Evaluation of Soybean–Navy Bean Emulsions Using Different Processing Technologies †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Starting Material
2.2. Emulsion Formulation and Cooking Methods
2.3. Evaluation of Physical Properties
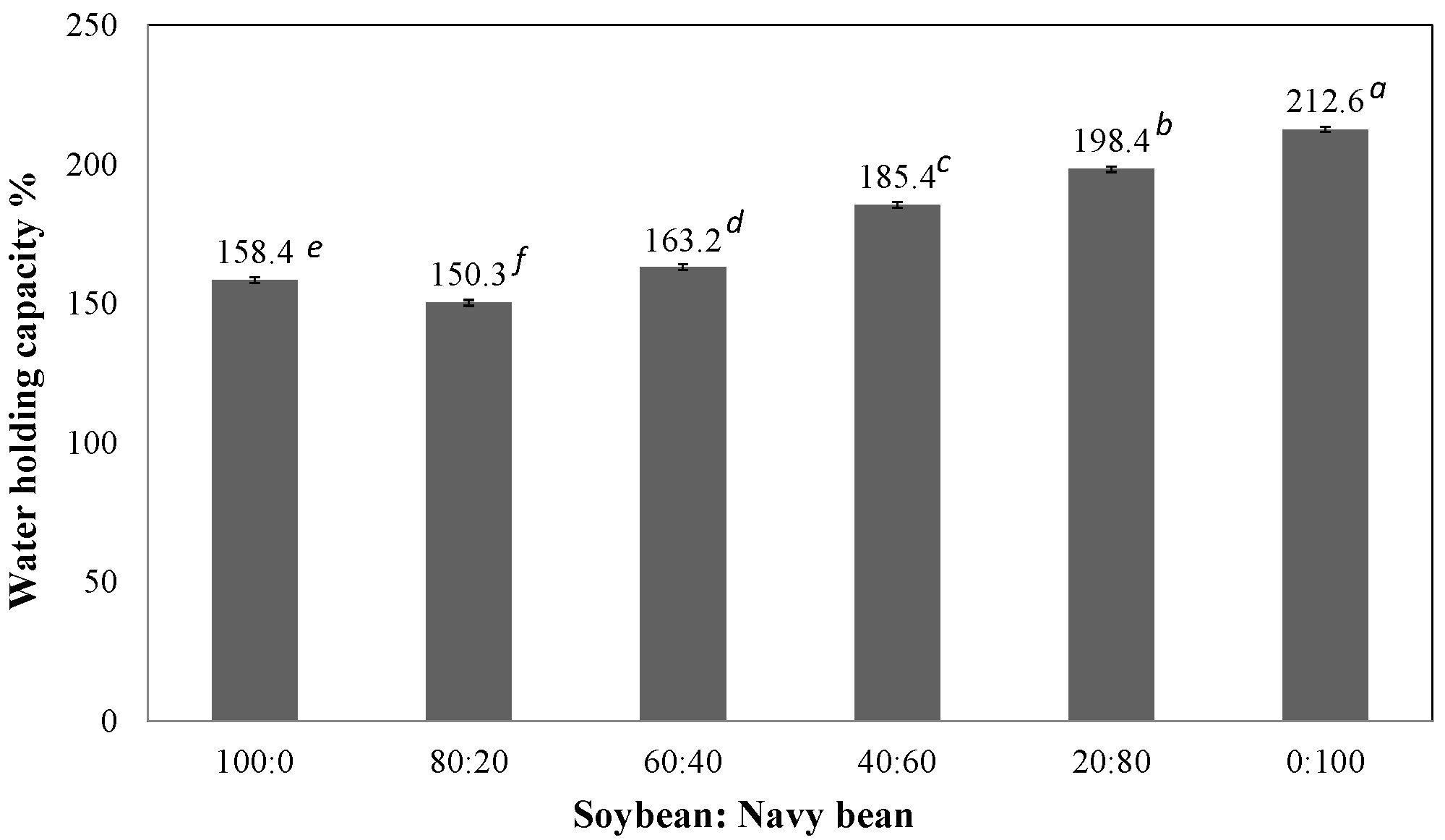
2.3.1. Water Holding Capacity
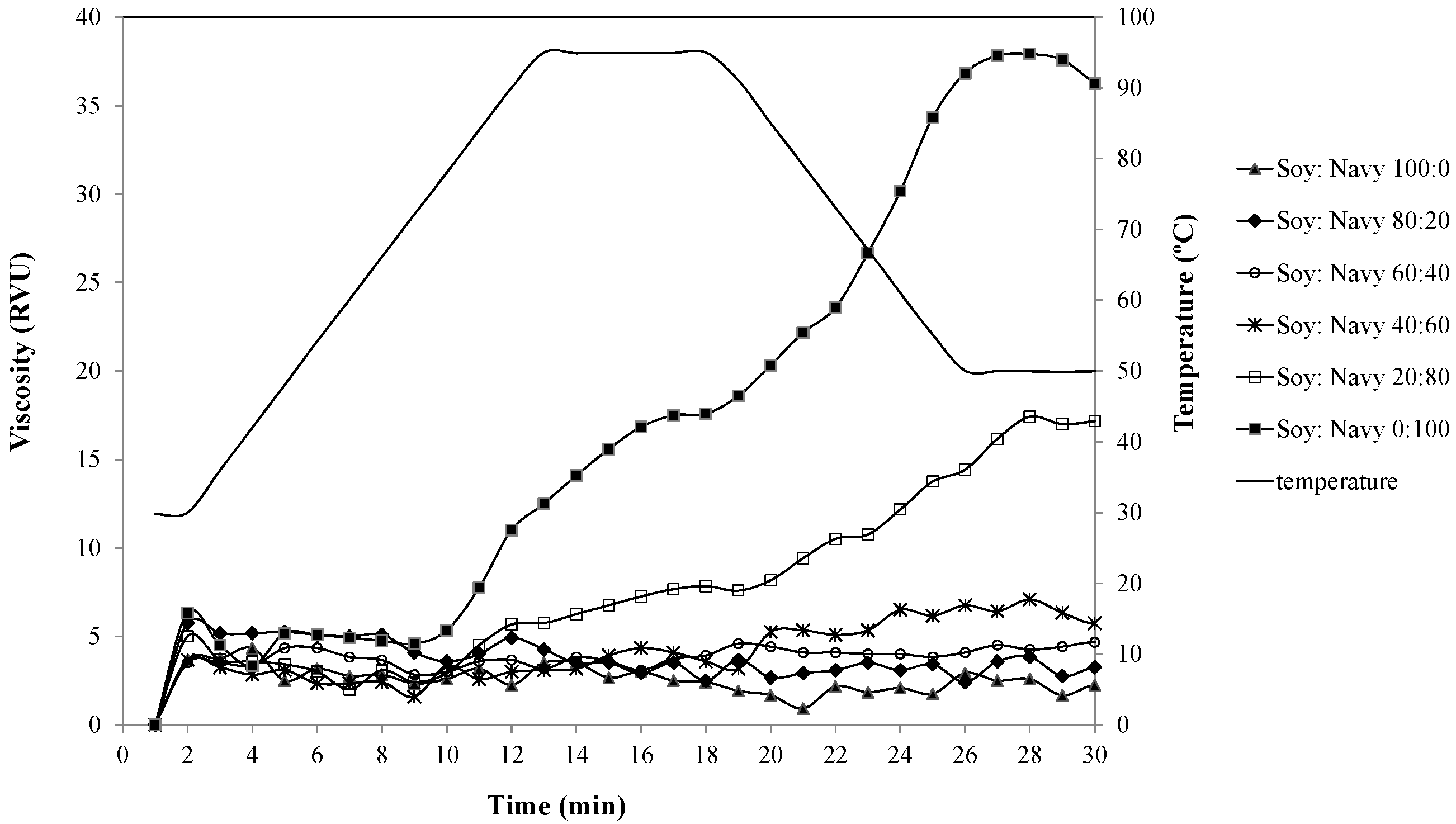
2.3.2. Pasting Property Measurement
2.3.3. Percentage of Solids in Emulsion
2.3.4. Viscosity
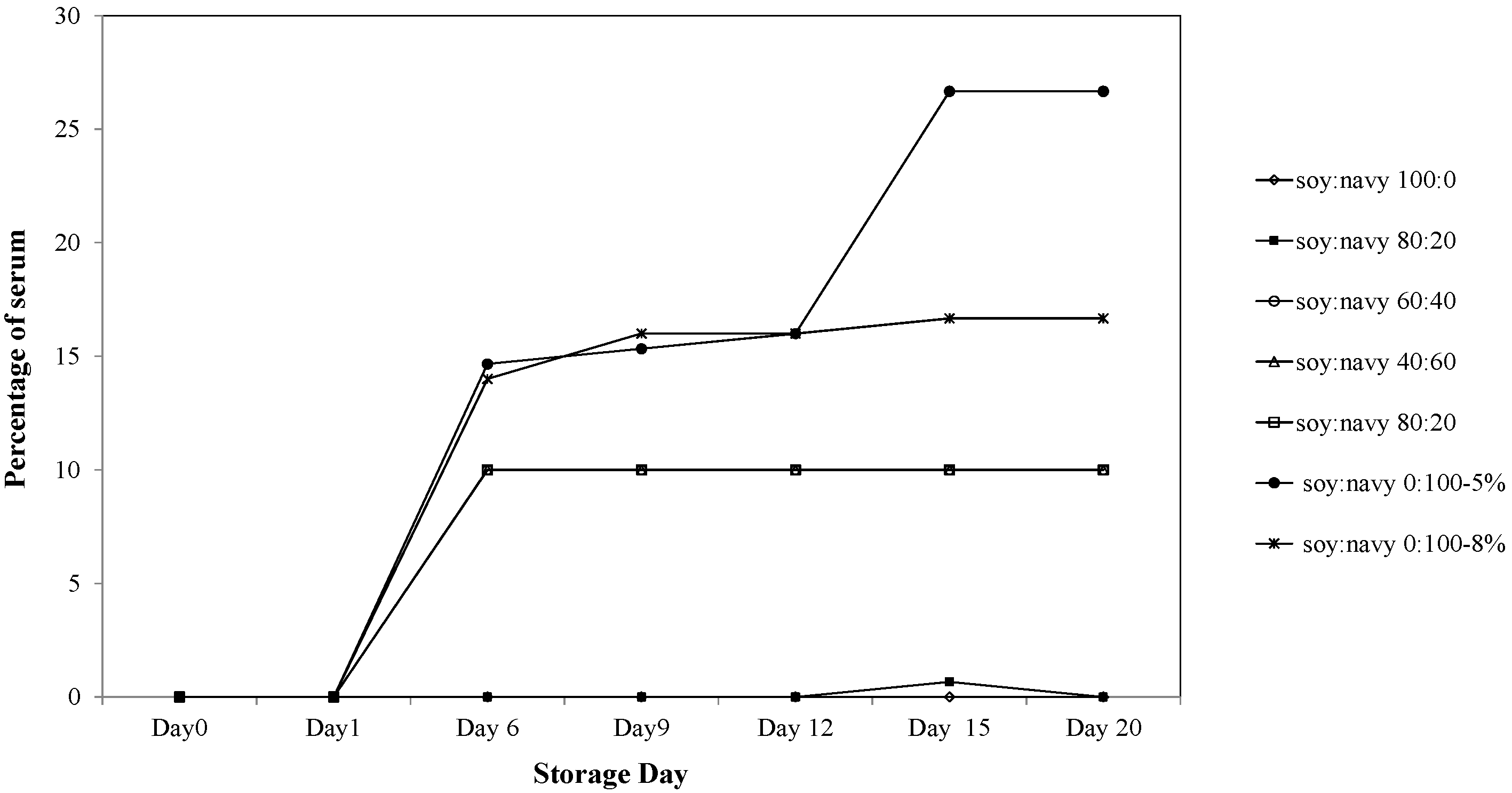
2.3.5. Storage Stability
2.3.6. Particle Size
2.4. Evaluation of Nutritional and Anti-nutritional Components
2.4.1. Total Starch
2.4.2. Protein Analysis
2.4.3. Fat Content
2.4.4. Dietary Fiber
2.4.5. Soluble Sugars
2.4.6. Trypsin Inhibitor
2.4.7. Phytohemagglutinin (PHA) Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Nutritional and Anti-Nutritional Components after Cooking
3.1.1. Protein, Crude Fat, Total Starch, and Dietary Fiber of Emulsions Made by Different Processing Methods
3.1.2. Soluble Sugars
3.1.3. Trypsin Inhibitor
3.1.4. Phytohemagglutinin (PHA) Activity
3.2. Physical Properties
3.2.1. Water Holding Capacity
3.2.2. RVA Pasting Properties
3.2.3. Percentage Solids in Emulsions
3.2.4. Emulsion Viscosity
3.2.5. Storage Stability
3.2.6. Particle Size
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- USDA National Nutrient Database for Standard Reference. Available online: http://ndb.nal.usda.gov/ndb/search/list (accessed on 15 November 2015).
