Biological Age in Congenital Heart Disease—Exploring the Ticking Clock
Abstract
:1. Introduction
2. Lifespan Research in CHD
2.1. Aging Research
2.2. Indications of Accelerated Aging in CHD
2.3. Young Patients with Old Hearts
2.4. CHD beyond Old Hearts
3. Measuring Biological Age in CHD
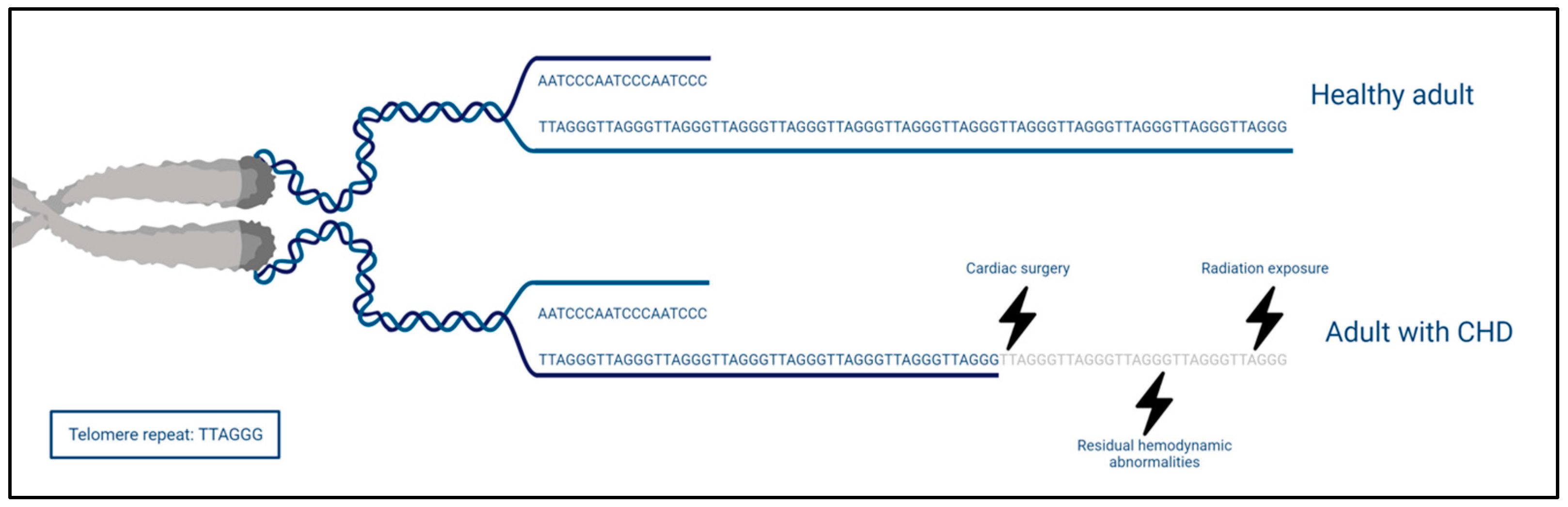
3.1. Telomere Length
3.2. Epigenetic Clock
3.3. Inflammaging
3.4. Frailty & Functional Parameters
4. Avenues for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Bovijn, L.; Budts, W.; Belmans, A.; Gewillig, M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010, 122, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.C.; Korones, S.B.; Berendes, H.W. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation 1971, 43, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Bouma, B.J.; Mulder, B.J. Changing Landscape of Congenital Heart Disease. Circ. Res. 2017, 120, 908–922. [Google Scholar] [CrossRef]
- Khairy, P.; Ionescu-Ittu, R.; Mackie, A.S.; Abrahamowicz, M.; Pilote, L.; Marelli, A.J. Changing mortality in congenital heart disease. J. Am. Coll. Cardiol. 2010, 56, 1149–1157. [Google Scholar] [CrossRef]
- Dellborg, M.; Giang, K.W.; Eriksson, P.; Liden, H.; Fedchenko, M.; Ahnfelt, A.; Rosengren, A.; Mandalenakis, Z. Adults With Congenital Heart Disease: Trends in Event-Free Survival Past Middle Age. Circulation 2023, 147, 930–938. [Google Scholar] [CrossRef]
- Marelli, A. Trajectories of care in congenital heart disease—The long arm of disease in the womb. J. Intern. Med. 2020, 288, 390–399. [Google Scholar] [CrossRef]
- Tutarel, O.; Kempny, A.; Alonso-Gonzalez, R.; Jabbour, R.; Li, W.; Uebing, A.; Dimopoulos, K.; Swan, L.; Gatzoulis, M.A.; Diller, G.P. Congenital heart disease beyond the age of 60: Emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur. Heart J. 2014, 35, 725–732. [Google Scholar] [CrossRef]
- Moons, P.; Marelli, A. Born to Age: When Adult Congenital Heart Disease Converges With Geroscience. JACC Adv. 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Diller, G.P.; Arvanitaki, A.; Opotowsky, A.R.; Jenkins, K.; Moons, P.; Kempny, A.; Tandon, A.; Redington, A.; Khairy, P.; Mital, S.; et al. Lifespan Perspective on Congenital Heart Disease Research: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2219–2235. [Google Scholar] [CrossRef]
- Afilalo, J.; Therrien, J.; Pilote, L.; Ionescu-Ittu, R.; Martucci, G.; Marelli, A.J. Geriatric congenital heart disease: Burden of disease and predictors of mortality. J. Am. Coll. Cardiol. 2011, 58, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Baker, D.W.; Cordina, R.L.; Gatzoulis, M.; Broberg, C.S. Common diagnostic errors in adults with congenital heart disease. Eur. Heart J. 2023, 44, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Walker, T.T.; Bove, K.; Veldtman, G. Fontan-associated liver disease: A review. J. Cardiol. 2019, 74, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Emamaullee, J.; Zaidi, A.N.; Schiano, T.; Kahn, J.; Valentino, P.L.; Hofer, R.E.; Taner, T.; Wald, J.W.; Olthoff, K.M.; Bucuvalas, J.; et al. Fontan-Associated Liver Disease: Screening, Management, and Transplant Considerations. Circulation 2020, 142, 591–604. [Google Scholar] [CrossRef] [PubMed]
- de Lange, C.; Moller, T.; Hebelka, H. Fontan-associated liver disease: Diagnosis, surveillance, and management. Front. Pediatr. 2023, 11, 1100514. [Google Scholar] [CrossRef] [PubMed]
