Effects of Beraprost with or without NOS Inhibition on Plasma Aldosterone and Hemodynamics in Healthy Cats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Materials
2.4. Treatments
2.5. Methods
2.6. Data Management
3. Results
3.1. Clinical Observations and Body Weight
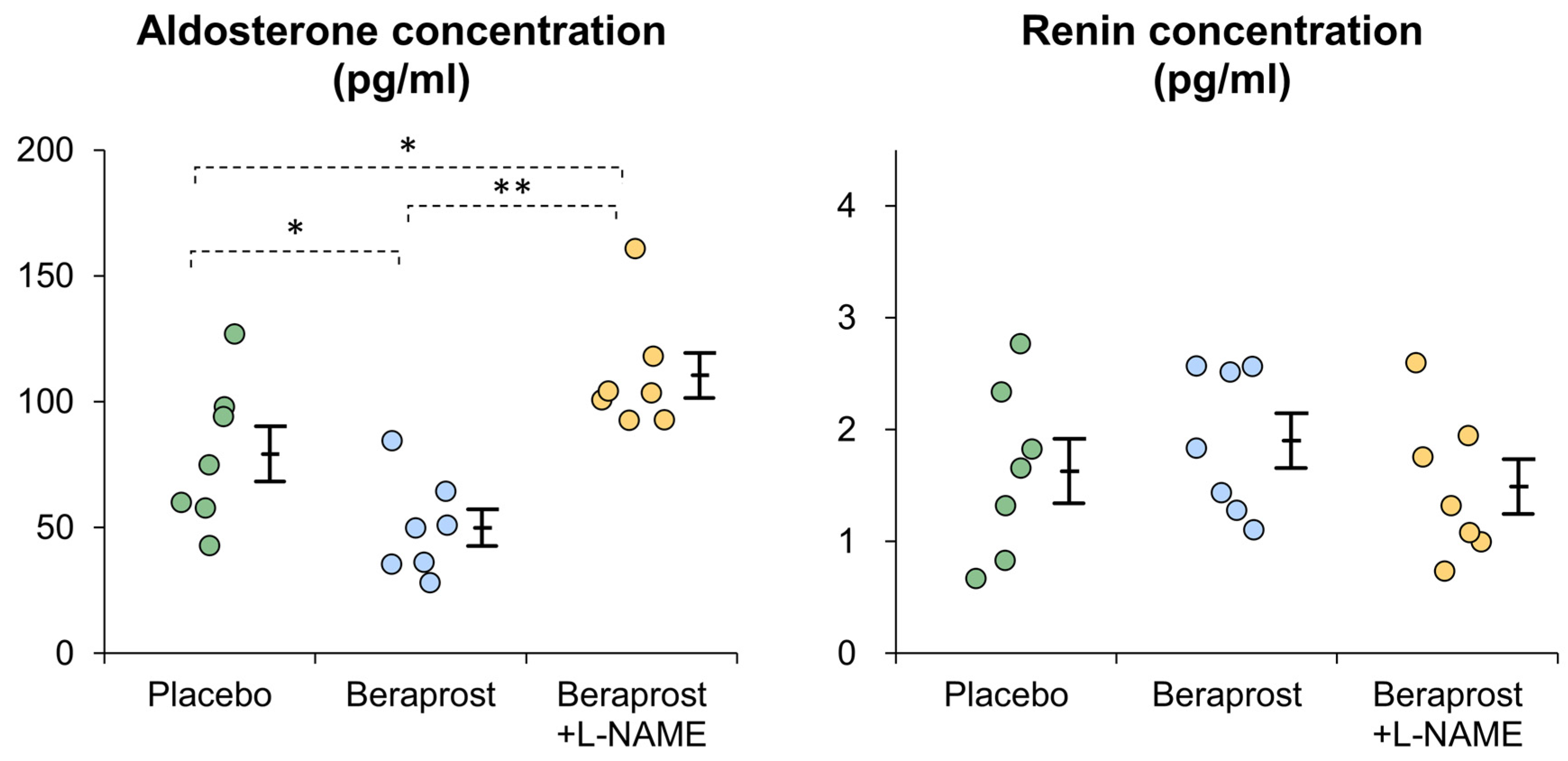
3.2. PAC and PRC
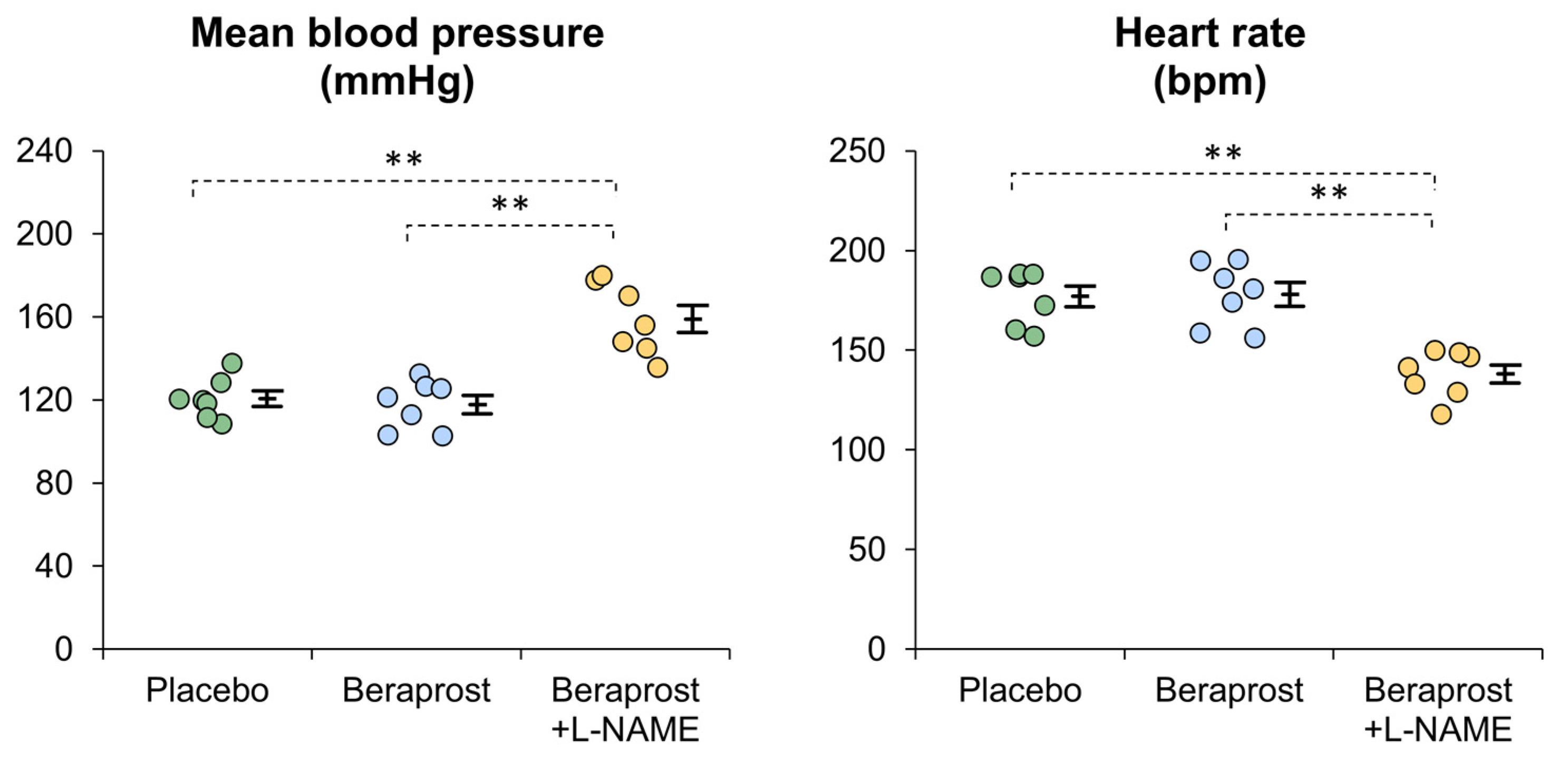
3.3. Measurements of Hemodynamics
3.4. Assessments of the Effects of Beraprost and Added L-NAME (NOS Inhibitor)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duprez, D.A. Aldosterone and the vasculature: Mechanisms mediating resistant hypertension. J. Clin. Hypertens. 2007, 9, 13–18. [Google Scholar] [CrossRef]
- Bertocchio, J.P.; Warnock, D.G.; Jaisser, F. Mineralocorticoid receptor activation and blockade: An emerging paradigm in chronic kidney disease. Kidney Int. 2011, 79, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Shavit, L.; Lifschitz, M.D.; Epstein, M. Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: Current concepts and emerging treatment paradigms. Kidney Int. 2012, 81, 955–968. [Google Scholar] [CrossRef]
- Spencer, S.; Wheeler-Jones, C.; Elliott, J. Aldosterone and the mineralocorticoid receptor in renal injury: A potential therapeutic target in feline chronic kidney disease. J. Vet. Pharmacol. Ther. 2020, 43, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Henik, R.A.; Brownfield, M.; Armstrong, J. Plasma renin activity and angiotensin I and aldosterone concentrations in cats with hypertension associated with chronic renal disease. Am. J. Vet. Res. 1997, 58, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Mishina, M.; Watanabe, T.; Fujii, K.; Maeda, H.; Wakao, Y.; Takahashi, M. Non-invasive blood pressure measurements in cats: Clinical significance of hypertension associated with chronic renal failure. J. Vet. Med. Sci. 1998, 60, 805–808. [Google Scholar] [CrossRef]