- Ha, V.; Sievenpiper, J.L.; de Souza, R.J.; Jayalath, V.H.; Mirrahimi, A.; Agarwal, A.; Chiavaroli, L.; Blanco Mejia, S.; Sacks, F.M.; Di Buono, M.; et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: A systematic review and meta-analysis of randomized controlled trials. Can. Med. Assoc. J. 2014, 186, E252–E262. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R. Dietary fiber—A perspective. In Dietary Fiber in Human Nutrition. CRC Handbook, 2nd ed.; Spiller, G.A., Ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 7–11. [Google Scholar]
- CGIAR. Improving Nutrition and Health. Available online: http://grainlegumes.cgiar.org/why-grain-legumes-matter/improving-nutrition-and-health/ (accessed on 15 September 2016).
- Fanta, G.F.; Felker, F.C.; Byars, J.A.; Kenar, J.A.; Shogren, R.L. Starch-soybean oil composites with high oil:starch ratios prepared by steam jet cooking. Starch/Stärke 2009, 61, 590–600. [Google Scholar] [CrossRef]
- Ade-Omowaye, B.I.O.; Taiwo, K.A.; Eshtiaghi, N.M.; Angersbach, A.; Knorr, D. Comparative Evaluation of the Effects of Pulsed Electric Field and Freezing on Cell Membrane Permeabilisation and Mass Transfer during Dehydration of Red Bell Peppers. Innov. Food Sci. Emerg. Technol. 2003, 4, 177–188. [Google Scholar] [CrossRef]
- AACC International. Total Starch. Approved Methods of Analysis, 11th ed.; Method 76–13.01.; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- AACC International. Total Dietary Fiber. Approved Methods of Analysis, 11th ed.; Method 32–05.01.; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- AACC International. Insoluble and Soluble Dietary Fiber in Oat Products—Enzymatic-Gravimetric Method. Approved Methods of Analysis, 11th ed.; Method 32–21.01.; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- Giannoccaro, E.; Wang, Y.J.; Chen, P. Effects of solvent, temperature, time, solvent-to-sample ratio, sample size, and defatting on the extraction of soluble sugars in soybean. J. Food Sci. 2006, 71, C59–C64. [Google Scholar] [CrossRef]
- Giannoccaro, E.; Wang, Y.J.; Chen, P. Comparison of two HPLC systems and an enzymatic method for quantification of soybean sugars. Food Chem. 2008, 106, 324–330. [Google Scholar] [CrossRef]
- Hou, A.; Chen, P.; Shi, A.; Zhang, B.; Wang, Y.-J. Sugar Variation in Soybean Seed Assessed with a Rapid Extraction and Quantification Method. Int. J. Agron. 2009, 1, 1–8. [Google Scholar] [CrossRef]
- Pareyt, B.; Wilderjans, E.; Goesaert, H.; Brijs, K.; Delcour, J. The role of gluten in a sugar-snap cookie system: A model approach based on gluten-starch blends. J. Cereal Sci. 2008, 48, 863–869. [Google Scholar] [CrossRef]
- AACC International. Measurement of Trypsin Inhibitor Activity of Soy Products-Spectrophotometric Method. Approved Methods of Analysis, 11th ed.; Method 22–40; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- Hammerstrand, G.E.; Black, L.T.; Glover, J.D. Trypsin inhibitors in soy products: Modification of the standard analytical procedure. Cereal Chem. 1981, 58, 42–45. [Google Scholar]