- Vecoli, C.; Pulignani, S.; Foffa, I.; Andreassi, M.G. Congenital heart disease: The crossroads of genetics, epigenetics and environment. Curr. Genom. 2014, 15, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hou, T.; Wang, Q.; He, J.; Wang, L.; Si, J.; Chen, S. An evaluation of aging measures: From biomarkers to clocks. Biogerontology 2023, 24, 303–328. [Google Scholar] [CrossRef]
- Lim, T.B.; Foo, S.Y.R.; Chen, C.K. The Role of Epigenetics in Congenital Heart Disease. Genes 2021, 12, 390. [Google Scholar] [CrossRef]
- Chang, S.; Wang, Y.; Xin, Y.; Wang, S.; Luo, Y.; Wang, L.; Zhang, H.; Li, J. DNA methylation abnormalities of imprinted genes in congenital heart disease: A pilot study. BMC Med. Genom. 2021, 14, 4. [Google Scholar] [CrossRef]
- Pierpont, M.E.; Brueckner, M.; Chung, W.K.; Garg, V.; Lacro, R.V.; McGuire, A.L.; Mital, S.; Priest, J.R.; Pu, W.T.; Roberts, A.; et al. Genetic Basis for Congenital Heart Disease: Revisited: A Scientific Statement From the American Heart Association. Circulation 2018, 138, e653–e711. [Google Scholar] [CrossRef]
- Muntean, I.; Togănel, R.; Benedek, T. Genetics of Congenital Heart Disease: Past and Present. Biochem. Genet. 2017, 55, 105–123. [Google Scholar] [CrossRef]
- Zaidi, S.; Brueckner, M. Genetics and Genomics of Congenital Heart Disease. Circ. Res. 2017, 120, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Gelb, B.D.; Seidman, J.G.; Seidman, C.E. Genetics of congenital heart disease: The glass half empty. Circ. Res. 2013, 112, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, Q.; Huang, Y.; Wang, L.; Su, Z.; Ye, H. The role of DNA methylation in syndromic and non-syndromic congenital heart disease. Clin. Epigenetics 2021, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Linglart, L.; Bonnet, D. Epigenetics and Congenital Heart Diseases. J. Cardiovasc. Dev. Dis. 2022, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, T.B. Understanding the odd science of aging. Cell 2005, 120, 437–447. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Schnabel, F.; Kornak, U.; Wollnik, B. Premature aging disorders: A clinical and genetic compendium. Clin. Genet. 2021, 99, 3–28. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Sinha, J.K.; Ghosh, S.; Raghunath, M. Progeria: A rare genetic premature ageing disorder. Indian. J. Med. Res. 2014, 139, 667–674. [Google Scholar]
- Peng, L.; Baradar, A.A.; Aguado, J.; Wolvetang, E. Cellular senescence and premature aging in Down Syndrome. Mech. Ageing Dev. 2023, 212, 111824. [Google Scholar] [CrossRef]
- Gensous, N.; Bacalini, M.G.; Franceschi, C.; Garagnani, P. Down syndrome, accelerated aging and immunosenescence. Semin. Immunopathol. 2020, 42, 635–645. [Google Scholar] [CrossRef]
- Horvath, S.; Garagnani, P.; Bacalini, M.G.; Pirazzini, C.; Salvioli, S.; Gentilini, D.; Di Blasio, A.M.; Giuliani, C.; Tung, S.; Vinters, H.V.; et al. Accelerated epigenetic aging in Down syndrome. Aging Cell 2015, 14, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Zigman, W.B. Atypical aging in Down syndrome. Dev. Disabil. Res. Rev. 2013, 18, 51–67. [Google Scholar] [CrossRef]
