Genistein Alleviates Intestinal Oxidative Stress by Activating the Nrf2 Signaling Pathway in IPEC-J2 Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Selection of Genistein Concentration
2.4. Determination of Intracellular ROS
2.5. Determination of Antioxidant Indices
2.6. RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR (qPCR)
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Protective Effect of Genistein on Cell Viability
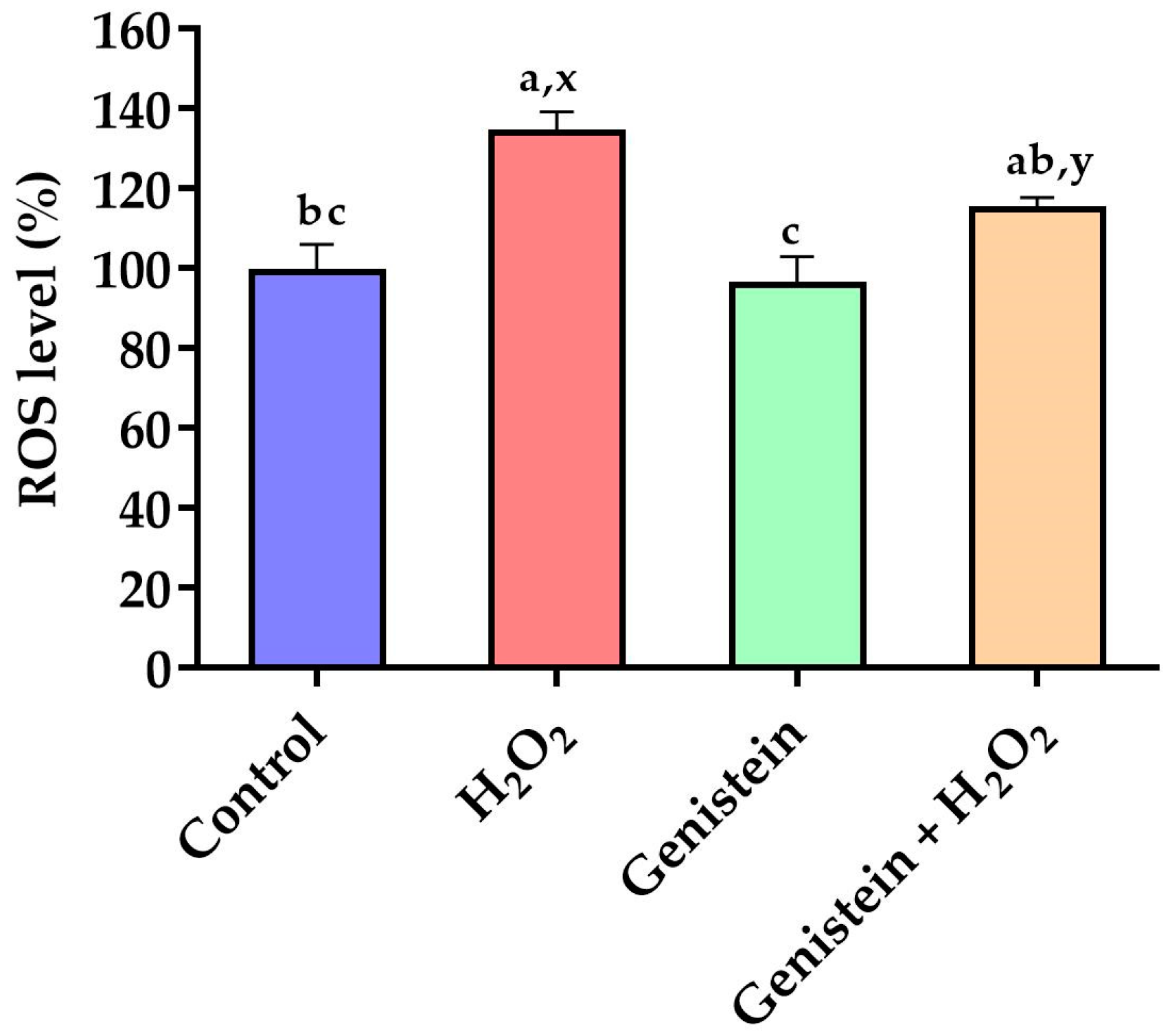
3.2. Intracellular ROS Levels
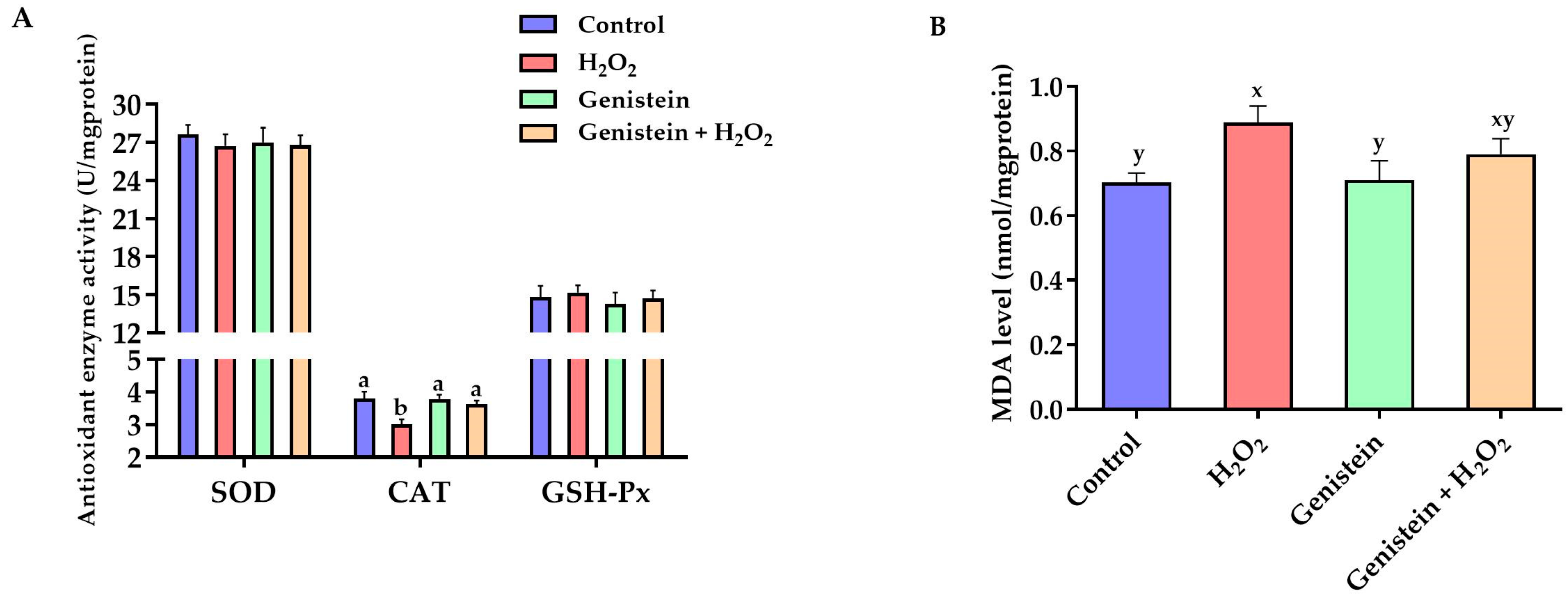
3.3. Antioxidant Enzyme Activities and MDA Level
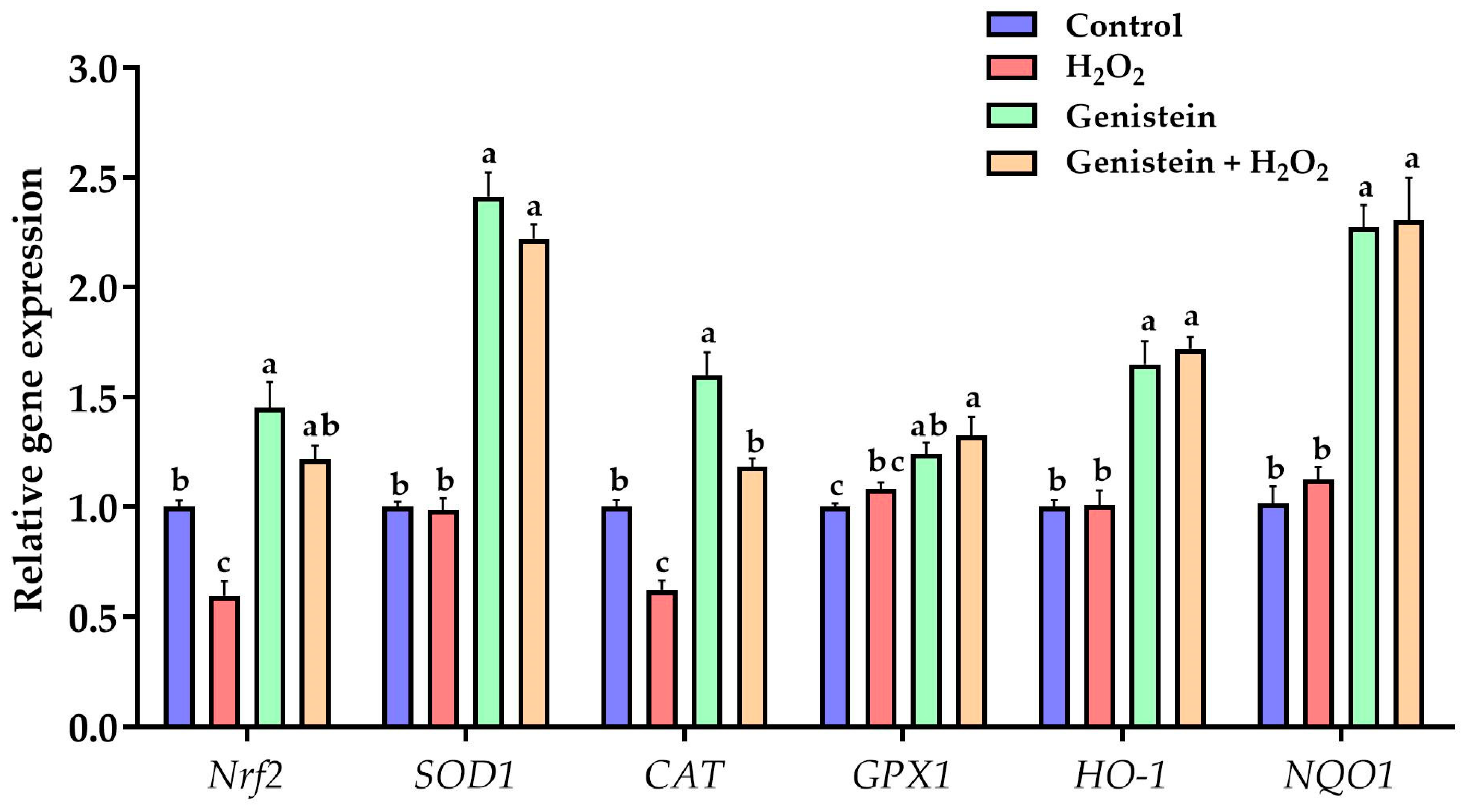
3.4. Expression of Key Genes in Nrf2 Signaling Pathway
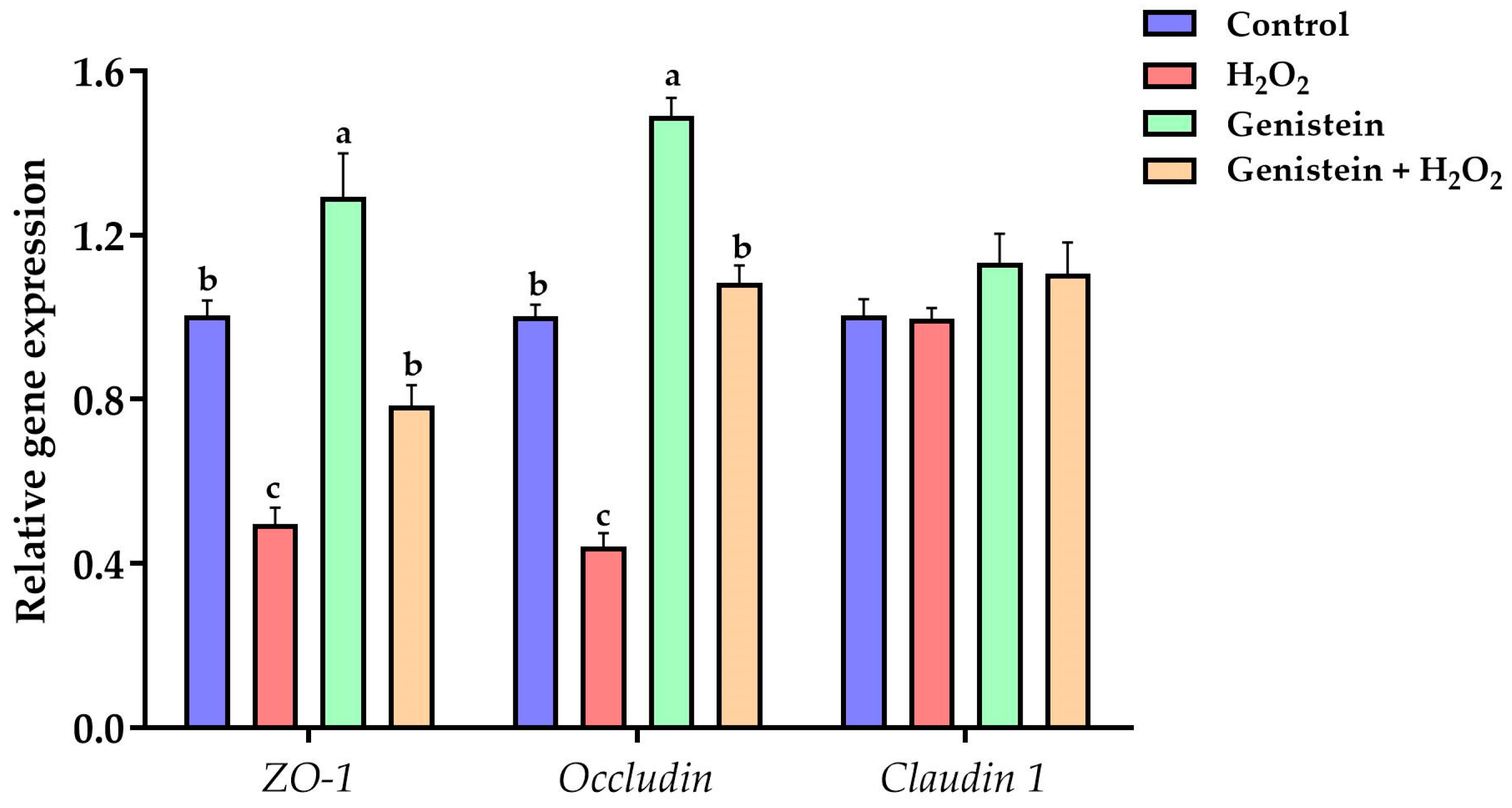
3.5. Tight Junction Gene Expression
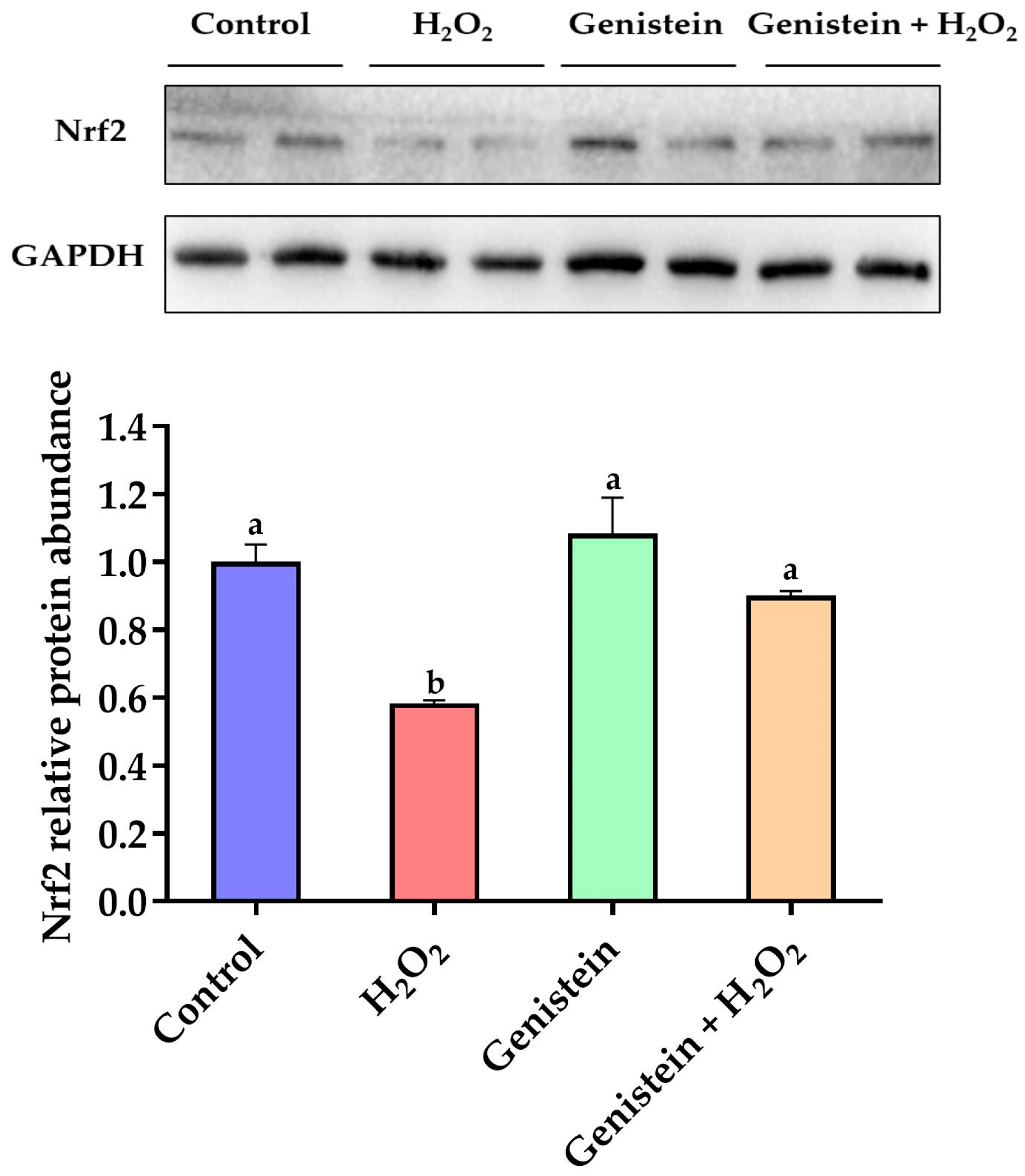
3.6. Nrf2 Protein Expression
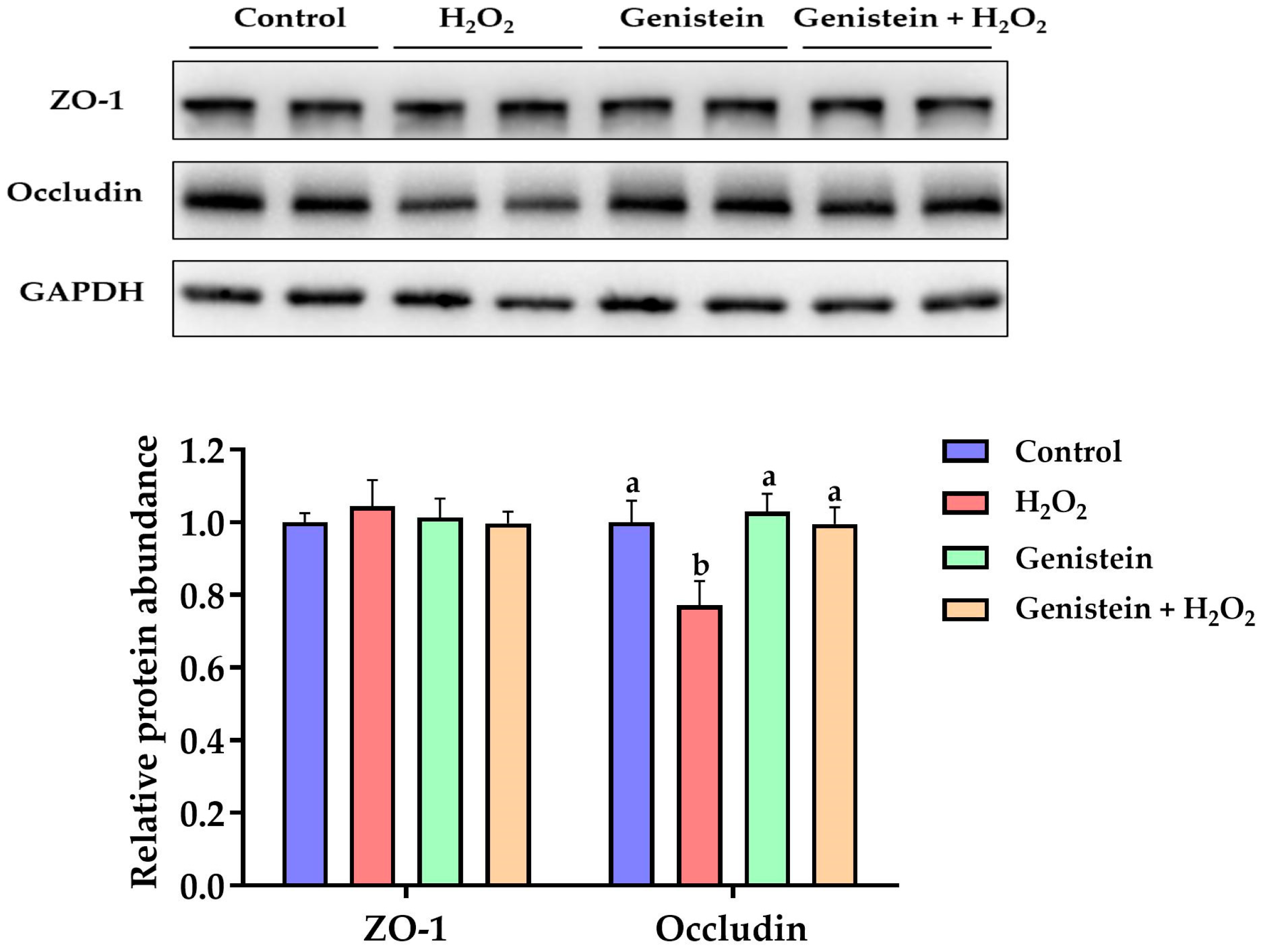
