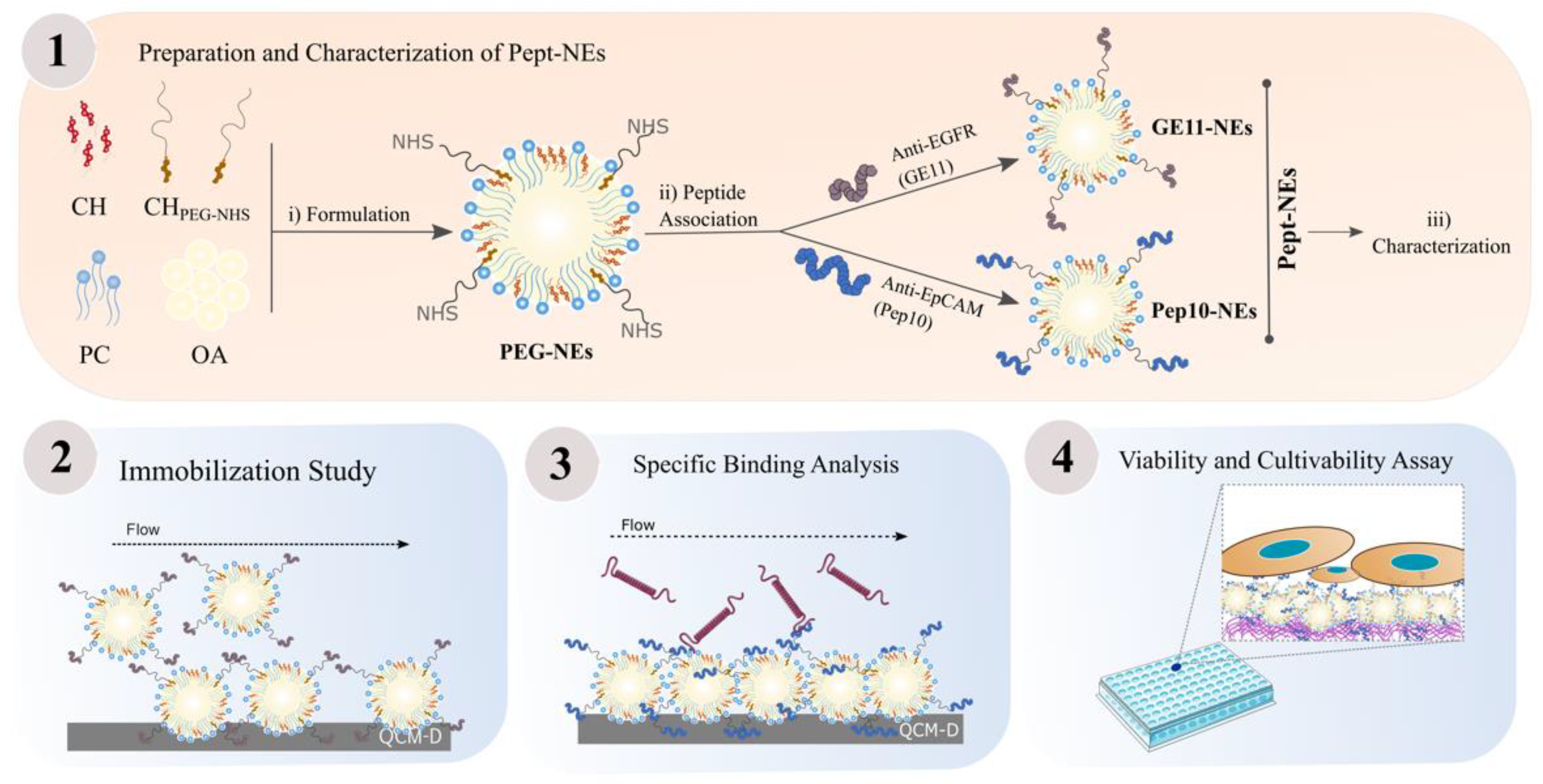
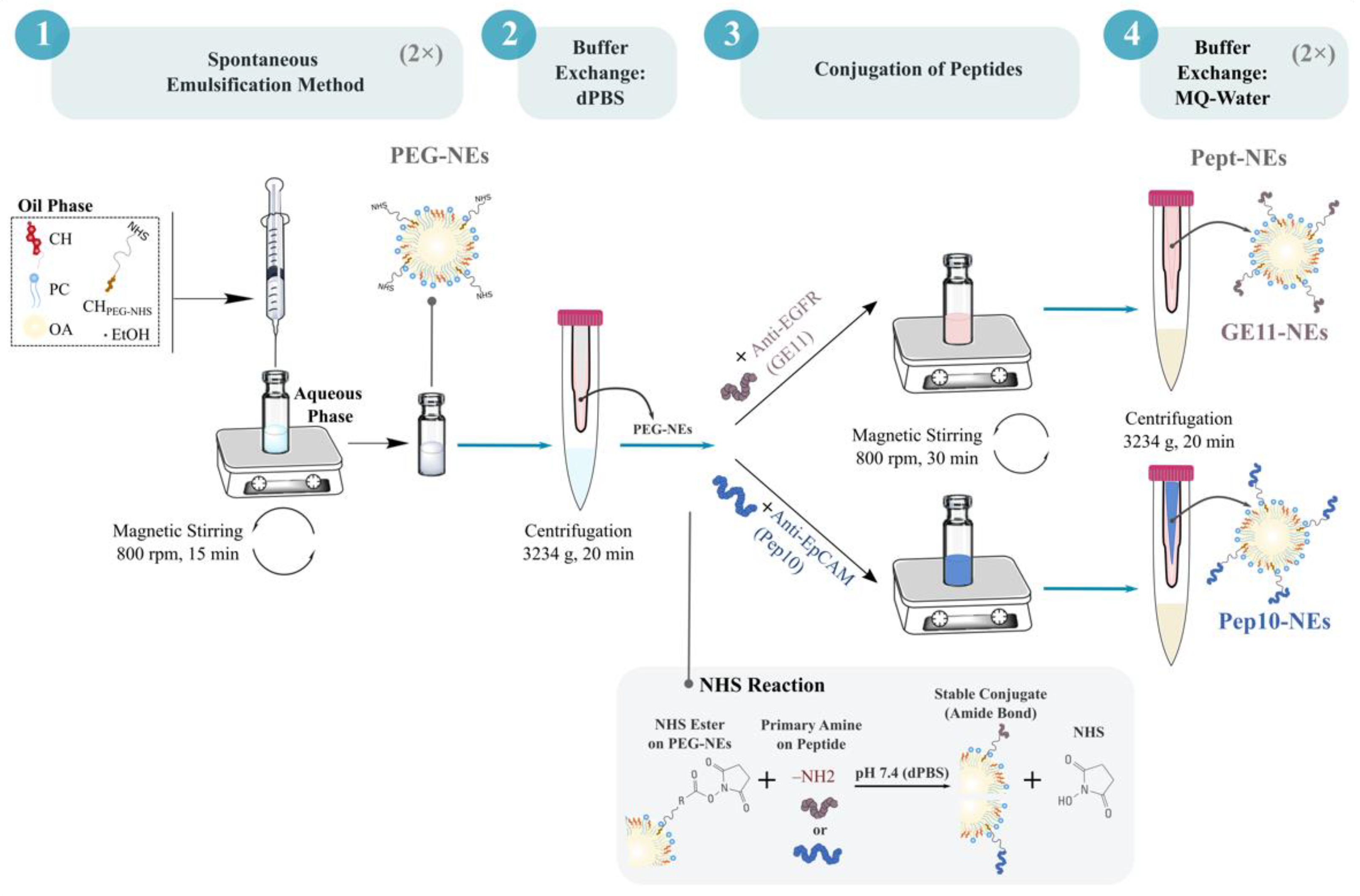
2.2.1. Formulation of PEGylated Nanoemulsions (PEG-NEs)
2.2.2. Conjugation of GE11 and Pep10 Peptides onto Surface of PEG-NEs
2.2.3. Characterization of Pept-NEs
Dynamic Light Scattering (DLS) and Nanoparticle Tracking Analysis (NTA)
Transmission Electron Microscopy (TEM) Analysis
2.2.4. Efficiency of Peptide Functionalization on Pept-NEs Formulations
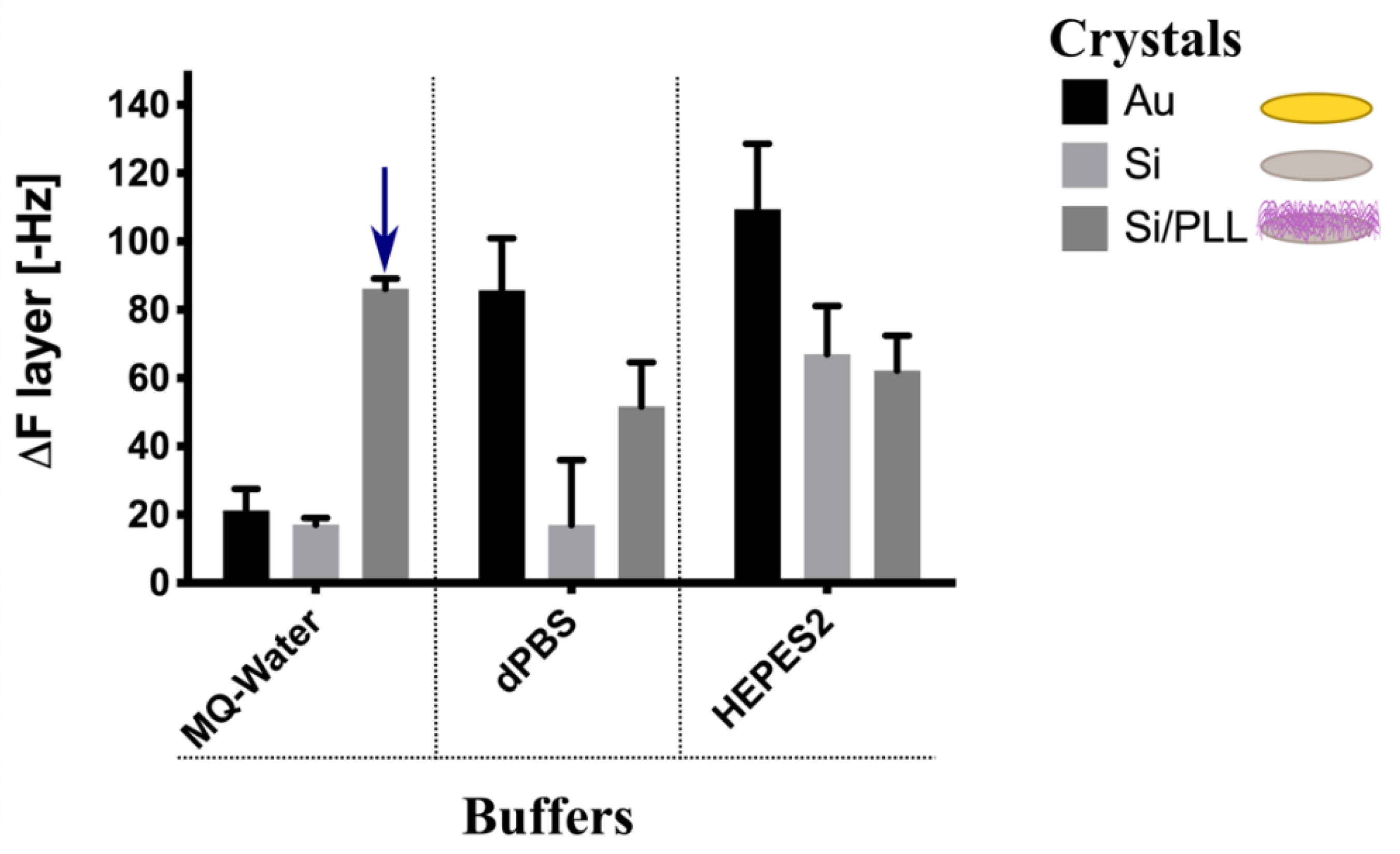
2.2.5. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D) Experiments
2.2.6. Fluorescence Microscopy
2.2.7. Cell Viability Analysis
2.2.8. Statistical Analysis
3. Results
3.1. PEG-NEs Immobilization Analysis onto Different Surfaces by QCM-D
3.2. Assessment of Pept-NEs Binding to Their Specific Proteins under Continuous Flow, Analyzed by QCM-D
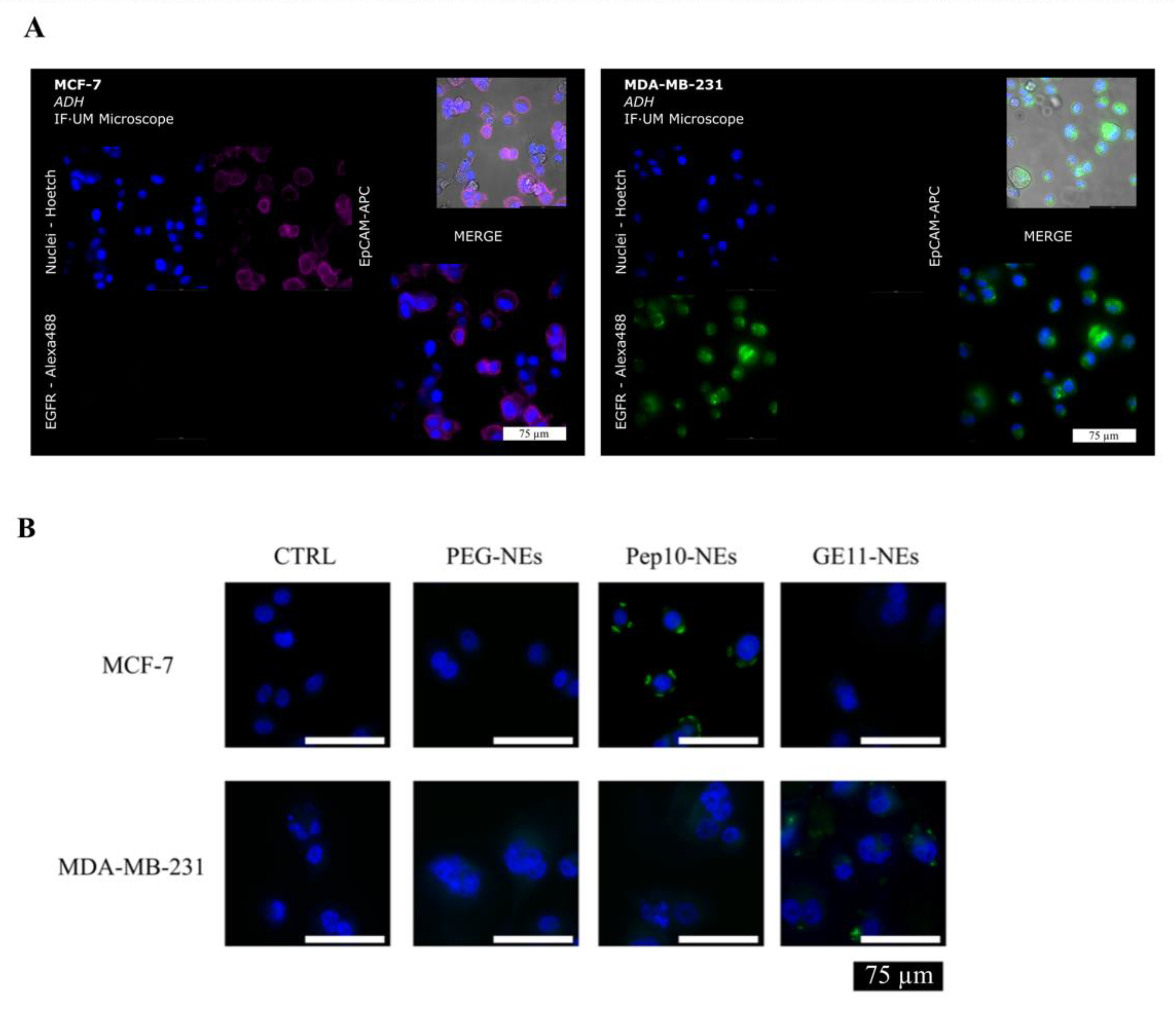
3.3. Specific Binding Analysis between Pept-NEs and Cells by Fluorescence Microscopy
3.4. Cell Viability Assessment of Immobilized Pept-NEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [Green Version]
- Micalizzi, D.S.; Maheswaran, S.; Haber, D.A. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017, 31, 1827–1840. [Google Scholar] [CrossRef]
- Larson, M.H.; Pan, W.; Kim, H.J.; Mauntz, R.E.; Stuart, S.M.; Pimentel, M.; Zhou, Y.; Knudsgaard, P.; Demas, V.; Aravanis, A.M.; et al. A comprehensive characterization of the cell-free transcriptome reveals tissue- and subtype-specific biomarkers for cancer detection. Nat. Commun. 2021, 12, 2357. [Google Scholar] [CrossRef]