- Boniglia, C.; Fedele, E.; Sanzini, E. Measurement by ELISA of active lectin in dietary supplements containing kidney bean protein. J. Food Sci. 2003, 68, 1283–1286. [Google Scholar] [CrossRef]
- Johnson, L.A.; Deyoe, C.W.; Hoover, W.J. Yield and quantity of soymilk processed by steam-infusion cooking. J. Food Sci. 1981, 46, 239–248. [Google Scholar] [CrossRef]
- Fanta, G.F.; Christianson, D.D. Starch-hydrocolloid composites prepared by steam jet cooking. Food Hydrocoll. 1996, 10, 173–178. [Google Scholar] [CrossRef]
- Fanta, G.F.; Felker, F.C.; Eskins, K.; Baker, F.L. Aqueous starch-oil dispersions prepared by steam jet cooking. Starch films at the oil-water interface. Carbohydr. Polym. 1999, 39, 25–35. [Google Scholar] [CrossRef]
- Anderson, R.L.; Wolf, J.W. Compositional changes in trypsin inhibitors, phytic acid, saponins, and isoflavones related to soybean processing. J. Nutr. 1995, 125, 581S–587S. [Google Scholar] [PubMed]
- Coffey, D.G.; Uebersax, M.A.; Hosfield, G.L.; Brunner, J.R. Evaluation of the hemaggultinating activity of low-temperature cooked kidney beans. J. Food Sci. 1985, 50, 78–81 and 87. [Google Scholar] [CrossRef]
- Noah, N.D.; Bender, A.E.; Reaidi, G.B.; Gilbert, R.J. Food poisoning from raw red kidney beans. Bri. Med. J. 1980, 6234, 236–237. [Google Scholar]
- Qiu, S.; Yadav, M.P.; Yin, L. Characterization and functionalities study of hemicellulose and cellulose components isolated from sorghum bran, bagasse and biomass. Food Chem. 2017, 230, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Byars, J.A.; Singh, M. Rheological and textural properties of pulse starch gels. Starch-Starke 2016, 68, 778–784. [Google Scholar] [CrossRef]
- Nakamura, A.; Takahashi, T.; Yoshida, R.; Maeda, H.; Corredig, M. Emulsifying properties of soybean soluble polysaccharide. Food Hydrocoll. 2004, 18, 795–803. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Rodríguez-García, J.; Chiralt, A.; González-Martínez, C. Effect of high pressure homogenisation and heat treatment on physical properties and stability of almond and hazelnut milks. LWT Food Sci. Technol. 2015, 62, 488–496. [Google Scholar] [CrossRef]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum Arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar] [CrossRef]


| Percentage (by Weight) of Soybean in Emulsion | Percentage (by Weight) of Navy Bean in Emulsion | Protein | Total Starch | Crude Fat | Soluble Fiber | Insoluble Fiber |
|---|---|---|---|---|---|---|
| Traditionally Cooked | ||||||
| 100 | 0 | 52.34 a | 0.22 g | 17.24 a | 4.71 a | 10.94 d,e |
| 80 | 20 | 47.72 c | 4.92 f | 14.48 b | 4.96 a | 10.85 d,e |
| 60 | 40 | 39.59 f | 11.18 e | 11.51 d | 2.97 a | 12.23 c,d |
| 40 | 60 | 32.20 h | 17.13 d | 7.52 g | 4.00 a | 19.10 a |
| 20 | 80 | 27.44 i | 29.15 b | 4.68 h | 4.94 a | 16.11 b |