- Wolf, E.J.; Maniates, H.; Nugent, N.; Maihofer, A.X.; Armstrong, D.; Ratanatharathorn, A.; Ashley-Koch, A.E.; Garrett, M.; Kimbrel, N.A.; Lori, A.; et al. Traumatic stress and accelerated DNA methylation age: A meta-analysis. Psychoneuroendocrinology 2018, 92, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Colich, N.L.; Rosen, M.L.; Williams, E.S.; McLaughlin, K.A. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol. Bull. 2020, 146, 721–764. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Cohen, H.J.; Kraus, W.E.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Impact of early personal-history characteristics on the Pace of Aging: Implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 2017, 16, 644–651. [Google Scholar] [CrossRef]
- Kruseova, J.; Zichova, A.; Eckschlager, T. Premature aging in childhood cancer survivors. Oncol. Lett. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, D.; Alvino, V.V.; Caputo, M.; Madeddu, P. Accelerated Cardiac Aging in Patients With Congenital Heart Disease. Front. Cardiovasc. Med. 2022, 9, 892861. [Google Scholar] [CrossRef]
- Verheugt, C.L.; Uiterwaal, C.S.; van der Velde, E.T.; Meijboom, F.J.; Pieper, P.G.; van Dijk, A.P.; Vliegen, H.W.; Grobbee, D.E.; Mulder, B.J. Mortality in adult congenital heart disease. Eur. Heart J. 2010, 31, 1220–1229. [Google Scholar] [CrossRef]
- Goldstein, S.A.; D′Ottavio, A.; Spears, T.; Chiswell, K.; Hartman, R.J.; Krasuski, R.A.; Kemper, A.R.; Meyer, R.E.; Hoffman, T.M.; Walsh, M.J.; et al. Causes of Death and Cardiovascular Comorbidities in Adults With Congenital Heart Disease. J. Am. Heart Assoc. 2020, 9, e016400. [Google Scholar] [CrossRef] [PubMed]
- El-Chouli, M.; Meddis, A.; Christensen, D.M.; Gerds, T.A.; Sehested, T.; Malmborg, M.; Phelps, M.; Bang, C.N.; Ahlehoff, O.; Torp-Pedersen, C.; et al. Lifetime risk of comorbidity in patients with simple congenital heart disease: A Danish nationwide study. Eur. Heart J. 2023, 44, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.J.; Bauer, U.M.M.; Baumgartner, H.; Uebing, A.; Walther, C.; Tutarel, O. Acquired Comorbidities in Adults with Congenital Heart Disease: An Analysis of the German National Register for Congenital Heart Defects. J. Clin. Med. 2021, 10, 314. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Yang, T.; Huang, P.; Wang, L.; Zhao, L.; Zhang, S.; Ye, Z.; Chen, L.; Zheng, Z.; et al. Congenital Heart Disease and Risk of Cardiovascular Disease: A Meta-Analysis of Cohort Studies. J. Am. Heart Assoc. 2019, 8, e012030. [Google Scholar] [CrossRef] [PubMed]
- Budts, W.; Roos-Hesselink, J.; Rädle-Hurst, T.; Eicken, A.; McDonagh, T.A.; Lambrinou, E.; Crespo-Leiro, M.G.; Walker, F.; Frogoudaki, A.A. Treatment of heart failure in adult congenital heart disease: A position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 2016, 37, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Bergh, N.; Skoglund, K.; Fedchenko, M.; Bollano, E.; Eriksson, P.; Dellborg, M.; Wai Giang, K.; Mandalenakis, Z. Risk of Heart Failure in Congenital Heart Disease: A Nationwide Register-Based Cohort Study. Circulation 2023, 147, 982–984. [Google Scholar] [CrossRef]
- Brida, M.; Lovric, D.; Griselli, M.; Riesgo Gil, F.; Gatzoulis, M.A. Heart failure in adults with congenital heart disease. Int. J. Cardiol. 2022, 357, 39–45. [Google Scholar] [CrossRef]
- Authors/Task Force, M.; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Bouchardy, J.; Therrien, J.; Pilote, L.; Ionescu-Ittu, R.; Martucci, G.; Bottega, N.; Marelli, A.J. Atrial arrhythmias in adults with congenital heart disease. Circulation 2009, 120, 1679–1686. [Google Scholar] [CrossRef]