- Katelyn, N.S.; Katherine, H.R.T. Circulating Biomarkers in Breast Cancer. Clin. Breast Cancer 2022, 22, 319–331. [Google Scholar] [CrossRef]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid biopsy of cancer: A multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef]
- Brock, G.; Castellanos-Rizaldos, E.; Hu, L.; Coticchia, C.; Skog, J. Liquid biopsy for cancer screening, patient stratification and monitoring. Transl. Cancer Res. 2015, 4, 280–290. [Google Scholar] [CrossRef]
- Zhongyi, Z.; Si, Q.; Kang, S.; Yong, H. Progress and challenges of sequencing and analyzing circulating tumor cells. Cell Biol. Toxicol. 2018, 34, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Panabières, C.A.; Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Sparano, J.; O’Neill, A.; Alpaugh, K.; Wolff, A.C.; Northfelt, D.W.; Dang, C.T.; Sledge, G.W.; Miller, K.D. Association of Circulating Tumor Cells with Late Recurrence of Estrogen Receptor—Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1700–1706. [Google Scholar] [CrossRef]
- Bidard, F.C.; Proudhon, C.; Pierga, J.Y. Circulating tumor cells in breast cancer. Mol. Oncol. 2016, 10, 418–430. [Google Scholar] [CrossRef] [Green Version]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabières, C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 2015, 9, 1773–1782. [Google Scholar] [CrossRef] [Green Version]
- Cimadamore, A.; Aurilio, G.; Nolé, F.; Massari, F.; Scarpelli, M.; Santoni, M.; Lopez-Beltran, A.; Cheng, L.; Montironi, R. Update on Circulating Tumor Cells in Genitourinary Tumors with Focus on Prostate Cancer. Cells 2020, 9, 1495. [Google Scholar] [CrossRef]