| 0 | 100 | 22.84 j | 36.11 a | 1.89 j | 3.74 a | 16.75 b |
| Jet-Cooked | ||||||
| 100 | 0 | 50.37 b | 0.20 g | 13.81 c | 3.19 a | 11.78 c,d |
| 80 | 20 | 43.93 d | 10.10 e | 8.81 e | 2.29 a | 9.97 e,f |
| 60 | 40 | 40.50 e | 14.45 d | 7.63 f | 2.63 a | 9.09 f |
| 40 | 60 | 34.35 g | 17.09 d | 3.43 i | 4.48 a | 13.18 c |
| 20 | 80 | 27.85 i | 22.25 c | 1.75 k | 4.23 a | 16.58 b |
| 0 | 100 | 23.14 j | 21.52 c | 0.20 l | 5.23 a | 18.53 a |
| Percentage (by Weight) of Soybean in Emulsion | Percentage (by Weight) of Navy Bean in Emulsion | Glucose | Fructose | Sucrose | Raffinose | Stachyose | Verbascose |
|---|---|---|---|---|---|---|---|
| Raw Flour | |||||||
| 100 | 0 | 0.27 e,f,g | 0.12 a | 103.00 c,d | 21.13 c,d,e | 41.62 c,d | 3.12 g |
| 80 | 20 | 0.28 e,f,g | 0.08 c | 88.21 e,f | 17.45 e,f,g | 35.03 e,f,g | 10.95 f,g |
| 60 | 40 | 0.31 d,e,f | 0.04 e,f,g | 78.00 f,g | 14.81 g,h,i | 30.47 f,g,h,i | 24.04 e,f |
| 40 | 60 | 0.35 c,d | 0.03 f,g,h | 73.07 g | 14.16 g,h,i,j | 29.44 g,h,i,j | 37.92 d |
| 20 | 80 | 0.32 c,d,e | 0.02 g,h | 58.17 h | 11.37 i,j,k | 24.25 i,j,k,l | 41.37 d |
| 0 | 100 | 0.17 h,i | 0.02 g,h | 41.14 i | 8.83 k | 18.75 l | 4.18 g |
| Traditionally Cooked | |||||||
| 100 | 0 | 0.22 g,h | 0.10 b | 120.83 a,b | 26.62 a,b | 48.81 a,b | 5.56 g |
| 80 | 20 | 0.38 b,c | 0.07 c,d | 127.61 a | 30.21 a | 53.05 a | 67.90 c |
| 60 | 40 | 0.50 a | 0.04 e,f | 107.46 c,d | 24.62 b,c | 44.41 b,c | 113.78 a |
| 40 | 60 | 0.42 b | 0.05 d,e | 98.15 d,e | 24.73 b,c | 42.68 b,c,d | 125.24 a |
| 20 | 80 | 0.49 a | 0.05 e,f | 76.00 f,g | 17.91 e,f,g | 33.23 f,g | 99.31 b |
| 0 | 100 | 0.24 f,g | 0.03 f,g,h | 51.73 h,i | 11.07 i,j,k | 23.48 j,k,l | 4.66 g |
| Jet-Cooked | |||||||
| 100 | 0 | 0.03 k | 0.01 h | 111.51 b,c | 26.05 b | 46.15 b,c | 3.64 g |
| 80 | 20 | 0.07 j,k | 0.02 h | 98.99 d,e | 22.54 b,c,d | 40.64 c,d,e | 16.40 e,f,g |
| 60 | 40 | 0.08 j,k | 0.01 h | 87.78 e,f | 19.78 d,e,f | 36.71 d,e,f | 16.13 e,f,g |
| 40 | 60 | 0.11 i,j | 0.02 g,h | 74.10 g | 16.56 f,g,h | 32.32 f,g,h | 27.69 d,e |
| 20 | 80 | 0.14 i,j | 0.02 g,h | 58.37 h | 12.82 h,i,j,k | 26.15 h,i,j,k | 28.73 d,e |
| 0 | 100 | 0.14 i,j | 0.02 g,h | 45.49 i | 10.35 j,k | 21.62 k,l | 1.95 g |
| Percentage (by Weight) of Soybean in Emulsion | Percentage (by Weight) of Navy Bean in Emulsion | Trypsin Inhibitor (mg TI/g Sample) | Phytohemagglutinin (μg PHA/g Sample) |
|---|---|---|---|
| Raw Flour | |||
| 100 | 0 | 19.23 a | 87.73 g,h |
| 80 | 20 | 19.00 a | 281.72 b,c,d |
| 60 | 40 | 17.08 b | 226.75 d,e |
| 40 | 60 | 12.89 c,d,e | 346.31 a,b |
| 20 | 80 | 14.44 c | 289.07 b,c,d |
| 0 | 100 | 12.03 d,e,f | 254.74 c,d,e |
| Traditionally Cooked | |||
| 100 | 0 | 8.23 i | 0.00 i |
| 80 | 20 | 9.69 g,h,i | 36.72 h,i |
| 60 | 40 | 9.28 h,i | 37.96 h,i |
| 40 | 60 | 10.03 f,g,h,i | 138.43 f,g |
| 20 | 80 | 10.46 f,g,h | 349.22 a,b |
| 0 | 100 | 13.28 c,d | 382.83 a |