- Tutarel, O. Acquired heart conditions in adults with congenital heart disease: A growing problem. Heart 2014, 100, 1317–1321. [Google Scholar] [CrossRef]
- Bessiere, F.; Mondesert, B.; Chaix, M.A.; Khairy, P. Arrhythmias in adults with congenital heart disease and heart failure. Heart Rhythm O2 2021, 2, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Mandalenakis, Z.; Rosengren, A.; Lappas, G.; Eriksson, P.; Hansson, P.O.; Dellborg, M. Ischemic Stroke in Children and Young Adults With Congenital Heart Disease. J. Am. Heart Assoc. 2016, 5, e003071. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.G.B.; Olsen, M.S.; Schmidt, M.; Johnsen, S.P.; Learn, C.; Laursen, H.B.; Madsen, N.L. Ischemic Stroke in Adults With Congenital Heart Disease: A Population-Based Cohort Study. J. Am. Heart Assoc. 2019, 8, e011870. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K. Metabolic syndrome and coronary artery disease in adults with congenital heart disease. Cardiovasc. Diagn. Ther. 2021, 11, 563–576. [Google Scholar] [CrossRef]
- Giannakoulas, G.; Dimopoulos, K.; Engel, R.; Goktekin, O.; Kucukdurmaz, Z.; Vatankulu, M.A.; Bedard, E.; Diller, G.P.; Papaphylactou, M.; Francis, D.P.; et al. Burden of coronary artery disease in adults with congenital heart disease and its relation to congenital and traditional heart risk factors. Am. J. Cardiol. 2009, 103, 1445–1450. [Google Scholar] [CrossRef]
- Meijs, T.A.; Minderhoud, S.C.S.; Muller, S.A.; de Winter, R.J.; Mulder, B.J.M.; van Melle, J.P.; Hoendermis, E.S.; van Dijk, A.P.J.; Zuithoff, N.P.A.; Krings, G.J.; et al. Cardiovascular Morbidity and Mortality in Adult Patients With Repaired Aortic Coarctation. J. Am. Heart Assoc. 2021, 10, e023199. [Google Scholar] [CrossRef]
- Fedchenko, M.; Mandalenakis, Z.; Rosengren, A.; Lappas, G.; Eriksson, P.; Skoglund, K.; Dellborg, M. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int. J. Cardiol. 2017, 248, 143–148. [Google Scholar] [CrossRef]
- Saha, P.; Potiny, P.; Rigdon, J.; Morello, M.; Tcheandjieu, C.; Romfh, A.; Fernandes, S.M.; McElhinney, D.B.; Bernstein, D.; Lui, G.K.; et al. Substantial Cardiovascular Morbidity in Adults With Lower-Complexity Congenital Heart Disease. Circulation 2019, 139, 1889–1899. [Google Scholar] [CrossRef]
- Agarwal, A.; Thombley, R.; Broberg, C.S.; Harris, I.S.; Foster, E.; Mahadevan, V.S.; John, A.; Vittinghoff, E.; Marcus, G.M.; Dudley, R.A. Age- and Lesion-Related Comorbidity Burden Among US Adults With Congenital Heart Disease: A Population-Based Study. J. Am. Heart Assoc. 2019, 8, e013450. [Google Scholar] [CrossRef]
- Moons, P.; Van Deyk, K.; Dedroog, D.; Troost, E.; Budts, W. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 612–616. [Google Scholar] [CrossRef]
- Levene, J.; Cambron, C.; McGrath, L.; Garcia, I.C.; Broberg, C.; Ramsey, K.; Khan, A. Prevalence of traditional and non-traditional cardiovascular risk factors in adults with congenital heart disease. Int. J. Cardiol. Congenit. Heart Dis. 2023, 11, 100424. [Google Scholar] [CrossRef]