- Pantel, K.; Denève, E.; Nocca, D.; Coffy, A.; Vendrell, J.P.; Maudelonde, T.; Riethdorf, S.; Alix-Panabières, C. Circulating epithelial cells in patients with benign colon diseases. Clin. Chem. 2012, 58, 936–940. [Google Scholar] [CrossRef]
- Schulze, K.; Gasch, C.; Staufer, K.; Nashan, B.; Lohse, A.W.; Pantel, K.; Riethdorf, S.; Wege, H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int. J. Cancer 2013, 133, 2165–2171. [Google Scholar] [CrossRef]
- Rink, M.; Chun, F.K.; Dahlem, R.; Soave, A.; Minner, S.; Hansen, J.; Stoupiec, M.; Coith, C.; Kluth, L.A.; Ahyai, S.A.; et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: A prospective study. Eur. Urol. 2012, 6, 1810–1817. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Maheswaran, Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 80, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.C.; Doyle, G.V.; Terstappen, L.W.M.M. Significance of Circulating Tumor Cells Detected by the Cell Search System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J. Oncol. 2010, 2010, 617421. [Google Scholar] [CrossRef]
- Yasuhiro, C.; Kazue, Y.; Takashi, O.; Fumihiro, T. EpCAM-independent capture of circulating tumor cells with a ‘universal CTC-chip’. Oncol. Rep. 2017, 37, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [Green Version]
- Subham, P.; Bishal, K.; Nahaka, S.; Patra, D.; Cristina, T.; Sukho, P.; Mikael, S.; Gorka, O.; Ashutosh, T. Emergence of microfluidics for next generation biomedical devices. Biosens. Bioelectron. 2022, 10, 100106. [Google Scholar] [CrossRef]