| Jet-Cooked | |||
| 100 | 0 | 10.68 f,g,h | 29.18 h,i |
| 80 | 20 | 11.43 d,e,f,g | 182.18 e,f |
| 60 | 40 | 11.63 d,e,f,g | 326.69 a,b,c |
| 40 | 60 | 11.14 e,f,g,h | 144.26 f,g |
| 20 | 80 | 11.48 d,e,f,g | 250.71 c,d,e |
| 0 | 100 | 9.62 g,h,i | 389.13 a |
| Percentage (by Weight) of Soybean in Emulsion | Percentage (by Weight) of Navy Bean in Emulsion | Percentage Solids |
|---|---|---|
| Traditionally Cooked | ||
| 100 | 0 | 3.6 ± 0.24 e,f |
| 80 | 20 | 3.5 ± 0.18 e,f,g |
| 60 | 40 | 3.8 ± 0.15 c,d,e |
| 40 | 60 | 3.6 ± 0.05 e,f |
| 20 | 80 | 4.0 ± 0.14 a,b,c |
| 0 | 100 | 4.1 ± 0.04 a,b |
| Jet-Cooked | ||
| 100 | 0 | 3.2 ± 0.08 g,h |
| 80 | 20 | 3.2 ± 0.20 h |
| 60 | 40 | 3.3 ± 0.02 f,g,h |
| 40 | 60 | 3.7 ± 0.01 d,e |
| 20 | 80 | 4.0 ± 0.10 b,c,d |
| 0 | 100 | 4.3 ± 0.01 a |
| Percentage (by Weight) of Soybean in Emulsion | Percentage (by Weight) of Navy Bean in Emulsion | Viscosity (mPa s) at 25 °C | Viscosity (mPa s) at 4 °C |
|---|---|---|---|
| Traditionally Cooked | |||
| 100 | 0 | 3.9 f | 5.7 g |
| 80 | 20 | 4.8 e,f | 6.2 g |
| 60 | 40 | 15.0 c | 15.2 e,d |
| 40 | 60 | 15.6 c | 16.9 c,d |
| 20 | 80 | 19.4 b | 17.8 c,d |
| 0 | 100 | 35.0 a | 48.3 a |
| Jet-Cooked | |||
| 100 | 0 | 4.5 f | 7.1 f,g |
| 80 | 20 | 6.1 e,f | 7.4 f,g |
| 60 | 40 | 7.7 e | 9.9 f |
| 40 | 60 | 10.6 d | 13.4 e |
| 20 | 80 | 15.8 c | 20.1 c |
| 0 | 100 | 34.8 a | 44.2 b |
| Soybean:Navy Bean | Day 1 | Day 7 | Day 10 | Day 14 | Day 21 |
|---|---|---|---|---|---|
| Traditionally Cooked | |||||
| 100:0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 4.8 ± 0.0 | 4.8 ± 0.0 | 4.8 ± 0.0 |
| 80:20 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 60:40 | 10.0 ± 0.0 | 16.3 ± 1.7 | 15.5 ± 1.7 | 16.7 ±.0.0 | 19.0 ± 0.0 |
| 40:60 | 21.4 ± 3.4 | 23.8 ± 0.0 | 23.8 ± 0.0 | 23.8 ± 0.0 | 28.6 ± 0.0 |
| 20:80 | 28.6 ± 0.0 | 33.3 ± 0.0 | 35.0 ± 0.0 | 35.0 ± 0.0 | 35.0 ± 0.0 |
| 0:100 | 52.4 ± 0.0 | 61.9 ± 0.0 | 59.5 ± 3.7 | 61.9 ± 0.0 | 60.0 ± 0.0 |
| Jet-Cooked | |||||
| 100:0 | 0 | 2.5 ± 0.0 | 5.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 |
| 80:20 | 0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 3.8 ± 1.8 | 5.0 ± 0.0 |
| 60:40 | 0 | 5.3 ± 0.0 | 5.0 ± 0.0 | 0 | 5.0 ± 0.0 |
| 40:60 | 0 | 0 | 0 | 0 | 5.0 ± 0.0 |
| 20:80 | 0 | 0 | 0 | 2.5 ± 3.5 | 0 |
| 0:100 | 0 | 0 | 1.3 ± 1.8 | 0 | 0 |
| Soybean:Navy Bean | Day 1 | Day 3 | Day 7 | Day 10 |
|---|---|---|---|---|
| Traditionally Cooked | ||||
| 100:0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ±0.0 | 5.0 ± 0.0 |
| 80:20 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 60:40 | 20.0 ± 0.0 | 14.3 ± 0.0 | 16.7 ± 0.0 | 15.5 ± 1.7 |
| 40:60 | 26.2 ± 3.4 | 25.0 ± 1.7 | 26.2 ± 3.4 | 26.2 ± 3.4 |
| 20:80 | 30.0 ± 0.0 | 30.0 ± 0.0 | 30.0 ± 0.0 | 30.0 ± 0.0 |
| 0:100 | 60.0 ± 0.0 | 57.1 ± 0.0 | 57.5 ± 3.5 | 65.0 ± 7.1 |
| Jet-Cooked | ||||
| 100:0 | 0 | 0 | 3.8 ± 1.8 | 2.5 ± 0.0 |
| 80:20 | 0 | 0 | 5.0 ± 0.0 | 5.0 ±0.0 |