3.7. Tight Junction Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Catalioto, R.M.; Maggi, C.A.; Giuliani, S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr. Med. Chem. 2011, 18, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Wang, S.; Tu, Z.; Zhang, L.; Wang, X.; Hou, Y.; Wang, C.; Chen, J.; Liu, Y. Flaxseed oil attenuates intestinal damage and inflammation by regulating necroptosis and TLR4/NOD signaling pathways following lipopolysaccharide challenge in a piglet model. Mol. Nutr. Food Res. 2018, 62, e1700814. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, S.; Wang, Y.; Chu, X.; Ji, H. Lactobacillus plantarum exhibits antioxidant and cytoprotective activities in porcine intestinal epithelial cells exposed to hydrogen peroxide. Oxid. Med. Cell. Longev. 2021, 2021, 8936907. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Jiang, X.R.; Wei, Z.X.; Cai, L.; Yin, J.D.; Li, X.L. Effects of soybean isoflavones on the growth performance, intestinal morphology and antioxidative properties in pigs. Animal 2020, 14, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.R.; Haak, S.J.; Fastinger, N.D.; Bohn, T.; Tian, Q.; Mahan, D.C.; Schwartz, S.J.; Failla, M.L. Gastrointestinal absorption and metabolism of soy isoflavonoids in ileal-canulated swine. Mol. Nutr. Food Res. 2009, 53, 277–286. [Google Scholar] [CrossRef]
- Cools, S.; Van den Broeck, W.; Vanhaecke, L.; Heyerick, A.; Bossaert, P.; Hostens, M.; Opsomer, G. Feeding soybean meal increases the blood level of isoflavones and reduces the steroidogenic capacity in bovine corpora lutea, without affecting peripheral progesterone concentrations. Anim. Reprod. Sci. 2014, 144, 79–89. [Google Scholar] [CrossRef]
- Li, Y.P.; Jiang, X.R.; Cai, L.; Zhang, Y.L.; Ding, H.B.; Yin, J.D.; Li, X.L. Effects of daidzein on antioxidant capacity in weaned pigs and IPEC-J2 cells. Anim. Nutr. 2022, 11, 48–59. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, Z. Genistein protects against doxorubicin-induced cardiotoxicity through Nrf-2/HO-1 signaling in mice model. Environ. Toxicol. 