- Jepson, R.E.; Syme, H.M.; Elliott, J. Plasma renin activity and aldosterone concentrations in hypertensive cats with and without azotemia and in response to treatment with amlodipine besylate. J. Vet. Intern. Med. 2014, 28, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Arima, S.; Kohagura, K.; Xu, H.L.; Sugawara, A.; Uruno, A.; Satoh, F.; Takeuchi, K.; Ito, S. Endothelium-derived nitric oxide modulates vascular action of aldosterone in renal arteriole. Hypertension 2004, 43, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Nakanishi, S.; Tanabe, S. Aldosterone affects blood flow and vascular tone regulated by endothelium-derived NO: Therapeutic implications. Br. J. Pharmacol. 2013, 168, 519–533. [Google Scholar] [CrossRef]
- Toda, N. Beraprost sodium. Cardiovasc. Drug Rev. 1988, 6, 222–238. [Google Scholar] [CrossRef]
- Niwano, K.; Arai, M.; Tomaru, K.; Uchiyama, T.; Ohyama, Y.; Kurabayashi, M. Transcriptional stimulation of the eNOS gene by the stable prostacyclin analogue beraprost is mediated through cAMP-responsive element in vascular endothelial cells: Close link between PGI2 signal and NO pathways. Circ. Res. 2003, 93, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Morishita, R.; Tomita, N.; Moriguchi, A.; Yamasaki, K.; Aoki, M.; Matsumoto, K.; Nakamura, T.; Higaki, J.; Ogihara, T. Impaired endothelial dysfunction in diabetes mellitus rats was restored by oral administration of prostaglandin I2 analogue. J. Endocrinol. 2002, 175, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fuchikami, C.; Murakami, K.; Tajima, K.; Homan, J.; Kosugi, K.; Kuramoto, K.; Oka, M.; Kuwano, K. A comparison of vasodilation mode among selexipag (NS-304; [2-4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy-N-(methylsulfonyl)acetamide]), its active metabolite MRE-269 and various prostacyclin receptor agonists in rat, porcine and human pulmonary arteries. Eur. J. Pharmacol. 