- Hogyeong, G.; Junmoo, K.; Leila, K.-K.; Bongseop, K.; Kyung-A, H.; Hyo-II, J. Progress in Circulating Tumor Cell Research Using Microfluidic Devices. Micromachines 2018, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Ule, N.; González-Conde, M.; Abuín, C.; Cueva, J.F.; Palacios, P.; López-López, R.; Costa, C.; Dávila-Ibáñez, A.B. Short-Term Ex Vivo Culture of CTCs from Advance Breast Cancer Patients: Clinical Implications. Cancers 2021, 13, 2668. [Google Scholar] [CrossRef]
- Carmona-Ule, N.; Abuín-Redondo, C.; Costa, C.; Piñeiro, R.; Pereira-Veiga, T.; Martínez-Pena, I.; Hurtado, P.; López-López, R.; de la Fuente, M.; Dávila-Ibáñez, A.B. Nanoemulsions to support ex vivo cell culture of breast cancer circulating tumor cells. Mater. Today Chem. 2020, 16, 100265–100370. [Google Scholar] [CrossRef]
- Keller, L. Biology and clinical relevance of EpCAM. Cell Stress 2019, 3, 165–180. [Google Scholar] [CrossRef] [Green Version]
- Payne, R.E.; Yague, E.; Slade, M.J.; Christos, A.; Jiao, L.R.; Ward, B.; Stebbing, J. Measurements of EGFR expression on circulating tumor cells are reproducible over time in metastatic breast cancer patients. Pharmacogenomics 2008, 10, 51–57. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Lee, A.H.S.; Robertson, J.F.; Ellis, I.O. Prognostic markers in triple-negative breast cancer. Cancer 2007, 109, 25–32. [Google Scholar] [CrossRef]
- Vila, A.; Abal, M.; Muinelo-romay, L.; Rodriguez-Abreu, C.; Lo, R. EGFR-Based Immunoisolation as a Recovery Target for Low-EpCAM CTC Subpopulation. PLoS ONE 2016, 6, e0163705. [Google Scholar] [CrossRef] [Green Version]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Bai, L.; Du, Y.; Peng, J.; Liu, Y.; Wang, Y.; Yang, Y.; Wang, C. Peptide-based isolation of circulating tumor cells by magneticnan particles. J. Mater. Chem. B 2014, 2, 4080–4088. [Google Scholar] [CrossRef]