2019, 34, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Aresta, A.; Trapani, A.; Zambonin, C.; Cianciulli, A.; Salvatore, R.; Clodoveo, M.L.; Corbo, F.; Franchini, C.; Panaro, M.A. Bovine and soybean milk bioactive compounds: Effects on inflammatory response of human intestinal Caco-2 cells. Food Chem. 2016, 210, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yang, Z.Q.; Huang, J.C.; Wang, Y.S.; Guo, B.; Yue, Z.P. Genistein protects ovarian granulosa cells from oxidative stress via cAMP-PKA signaling. Cell Biol. Int. 2020, 44, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pang, X.; Yu, H.; Zhou, H. Genistein suppresses ox-LDL-elicited oxidative stress and senescence in HUVECs through the SIRT1-p66shc-Foxo3a pathways. J. Biochem. Mol. Toxicol. 2022, 36, e22939. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet. Microbiol. 2012, 156, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Yan, S.; Xiao, H.; Yi, J.; Li, R.; Wu, J.; Wen, L. Phenethyl isothiocyanate induces IPEC-J2 cells cytotoxicity and apoptosis via S-G2/M phase arrest and mitochondria-mediated Bax/Bcl-2 pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 226, 108574. [Google Scholar] [CrossRef] [PubMed]
- Kaschubek, T.; Mayer, E.; Rzesnik, S.; Grenier, B.; Bachinger, D.; Schieder, C.; König, J.; Teichmann, K. Effects of phytogenic feed additives on cellular oxidative stress and inflammatory reactions in intestinal porcine epithelial cells. J. Anim. Sci. 2018, 96, 3657–3669. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wei, H.; Yu, H.; Xing, Q.; Zou, Y.; Zhou, Y.; Peng, J. Fish skin gelatin hydrolysate production by ginger powder induces glutathione synthesis to prevent hydrogen peroxide induced intestinal oxidative stress via the Pept1-p62-Nrf2 Cascade. J. Agric. Food Chem. 2018, 66, 11601–11611. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.; He, C. An isoflavonoid-enriched extract from Pueraria lobata (kudzu) root protects human umbilical vein endothelial cells against oxidative stress induced apoptosis. J. Ethnopharmacol. 2016, 193, 524–530. [Google Scholar] [CrossRef]