2017, 795, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Yamaguchi, S.; Tamura, M.; Mochizuki, H.; Kurumatani, H.; Okano, K.; Miyamoto, M. A prostacyclin analog prevents the regression of renal microvascular network by inhibiting mitochondria-dependent apoptosis in the kidney of rat progressive glomerulonephritis. Prostaglandins Other Lipid Mediat. 2014, 112, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Inada, C.; Tamura, M.; Sato, N.; Yamada, M.; Itaba, S.; Okazaki, S.; Matsuura, H.; Fujii, S.; Matsuda, F.; et al. Beraprost sodium improves survival rates in anti-glomerular basement membrane glomerulonephritis and 5/6 nephrectomized chronic kidney disease rats. Eur. J. Pharmacol. 2013, 714, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Iio, A.; Sato, R.; Sakamoto, T.; Kurumatani, H.; KT-140 Clinical Study Group. A Double-blind, Placebo-controlled, Multicenter, Prospective, Randomized Study of Beraprost Sodium Treatment for Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2018, 32, 236–248. [Google Scholar] [CrossRef]
- Ito, H.; Matsuura, T.; Sano, T. Beraprost and Overall Survival in Cats with Chronic Kidney Disease. Vet. Sci. 2023, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, A.H.; Caney, S.; Chalhoub, S.; Elliott, J.; Finch, N.; Gajanayake, I.; Langston, C.; Lefebvre, H.P.; White, J.; Quimby, J. ISFM Consensus Guidelines on the Diagnosis and Management of Feline Chronic Kidney Disease. J. Feline Med. Surg. 2016, 18, 219–239. [Google Scholar] [CrossRef]
- Williams, T.L.; Peak, K.J.; Brodbelt, D.; Elliott, J.; Syme, H.M. Survival and the development of azotemia after treatment of hyperthyroid cats. J. Vet. Intern. Med. 2010, 24, 863–869. [Google Scholar] [CrossRef]
- Perez-Lopez, L.; Boronat, M.; Melian, C.; Saavedra, P.; Brito-Casillas, Y.; Wagner, A.M. Assessment of the association between diabetes mellitus and chronic kidney disease in adult cats. J. Vet. Intern. Med. 2019, 33, 1921–1925. [Google Scholar] [CrossRef]