| 60:40 | 0 | 0 | 6.3 ± 1.8 | 5.0 ± 0.0 |
| 40:60 | 0 | 0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 20:80 | 0 | 0 | 0 | 0 |
| 0:100 | 0 | 0 | 0 | 0 |
| Soybean:Navy Bean | Day 1 | Day 7 | Day 10 | Day 14 | Day 21 |
|---|---|---|---|---|---|
| Traditionally Cooked | |||||
| 100:0 | 0 | 0 | 0 | 0 | 0 |
| 80:20 | 0 | 0 | 0 | 0 | 0 |
| 60:40 | 0 | 0 | 0 | 0 | 0 |
| 40:60 | 0 | 0 | 0 | 0 | 0 |
| 20:80 | 0 | 0 | 5.0 ± 0.0 | 10.0 ± 0.0 | 17.5 ± 3.5 |
| 0:100 | 0 | 0 | 9.5 ± 0.0 | 9.5 ± 0.0 | 40.0 ± 0.0 |
| Jet-Cooked | |||||
| 100:0 | 0 | 0 | 0 | 0 | 0 |
| 80:20 | 0 | 0 | 0 | 0 | 0 |
| 60:40 | 0 | 0 | 0 | 0 | 0 |
| 40:60 | 0 | 0 | 0 | 0 | 0 |
| 20:80 | 0 | 5.0 ± 0.0 | 7.3 ± 0.0 | 7.5 ± 3.5 | 10.0 ± 0.0 |
| 0:100 | 0 | 2.5 ± 0.0 | 5.0 ± 0.0 | 7.7 ± 3.6 | 7.9 ± 0.0 |
| Soybean:Navy Bean | Day 1 | Day 3 | Day 7 | Day 10 |
|---|---|---|---|---|
| Traditionally Cooked | ||||
| 100:0 | 0 | 0 | 0 | 0 |
| 80:20 | 0 | 0 | 0 | 0 |
| 60:40 | 0 | 0 | 0 | 1.25 ± 1.7 |
| 40:60 | 0 | 0 | 0 | 0 |
| 20:80 | 0 | 0 | 5.0 ± 0.0 | 7.5 ± 3.5 |
| 0:100 | 0 | 0 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| Jet-Cooked | ||||
| 100:0 | 0 | 0 | 0 | 0 |
| 80:20 | 0 | 0 | 0 | 0 |
| 60:40 | 0 | 0 | 0 | 0 |
| 40:60 | 0 | 0 | 0 | 0 |
| 20:80 | 0 | 0 | 5.0 ± 0.0 | 5.0 ± 0.0 |
| 0:100 | 0 | 0 | 0 | 5.0 ± 0.0 |
| Soybean:Navy Bean | Traditional Cooked | Jet-Cooked |
|---|---|---|
| Particle Size (μm) | Particle Size (μm) | |
| by volume | ||
| 100:0 | 0.229 ± 0.14 c | 0.180 ± 0.10 b |
| 80:20 | 0.269 ± 0.18 c | 0.196 ± 0.08 b |
| 60:40 | 0.366 ± 0.33 c | 0.197 ± 0.09 b |
| 40:60 | 0.395 ± 0.37 c | 0.218 ± 0.07 b |
| 20:80 | 0.638 ± 0.62 b,c | 0.271 ± 0.14 b |
| 0:100 | 2.403 ± 1.02 a | 2.281 ± 1.21 a |
| by number | ||
| 100:0 | 0.118 ± 0.05 c | 0.094 ± 0.04 b |
| 80:20 | 0.133 ± 0.06 c | 0.118 ± 0.05 b |
| 60:40 | 0.148 ± 0.06 c | 0.112 ± 0.05 b |
| 40:60 | 0.136 ± 0.06 c | 0.145 ± 0.06 b |
| 20:80 | 0.183 ± 0.08 c | 0.145 ± 0.06 b |
| 0:100 | 1.277 ± 0.61 c | 0.304 ± 0.17 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.X.; Singh, M.; Wayman, A.E.; Chen, D.; Kenar, J.A. Evaluation of Soybean–Navy Bean Emulsions Using Different Processing Technologies. Beverages 2017, 3, 23. https://doi.org/10.3390/beverages3020023
Liu SX, Singh M, Wayman AE, Chen D, Kenar JA. Evaluation of Soybean–Navy Bean Emulsions Using Different Processing Technologies. Beverages. 2017; 3(2):23. https://doi.org/10.3390/beverages3020023
Chicago/Turabian StyleLiu, Sean X., Mukti Singh, Ashley E. Wayman, Diejun Chen, and James A. Kenar. 2017. "Evaluation of Soybean–Navy Bean Emulsions Using Different Processing Technologies" Beverages 3, no. 2: 23. https://doi.org/10.3390/beverages3020023
APA StyleLiu, S. X., Singh, M., Wayman, A. E., Chen, D., & Kenar, J. A. (2017). Evaluation of Soybean–Navy Bean Emulsions Using Different Processing Technologies. Beverages, 3(2), 23. https://doi.org/10.3390/beverages3020023