- Wijeratne, S.S.; Cuppett, S.L. Soy isoflavones protect the intestine from lipid hydroperoxide mediated oxidative damage. J. Agric. Food Chem. 2007, 55, 9811–9816. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Zhou, L.L.; Weng, Q.; Xiao, L.X.; Li, Q.Y. Curcumin analogues attenuate Aβ25-35-induced oxidative stress in PC12 cells via Keap1/Nrf2/HO-1 signaling pathways. Chem. Biol. Interact. 2019, 305, 171–179. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Li, J.; Shan, A. DL-Selenomethionine alleviates oxidative stress induced by zearalenone via Nrf2/Keap1 signaling pathway in IPEC-J2 cells. Toxins 2021, 13, 557. [Google Scholar] [CrossRef]
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: An update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.Y.; Xia, X.; Che, L.; Song, Y.T. Genistein attenuates brain damage induced by transient cerebral ischemia through up-regulation of Nrf2 expression in ovariectomized rats. Neurol. Res. 2018, 40, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- He, C.M.; Deng, J.; Hu, X.; Zhou, S.C.; Wu, J.T.; Xiao, D.; Darko, K.O.; Huang, Y.J.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef]

| Gene | Forward (5′-3′) | Reverse (5′-3′) | Product Length, bp | Accession No. |
|---|---|---|---|---|
| GAPDH | GCTTGTCATCAATGGAAAGG | CATACGTAGCACCAGCATCA | 86 | NM_001206359.1 |
| SOD1 | GAAGACAGTGTTAGTAACGG | CAGCCTTGTGTATTATCTCC | 93 | NM_001190422.1 |
| CAT | CCTGCAACGTTCTGTAAGGC | GCTTCATCTGGTCACTGGCT | 72 | NM_214301.2 |
| GPX1 | TCTCCAGTGTGTCGCAATGA | TCGATGGTCAGAAAGCGACG | 104 | NM_214201.1 |
| Nrf2 | GACCTTGGAGTAAGTCGAGA | GGAGTTGTTCTTGTCTTTCC | 103 | XM_005671981.3 |
| HO-1 | GAGAAGGCTTTAAGCTGGTG | GTTGTGCTCAATCTCCTCCT | 74 | NM_001004027.1 |
| NQO1 | GGACATCACAGGTAAACTGA | TATAAGCCAGAGCAGTCTCG | 68 | NM_001159613.1 |
| Occludin | TCAGGTGCACCCTCCAGATT | TGGACTTTCAAGAGGCCTGG | 112 | NM_001163647.2 |
| ZO-1 | CGATCACTCCAGCATACAAT | CACTTGGCAGAAGATTGTGA | 111 | CV870309 |
| Claudin 1 | CCTCAATACAGGAGGGAAGC | CTCTCCCCACATTCGAGATGATT | 76 | NM_001244539.1 |
| Antibody | Source | Dilution | Company | Cat# |
|---|---|---|---|---|
| Nrf2 | Rabbit | 1:1000 | Abcam, Cambridge, UK | ab92946 |