- Moons, P.; Luyckx, K.; Kovacs, A.H.; Holbein, C.E.; Thomet, C.; Budts, W.; Enomoto, J.; Sluman, M.A.; Yang, H.L.; Jackson, J.L.; et al. Prevalence and Effects of Cigarette Smoking, Cannabis Consumption, and Co-use in Adults From 15 Countries With Congenital Heart Disease. Can. J. Cardiol. 2019, 35, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Madsen, N.L.; Marino, B.S.; Woo, J.G.; Thomsen, R.W.; Videbœk, J.; Laursen, H.B.; Olsen, M. Congenital Heart Disease With and Without Cyanotic Potential and the Long-term Risk of Diabetes Mellitus: A Population-Based Follow-up Study. J. Am. Heart Assoc. 2016, 5, e003076. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gonzalez, R.; Borgia, F.; Diller, G.P.; Inuzuka, R.; Kempny, A.; Martinez-Naharro, A.; Tutarel, O.; Marino, P.; Wustmann, K.; Charalambides, M.; et al. Abnormal lung function in adults with congenital heart disease: Prevalence, relation to cardiac anatomy, and association with survival. Circulation 2013, 127, 882–890. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Diller, G.P.; Koltsida, E.; Pijuan-Domenech, A.; Papadopoulou, S.A.; Babu-Narayan, S.V.; Salukhe, T.V.; Piepoli, M.F.; Poole-Wilson, P.A.; Best, N.; et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation 2008, 117, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, C.; Johansson, K.; Christersson, C.; Hlebowicz, J.; Thilén, U.; Johansson, B. Sarcopenia is common in adults with complex congenital heart disease. Int. J. Cardiol. 2019, 296, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, C.; Johansson, K.; Christersson, C.; Hlebowicz, J.; Thilén, U.; Johansson, B. Low bone mineral density in adults with complex congenital heart disease. Int. J. Cardiol. 2020, 319, 62–66. [Google Scholar] [CrossRef]
- Marelli, A.; Miller, S.P.; Marino, B.S.; Jefferson, A.L.; Newburger, J.W. Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation 2016, 133, 1951–1962. [Google Scholar] [CrossRef]
- Bagge, C.N.; Henderson, V.W.; Laursen, H.B.; Adelborg, K.; Olsen, M.; Madsen, N.L. Risk of Dementia in Adults With Congenital Heart Disease: Population-Based Cohort Study. Circulation 2018, 137, 1912–1920. [Google Scholar] [CrossRef]
- Gurvitz, M.; Ionescu-Ittu, R.; Guo, L.; Eisenberg, M.J.; Abrahamowicz, M.; Pilote, L.; Marelli, A.J. Prevalence of Cancer in Adults With Congenital Heart Disease Compared With the General Population. Am. J. Cardiol. 2016, 118, 1742–1750. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Karazisi, C.; Skoglund, K.; Rosengren, A.; Lappas, G.; Eriksson, P.; Dellborg, M. Risk of Cancer Among Children and Young Adults With Congenital Heart Disease Compared With Healthy Controls. JAMA Netw. Open 2019, 2, e196762. [Google Scholar] [CrossRef] [PubMed]
- Abalo, K.D.; Malekzadeh-Milani, S.; Hascoet, S.; Dreuil, S.; Feuillet, T.; Cohen, S.; Dauphin, C.; Filippo, S.D.; Douchin, S.; Godart, F.; et al. Exposure to low-dose ionising radiation from cardiac catheterisation and risk of cancer: The COCCINELLE study cohort profile. BMJ Open 2021, 11, e048576. [Google Scholar] [CrossRef] [PubMed]
- Campolo, J.; Annoni, G.; Giaccardi, M.; Andreassi, M.G. Congenital Heart Disease and the Risk of Cancer: An Update on the Genetic Etiology, Radiation Exposure Damage, and Future Research Strategies. J. Cardiovasc. Dev. Dis. 2022, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Liu, A.; Gurvitz, M.; Guo, L.; Therrien, J.; Laprise, C.; Kaufman, J.S.; Abrahamowicz, M.; Marelli, A.J. Exposure to Low-Dose Ionizing Radiation from Cardiac Procedures and Malignancy Risk in Adults With Congenital Heart Disease. Circulation 2018, 137, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological Age Predictors. EBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. 2019, 20, 249. [Google Scholar] [CrossRef]