- Fox, P.R.; Keene, B.W.; Lamb, K.; Schober, K.E.; Chetboul, V.; Luis Fuentes, V.; Payne, J.R.; Wess, G.; Hogan, D.F.; Abbott, J.A.; et al. Long-term incidence and risk of noncardiovascular and all-cause mortality in apparently healthy cats and cats with preclinical hypertrophic cardiomyopathy. J. Vet. Intern. Med. 2019, 33, 2572–2586. [Google Scholar] [CrossRef] [PubMed]
- Wakeling, J.; Moore, K.; Elliott, J.; Syme, H. Diagnosis of hyperthyroidism in cats with mild chronic kidney disease. J. Small Anim. Pract. 2008, 49, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Geddes, R.; Aguiar, J. Feline Comorbidities: Balancing hyperthyroidism and concurrent chronic kidney disease. J. Feline Med. Surg. 2022, 24, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Guillot, E.; Domenig, O.; Ware, W.A.; Yuan, L.; Mochel, J.P. Circulating renin-angiotensin-aldosterone system activity in cats with systemic hypertension or cardiomyopathy. J. Vet. Intern. Med. 2022, 36, 897–909. [Google Scholar] [CrossRef]
- Williams, T.L.; Elliott, J.; Syme, H.M. Renin-angiotensin-aldosterone system activity in hyperthyroid cats with and without concurrent hypertension. J. Vet. Intern. Med. 2013, 27, 522–529. [Google Scholar] [CrossRef]
- Atkins, C.; Adin, D.; Glahn, C.; Domenig, O.; DeFrancesco, T.; Meurs, K. Renin-angiotensin-aldosterone system activation in hypertensive cats receiving amlodipine. J. Vet. Intern. Med. 2020, 34, 2846. [Google Scholar]
- National Veterinary Assay Laboratory (NVAL) in Japan. Veterinary Medicinal Products Database: RAPROS. Available online: https://www.vm.nval.go.jp/public/detail/16941 (accessed on the 21 November 2023). (In Japanese).
- Brown, S.A. Effects of inhibition of nitric oxide synthase on systemic arterial pressure and renal vascular resistance in cats. Res. Vet. Sci. 1993, 55, 398–400. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 2018, 32, 1803–1822. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceuticals and Medical Devices Agency (PMDA). Sodium Para-Aminohippurate Injection 10%. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/7225401 (accessed on the 12 February 2024). (In Japanese).
