Follow Me! A Tale of Avian Heart Development with Comparisons to Mammal Heart Development
Abstract
:1. Introduction
2. Avian Models
3. Avian Development and Staging
4. Tubular Heart Assembly
5. Cardiac Conduction
6. Heart Pumping and Tube Looping
7. Heart Septation
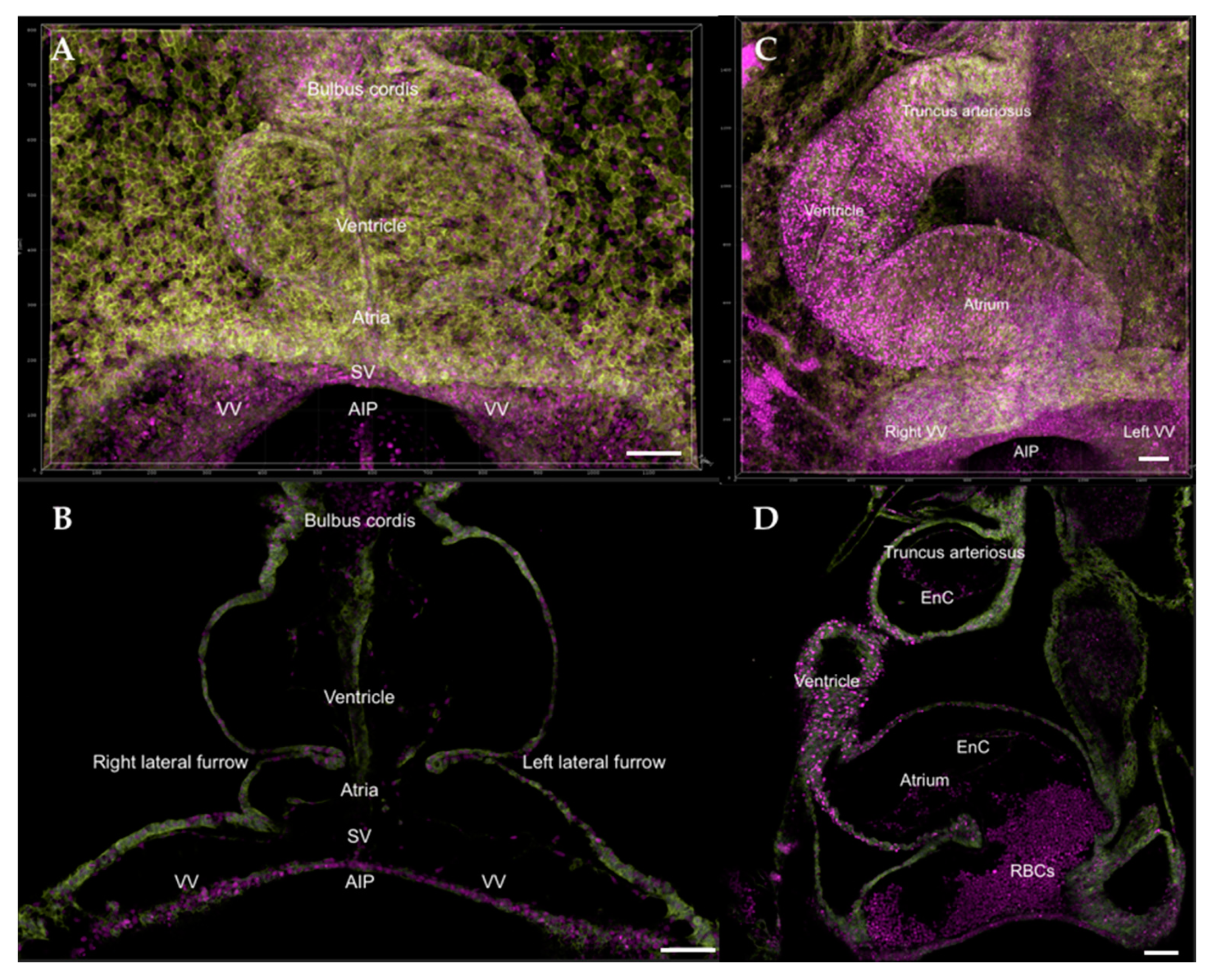
8. The Fully Formed Heart
9. Imaging Strategies to Capture the Heart Beating Motion
10. Summary and Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Gill, F.B.; Prum, R.O. Ornithology; WH Freeman: New York, NY, USA, 2019. [Google Scholar]
- Scott, G.R. Elevated performance: The unique physiology of birds that fly at high altitudes. J. Exp. Biol. 2011, 214, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Butler, P.J. The physiological basis of bird flight. Philos. Trans. R. Soc. B: Biol. Sci. 2016, 371, 20150384. [Google Scholar] [CrossRef] [Green Version]
- Gavrilov, V.M. Origin and development of homoiothermy: A case study of avian energetics. Adv. Biosci. Biotech. 2013, 4, 1–17. [Google Scholar]
- Tucker, V.A. Oxygen consumption of a flying bird. Science 1966, 154, 150–151. [Google Scholar] [CrossRef]
- Hartman, F.A. Heart Weight in Birds. Condor 1955, 57, 221–238. [Google Scholar] [CrossRef]
- Brush, A.H. Avian Heart Size and Cardiovascular Performance. Auk 1966, 83, 266–273. [Google Scholar] [CrossRef]
- Butler, P.J.; West, N.H.; Jones, D.R. Respiratory and cardiovascular responses of the pigeon to sustained, level flight in a wind tunnel. J. Exp. Biol. 1977, 71, 7–26. [Google Scholar]
- Bishop, C.M.; Butler, P.J. Chapter 39 - Flight. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 919–974. [Google Scholar]
- Prothero, J. Heart weight as a function of body weight in mammals. Growth 1979, 43, 139–150. [Google Scholar]
- Dzialowski, E.M.; Crossley, D.A. Chapter 11—The Cardiovascular System. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 193–283. [Google Scholar]