- Calado, R.T.; Young, N.S. Telomere diseases. N. Engl. J. Med. 2009, 361, 2353–2365. [Google Scholar] [CrossRef]
- De Meyer, T.; Nawrot, T.; Bekaert, S.; De Buyzere, M.L.; Rietzschel, E.R.; Andrés, V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Martínez, P.; Blasco, M.A. Heart-Breaking Telomeres. Circ. Res. 2018, 123, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Lulkiewicz, M.; Bajsert, J.; Kopczynski, P.; Barczak, W.; Rubis, B. Telomere length: How the length makes a difference. Mol. Biol. Rep. 2020, 47, 7181–7188. [Google Scholar] [CrossRef]
- DeBoy, E.A.; Tassia, M.G.; Schratz, K.E.; Yan, S.M.; Cosner, Z.L.; McNally, E.J.; Gable, D.L.; Xiang, Z.; Lombard, D.B.; Antonarakis, E.S.; et al. Familial Clonal Hematopoiesis in a Long Telomere Syndrome. N. Engl. J. Med. 2023, 388, 2422–2433. [Google Scholar] [CrossRef] [PubMed]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of telomere length across human tissues. Science 2020, 369, 1597. [Google Scholar] [CrossRef] [PubMed]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.; Granick, M.; Aviv, A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef] [PubMed]
- Schellnegger, M.; Lin, A.C.; Hammer, N.; Kamolz, L.P. Physical Activity on Telomere Length as a Biomarker for Aging: A Systematic Review. Sports Med. Open 2022, 8, 111. [Google Scholar] [CrossRef]
- D′Mello, M.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere length: A review of methods for measurement. Nurs. Res. 2014, 63, 289–299. [Google Scholar] [CrossRef]
- Willeit, P.; Willeit, J.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Brandstätter, A.; Kronenberg, F.; Kiechl, S. Telomere length and risk of incident cancer and cancer mortality. JAMA 2010, 304, 69–75. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [PubMed]
- Vecoli, C.; Borghini, A.; Foffa, I.; Ait-Ali, L.; Picano, E.; Andreassi, M.G. Leukocyte telomere shortening in grown-up patients with congenital heart disease. Int. J. Cardiol. 2016, 204, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Shelly, E.; Waldron, M.G.; Field, E.; Moore, N.; Young, R.; Scally, A.; England, A.; Maher, M.; McEntee, M.F. Cumulative Radiation Dose from Medical Imaging in Children with Congenital Heart Disease: A Systematic Review. Children 2023, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ali, L.; Andreassi, M.G.; Foffa, I.; Spadoni, I.; Vano, E.; Picano, E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart 2010, 96, 269–274. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- Chen, B.H.; Marioni, R.E.; Colicino, E.; Peters, M.J.; Ward-Caviness, C.K.; Tsai, P.C.; Roetker, N.S.; Just, A.C.; Demerath, E.W.; Guan, W.; et al. DNA methylation-based measures of biological age: Meta-analysis predicting time to death. Aging 2016, 8, 1844–1865. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Fransquet, P.D.; Wrigglesworth, J.; Woods, R.L.; Ernst, M.E.; Ryan, J. The epigenetic clock as a predictor of disease and mortality risk: A systematic review and meta-analysis. Clin. Epigenetics 2019, 11, 62. [Google Scholar] [CrossRef]