- Eggleton, M.G.; Habib, Y.A. Excretion of para-aminohippurate by the kidney of the cat. J. Physiol. 1949, 110, 458–467. [Google Scholar] [CrossRef]
- Osbaldiston, G.W.; Fuhrman, W. The clearance of creatinine, inulin, para-aminohippurate and phenosulphothalein in the cat. Can. J. Comp. Med. 1970, 34, 138–141. [Google Scholar]
- Blainey, P.; Krzywinski, M.; Altman, N. Points of significance: Replication. Nat. Methods 2014, 11, 879–880. [Google Scholar] [CrossRef]
- Jepson, R.; Syme, H.; Vallance, C.; Elliott, J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J. Vet. Intern. Med. 2008, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Paschall, R.E.; Quimby, J.M.; Cianciolo, R.E.; McLeland, S.M.; Lunn, K.F.; Elliott, J. Assessment of peritubular capillary rarefaction in kidneys of cats with chronic kidney disease. J. Vet. Intern. Med. 2023, 37, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Kainoh, M.N.S.; Nakadate, T. Cytoprotective Action of Beraprost Sodium against Peroxide-Induced Damage in Vascular Endothelial Cells. Pharmacology 1992, 45, 61–70. [Google Scholar] [CrossRef]
- Okamoto, M. Effects of alpha-human atrial natriuretic polypeptide, sodium nitroprusside and dibutyryl cyclic GMP on aldosterone production in bovine zona glomerulosa cells. Acta Endocrinol. 1988, 119, 358–366. [Google Scholar] [CrossRef]
- Natarajan, R.; Lanting, L.; Bai, W.; Bravo, E.L.; Nadler, J. The role of nitric oxide in the regulation of aldosterone synthesis by adrenal glomerulosa cells. J. Steroid Biochem. Mol. Biol. 1997, 61, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hanke, C.J.; Drewett, J.G.; Myers, C.R.; Campbell, W.B. Nitric oxide inhibits aldosterone synthesis by a guanylyl cyclase-independent effect. Endocrinology 1998, 139, 4053–4060. [Google Scholar] [CrossRef]
- Hanke, C.J.; O’Brien, T.; Pritchard, K.A., Jr.; Campbell, W.B. Inhibition of adrenal cell aldosterone synthesis by endogenous nitric oxide release. Hypertension 2000, 35, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Muldowney, J.A., 3rd; Davis, S.N.; Vaughan, D.E.; Brown, N.J. NO synthase inhibition increases aldosterone in humans. Hypertension 2004, 44, 739–745. [Google Scholar] [CrossRef]
- Linas, S.L. Role of prostaglandins in renin secretion in the isolated kidney. Am. J. Physiol. 1984, 246, F811–F818. [Google Scholar] [CrossRef]
- Beierwaltes, W.H.; Schryver, S.; Sanders, E.; Strand, J.; Romero, J.C. Renin release selectively stimulated by prostaglandin I2 in isolated rat glomeruli. Am. J. Physiol. 1982, 243, F276–F283. [Google Scholar] [CrossRef] [PubMed]
- Saji, T.; Ozawa, Y.; Ishikita, T.; Matsuura, H.; Matsuo, N. Short-term hemodynamic effect of a new oral PGI2 analogue, beraprost, in primary and secondary pulmonary hypertension. Am. J. Cardiol. 1996, 78, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Vizza, C.D.; Kneussl, M.; Manes, A.; Sitbon, O.; Torbicki, A.; Delcroix, M.; Naeije, R.; et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 2002, 39, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Takashima, K.; Kurumatani, H.; Ida, N.; Sato, R.; Yamane, Y. Safety assessment of a prostacyclin derivative (beraprost sodium) in healthy cats. J. Anim. Clin. Med. 2011, 20, 131–139. [Google Scholar]
- Basile, D.P.; Donohoe, D.; Roethe, K.; Osborn, J.L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Ren. Physiol. 2001, 281, F887–F899. [Google Scholar] [CrossRef]
- Nangaku, M. Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bieri, P. Laboratory diagnosis of kidney diseases in the cat with special reference to the para-amino-hippuric acid, PAH-clearance. Kleintierpraxis 1976, 21, 189–196. [Google Scholar]