- Ruoslahti, E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mater. 2012, 24, 3747–3756. [Google Scholar] [CrossRef]
- Zou, Y.; Xia, Y.; Meng, F.; Zhang, J.; Zhong, Z. GE11-Directed Functional Polymersomal Doxorubicin as an Advanced Alternative to Clinical Liposomal Formulation for Ovarian Cancer Treatment. Mol. Pharm. 2018, 15, 3664–3671. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Tang, W.J.; Li, D.; Wei, Y.Z.; Lu, Y.; Li, Z.-H.; Liang, X.F. Construction of Epidermal Growth Factor Receptor Peptide Magnetic Nanovesicles with Lipid Bilayers for Enhanced Capture of Liver Cancer Circulating Tumor Cells. Anal. Chem. 2016, 88, 8997–9003. [Google Scholar] [CrossRef] [Green Version]
- Khode, V.; Patil, S.; Kaveeshwar, V.; Ruikar, K.; Bargale, A.E.S.; Patil, S. Ubiquitin Mediated Degradation of EGFR by 17 β-estradiol in Triple Negative MDA-MB-231 (TNBC) Breast Cancer Cells Line. Curr. Mol. Med. 2022, 22, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sheng, W.; Al-Rawe, M.; Mohiuddin, T.M.; Niebert, M.; Zeppernick, F.; Meihold-Heerlein, I.; Hussain, A.F. EpCAM- and EGFR-Specific Antibody Drug Conjugates for Triple-Negative Breast Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 6122. [Google Scholar] [CrossRef]