- Roetker, N.S.; Pankow, J.S.; Bressler, J.; Morrison, A.C.; Boerwinkle, E. Prospective Study of Epigenetic Age Acceleration and Incidence of Cardiovascular Disease Outcomes in the ARIC Study (Atherosclerosis Risk in Communities). Circ. Genom. Precis. Med. 2018, 11, e001937. [Google Scholar] [CrossRef]
- Dugué, P.A.; Bassett, J.K.; Joo, J.E.; Jung, C.H.; Ming Wong, E.; Moreno-Betancur, M.; Schmidt, D.; Makalic, E.; Li, S.; Severi, G.; et al. DNA methylation-based biological aging and cancer risk and survival: Pooled analysis of seven prospective studies. Int. J. Cancer 2018, 142, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Uchehara, B.; Coulter Kwee, L.; Regan, J.; Chatterjee, R.; Eckstrand, J.; Swope, S.; Gold, G.; Schaack, T.; Douglas, P.; Mettu, P.; et al. Accelerated Epigenetic Aging Is Associated With Multiple Cardiometabolic, Hematologic, and Renal Abnormalities: A Project Baseline Health Substudy. Circ. Genom. Precis. Med. 2023, 16, 216–223. [Google Scholar] [CrossRef]
- Ammous, F.; Zhao, W.; Ratliff, S.M.; Mosley, T.H.; Bielak, L.F.; Zhou, X.; Peyser, P.A.; Kardia, S.L.R.; Smith, J.A. Epigenetic age acceleration is associated with cardiometabolic risk factors and clinical cardiovascular disease risk scores in African Americans. Clin. Epigenetics 2021, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Joyce, B.T.; Colicino, E.; Liu, L.; Zhang, W.; Dai, Q.; Shrubsole, M.J.; Kibbe, W.A.; Gao, T.; Zhang, Z.; et al. Blood Epigenetic Age may Predict Cancer Incidence and Mortality. EBioMedicine 2016, 5, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Nwanaji-Enwerem, J.C.; Samet, M.; Ward-Caviness, C.K. DNA Methylation Age-Environmental Influences, Health Impacts, and Its Role in Environmental Epidemiology. Curr. Environ. Health Rep. 2018, 5, 317–327. [Google Scholar] [CrossRef]
- Serra-Juhé, C.; Cuscó, I.; Homs, A.; Flores, R.; Torán, N.; Pérez-Jurado, L.A. DNA methylation abnormalities in congenital heart disease. Epigenetics 2015, 10, 167–177. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Schmauck-Medina, T.; Moliere, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Singampalli, K.L.; Jui, E.; Shani, K.; Ning, Y.; Connell, J.P.; Birla, R.K.; Bollyky, P.L.; Caldarone, C.A.; Keswani, S.G.; Grande-Allen, K.J. Congenital Heart Disease: An Immunological Perspective. Front. Cardiovasc. Med. 2021, 8, 701375. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, L.M.; Cohen, S.; Bauersachs, J.; Mebazaa, A.; Chousterman, B.G. Immunity and inflammation: The neglected key players in congenital heart disease? Heart Fail. Rev. 2022, 27, 1957–1971. [Google Scholar] [CrossRef]
- Geenen, L.W.; Baggen, V.J.M.; van den Bosch, A.E.; Eindhoven, J.A.; Kauling, R.M.; Cuypers, J.; Roos-Hesselink, J.W.; Boersma, E. Prognostic value of C-reactive protein in adults with congenital heart disease. Heart 2020, 107, 474–481. [Google Scholar] [CrossRef]