- Fujita, T.; Fuke, Y.; Satomura, A.; Hidaka, M.; Ohsawa, I.; Endo, M.; Komatsu, K.; Ohi, H.J.P. PGl2 analogue mitigates the progression rate of renal dysfunction improving renal blood flow without glomerular hyperfiltration in patients with chronic renal insufficiency. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2001, 65, 223–227. [Google Scholar] [CrossRef]
- Marcel van Gelderen, E.; Heiligers, J.P.; Saxena, P.R. Haemodynamic changes and acetylcholine-induced hypotensive responses after NG-nitro-L-arginine methyl ester in rats and cats. Br. J. Pharmacol. 1991, 103, 1899–1904. [Google Scholar] [CrossRef]
- Hirata-Dulas, C.A.; Awni, W.M.; Matzke, G.R.; Halstenson, C.E.; Guay, D.R. Evaluation of two intravenous single-bolus methods for measuring effective renal plasma flow. Am. J. Kidney Dis. 1994, 23, 374–381. [Google Scholar] [CrossRef]
- Brochner-Mortensen, J. A simple method for the determination of glomerular filtration rate. Scand. J. Clin. Lab. Investig. 1972, 30, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Javadi, S.; Slingerland, L.I.; van de Beek, M.G.; Boer, P.; Boer, W.H.; Mol, J.A.; Rijnberk, A.; Kooistra, H.S. Plasma renin activity and plasma concentrations of aldosterone, cortisol, adrenocorticotropic hormone, and alpha-melanocyte-stimulating hormone in healthy cats. J. Vet. Intern. Med. 2004, 18, 625–631. [Google Scholar] [CrossRef] [PubMed]

| No. | Age (years) | Weight (kg) | Sex | Breeds |
|---|---|---|---|---|
| 1 | 3.4 | 2.7 | Intact Female | Domestic Shorthair |
| 2 | 3.7 | 2.6 | ||
| 3 | 3.4 | 3.1 | ||
| 4 | 3.4 | 3.0 | ||
| 5 | 2.0 | 3.8 | Intact Male | |
| 6 | 3.4 | 2.8 | ||
| 7 | 2.0 | 3.6 |
| Phase 1 | Phase 2 | Phase 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Beraprost | Beraprost + L-NAME | ||||||||||||
| Day 1 | Day 7 | Day 8 | Day 14 | Day 15 | Day 16 | Day 22 | Day 23 | Day 29 | Day 30 | Day 36 | Day 37 | Day 42 | Day 43 | |
| Treatments | ||||||||||||||
| Placebo administration (oral) | Day 1 to 15 inclusive | |||||||||||||
| Beraprost administration (oral) | Day 16 to 43 inclusive | |||||||||||||
| L-NAME administration (intravenous) | X | X | X | X | ||||||||||
| Assessments | ||||||||||||||
| Clinical observations | Day 1 to 43 inclusive | |||||||||||||
| Body weight | X | X | X | X | X | X | ||||||||
| Plasma PAH clearance | X | X | X | X | X | X | ||||||||
| Blood pressure and heart rate | X | X | X | X | X | X | ||||||||
| Plasma aldosterone and renin | X | X | X | X | X | X | ||||||||
| Aldosterone (pg/mL) | Renin (ng/mL) | Mean Blood Pressure (mmHg) | Heart Rate (bpm) | Estimated Renal Plasma Flow (mL/kg/min) | Estimated Renal Vascular Resistance (mmHg-min/mL) | |
|---|---|---|---|---|---|---|
| Beraprost | −31.1 ± 12.0 | +0.3 ± 0.2 | −2.8 ± 3.5 | +0.9 ± 2.1 | +1.2 ± 0.4 | −0.4 ± 0.2 |
| Added L-NAME | +62.3 ± 10.0 | −0.4 ± 0.2 | +41.1 ± 6.3 | −39.9 ± 2.6 | −3.2 ± 0.6 | +2.9 ± 0.8 |
| Correlation ratio (η2) | 0.749 | 0.357 | 0.756 | 0.926 | 0.758 | 0.593 |
| p-value | ** p < 0.001 | 0.0241 * | ** p < 0.001 | ** p < 0.001 | ** p < 0.001 | 0.0013 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, T.; Yoshimura, A.; Fukushima, R. Effects of Beraprost with or without NOS Inhibition on Plasma Aldosterone and Hemodynamics in Healthy Cats. Vet. Sci. 2024, 11, 155. https://doi.org/10.3390/vetsci11040155
Matsuura T, Yoshimura A, Fukushima R. Effects of Beraprost with or without NOS Inhibition on Plasma Aldosterone and Hemodynamics in Healthy Cats. Veterinary Sciences. 2024; 11(4):155. https://doi.org/10.3390/vetsci11040155
Chicago/Turabian StyleMatsuura, Takumi, Aritada Yoshimura, and Ryuji Fukushima. 2024. "Effects of Beraprost with or without NOS Inhibition on Plasma Aldosterone and Hemodynamics in Healthy Cats" Veterinary Sciences 11, no. 4: 155. https://doi.org/10.3390/vetsci11040155