- Kalkhof, S.; Sinz, A. Chances and pitfalls of chemical cross-linking with amine-reactive N-hydroxysuccinimide esters. Anal. Bioanal. Chem. 2008, 392, 305–312. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L.; Michael, H.; Sapsford, K.E.; et al. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J. Surface engineering of nanomaterials with phospholipid-polyethylene glycol-derived functional conjugates for molecular imaging and targeted therapy. Biomaterials 2020, 230, 119646. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, F.; Cheng, L.; Zhu, Y.; Hu, Q.; Li, L.; Wei, L. GE11-modified liposomes for non-small cell lung cancer targeting: Preparation, ex vitro and in vivo evaluation. Int. J. Nanomed. 2014, 9, 921–935. [Google Scholar] [CrossRef] [Green Version]
- Akpinar, B.; Haynes, J.P.; Bell, N.A.W.; Brunner, K.; Pyne, A.L.B.; Hoogenboom, B.W. PEGylated surfaces for the study of DNA-protein interactions by atomic force microscopy. Nanoscale 2019, 11, 20072–20080. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Pan, M.; Zhang, W.; Lin, H.; Wu, S.; Lu, C.; Tang, S.; Liu, D.; Cai, J. Poly(α-l-lysine)-based nanomaterials for versatile biomedical applications: Current advances and perspectives. Bioact. Mater. 2021, 6, 1878–1909. [Google Scholar] [CrossRef]
- Wankowicz, S.A.; de Oliveira, S.H.; Hogan, D.W.; van den Bedem, H.; Fraser, J.S. Ligand binding remodels protein side-chain conformational heterogeneity. eLife 2022, 11, 74114. [Google Scholar] [CrossRef]
- Sanders, J.M.; Wampole, M.E.; Thakur, M.L.; Wickstrom, E. Molecular determinants of epidermal growth factor binding: A molecular dynamics study. PLoS ONE 2013, 8, 54136. [Google Scholar] [CrossRef] [Green Version]