- Opotowsky, A.R.; Valente, A.M.; Alshawabkeh, L.; Cheng, S.; Bradley, A.; Rimm, E.B.; Landzberg, M.J. Prospective cohort study of C-reactive protein as a predictor of clinical events in adults with congenital heart disease: Results of the Boston adult congenital heart disease biobank. Eur. Heart J. 2018, 39, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Baggen, V.J.M.; van den Bosch, A.E.; van Kimmenade, R.R.; Eindhoven, J.A.; Witsenburg, M.; Cuypers, J.; Leebeek, F.W.G.; Boersma, E.; Roos-Hesselink, J.W. Red cell distribution width in adults with congenital heart disease: A worldwide available and low-cost predictor of cardiovascular events. Int. J. Cardiol. 2018, 260, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bolger, A.P.; Li, W.; Davlouros, P.A.; Volk, H.D.; Poole-Wilson, P.A.; Coats, A.J.; Gatzoulis, M.A.; Anker, S.D. Elevated circulating levels of inflammatory cytokines and bacterial endotoxin in adults with congenital heart disease. Am. J. Cardiol. 2003, 92, 188–193. [Google Scholar] [CrossRef]
- Nassef, Y.E.; Hamed, M.A.; Aly, H.F. Inflammatory cytokines, apoptotic, tissue injury and remodeling biomarkers in children with congenital heart disease. Indian. J. Clin. Biochem. 2014, 29, 145–149. [Google Scholar] [CrossRef]
- Won, C.W. Diagnosis and Management of Frailty in Primary Health Care. Korean J. Fam. Med. 2020, 41, 207–213. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; Mitnitski, A. Changes in relative fitness and frailty across the adult lifespan: Evidence from the Canadian National Population Health Survey. CMAJ 2011, 183, E487–E494. [Google Scholar] [CrossRef] [PubMed]
- Van Bulck, L.; Kovacs, A.H.; Goossens, E.; Luyckx, K.; Zaidi, A.; Wang, J.K.; Yadeta, D.; Windram, J.; Van De Bruaene, A.; Thomet, C.; et al. Rationale, design and methodology of APPROACH-IS II: International study of patient-reported outcomes and frailty phenotyping in adults with congenital heart disease. Int. J. Cardiol. 2022, 363, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Belsky, D.W.; Moffitt, T.E.; Cohen, A.A.; Corcoran, D.L.; Levine, M.E.; Prinz, J.A.; Schaefer, J.; Sugden, K.; Williams, B.; Poulton, R.; et al. Eleven Telomere, Epigenetic Clock, and Biomarker-Composite Quantifications of Biological Aging: Do They Measure the Same Thing? Am. J. Epidemiol. 2018, 187, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Bonafè, M.; Sabbatinelli, J.; Olivieri, F. Exploiting the telomere machinery to put the brakes on inflamm-aging. Ageing Res. Rev. 2020, 59, 101027. [Google Scholar] [CrossRef]
- Bae, C.Y.; Kang, Y.G.; Piao, M.H.; Cho, B.; Cho, K.H.; Park, Y.K.; Yu, B.Y.; Lee, S.W.; Kim, M.J.; Lee, S.H.; et al. Models for estimating the biological age of five organs using clinical biomarkers that are commonly measured in clinical practice settings. Maturitas 2013, 75, 253–260. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Houts, R.; Cohen, H.J.; Corcoran, D.L.; Danese, A.; Harrington, H.; Israel, S.; Levine, M.E.; Schaefer, J.D.; et al. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci. USA 2015, 112, E4104–E4110. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tournoy, T.K.; Moons, P.; Daelman, B.; De Backer, J. Biological Age in Congenital Heart Disease—Exploring the Ticking Clock. J. Cardiovasc. Dev. Dis. 2023, 10, 492. https://doi.org/10.3390/jcdd10120492
Tournoy TK, Moons P, Daelman B, De Backer J. Biological Age in Congenital Heart Disease—Exploring the Ticking Clock. Journal of Cardiovascular Development and Disease. 2023; 10(12):492. https://doi.org/10.3390/jcdd10120492
Chicago/Turabian StyleTournoy, Tijs K., Philip Moons, Bo Daelman, and Julie De Backer. 2023. "Biological Age in Congenital Heart Disease—Exploring the Ticking Clock" Journal of Cardiovascular Development and Disease 10, no. 12: 492. https://doi.org/10.3390/jcdd10120492