| ZO-1 | Rabbit | 1:1000 | Thermo Fisher Scientific, Waltham, MA, USA | 61-7300 |
| Occludin | Rabbit | 1:1000 | Abcam, Cambridge, UK | ab31721 |
| GAPDH | Rabbit | 1:2000 | Cell Signaling Technology, Danvers, MA, USA | 2118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cai, L.; Bi, Q.; Sun, W.; Pi, Y.; Jiang, X.; Li, X. Genistein Alleviates Intestinal Oxidative Stress by Activating the Nrf2 Signaling Pathway in IPEC-J2 Cells. Vet. Sci. 2024, 11, 154. https://doi.org/10.3390/vetsci11040154
Li Y, Cai L, Bi Q, Sun W, Pi Y, Jiang X, Li X. Genistein Alleviates Intestinal Oxidative Stress by Activating the Nrf2 Signaling Pathway in IPEC-J2 Cells. Veterinary Sciences. 2024; 11(4):154. https://doi.org/10.3390/vetsci11040154
Chicago/Turabian StyleLi, Yanpin, Long Cai, Qingyue Bi, Wenjuan Sun, Yu Pi, Xianren Jiang, and Xilong Li. 2024. "Genistein Alleviates Intestinal Oxidative Stress by Activating the Nrf2 Signaling Pathway in IPEC-J2 Cells" Veterinary Sciences 11, no. 4: 154. https://doi.org/10.3390/vetsci11040154