- Gaber, A.; Lenarčič, B.; Pavšič, M. Current View on EpCAM Structural Biology. Cells 2020, 31, 1361. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Long, Y.; Liu, G.; Zang, T.; Zhang, G. Ion-Specific Conformational Behavior of Polyzwitterionic Brushes: Exploiting It for Protein Adsorption/Desorption Control. Langmuir 2013, 29, 6588–6596. [Google Scholar] [CrossRef]
- Scharpenseel, H.; Hanssen, A.; Loges, S.; Mohme, M.; Bernreuther, C.; Peine, S.; Lamzus, K.; Goy, Y.; Petersen, C.; Westphal, M.; et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non small cell lung cancer patients. Sci. Rep. 2019, 9, 7406. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Ule, N.; Gal, N.; Abuín Redondo, C.; De La Fuente Freire, M.; López López, R.; Dávila-Ibáñez, A.B. Peptide-Functionalized Nanoemulsions as a Promising Tool for Isolation and Ex Vivo Culture of Circulating Tumor Cells. Bioengineering 2022, 9, 380. https://doi.org/10.3390/bioengineering9080380
Carmona-Ule N, Gal N, Abuín Redondo C, De La Fuente Freire M, López López R, Dávila-Ibáñez AB. Peptide-Functionalized Nanoemulsions as a Promising Tool for Isolation and Ex Vivo Culture of Circulating Tumor Cells. Bioengineering. 2022; 9(8):380. https://doi.org/10.3390/bioengineering9080380
Chicago/Turabian StyleCarmona-Ule, Nuria, Noga Gal, Carmen Abuín Redondo, María De La Fuente Freire, Rafael López López, and Ana Belén Dávila-Ibáñez. 2022. "Peptide-Functionalized Nanoemulsions as a Promising Tool for Isolation and Ex Vivo Culture of Circulating Tumor Cells" Bioengineering 9, no. 8: 380. https://doi.org/10.3390/bioengineering9080380