- Hartman, F.A. Locomotor mechanisms of birds. Smithson. Misc. Collect. 1961, 143, 1–91. [Google Scholar]
- Smith, F.M.; West, N.H.; Jones, D.R. Chapter 9 - The Cardiovascular System. In Sturkie’s Avian Physiology, 5th ed.; Whittow, G.C., Ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 141–231. [Google Scholar]
- Lundgren, B.O.; Kiessling, K.-H. Comparative aspects of fibre types, areas, and capillary supply in the pectoralis muscle of some passerine birds with differing migratory behaviour. J. Comp. Physiol. B 1988, 158, 165–173. [Google Scholar]
- Mathieu-Costello, O.; Suarez, R.K.; Hochachka, P.W. Capillary-to-fiber geometry and mitochondrial density in hummingbird flight muscle. Respir. Physiol. 1992, 89, 113–132. [Google Scholar] [CrossRef]
- Lu, Y.; James, T.N.; Bootsma, M.; Terasaki, F. Histological organization of the right and left atrioventricular valves of the chicken heart and their relationship to the atrioventricular Purkinje ring and the middle bundle branch. Anat. Rec. 1993, 235, 74–86. [Google Scholar] [CrossRef]
- Needham, J. A History of Embryology; Cambridge University Press: Cambridge, UK, 1959. [Google Scholar]
- Aristotle; Balme, D.M.; Peck, A.L. The History of Animals; Harvard University Press: Cambridge, MA, USA, 1965. [Google Scholar]
- Harvey, W. Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus; Guiliemi Fitzeri: Frankfurt, Germany, 1628. [Google Scholar]
- Malpighi, M. Dissertatio Epistolica de Formatione Pulli in Ovo: Regiae Societati, Londini ad Scientiam Naturalem; Promovendam Institutae: Martyn, London, UK, 1673. [Google Scholar]
- Malpighi, M. Repetitas Auctasque de Ovo Incubato Observationes Continens; Johannis Martyn: London, UK, 1675. [Google Scholar]
- Gilbert, S.F.; Barresi, M.J. Developmental Biology, 11th ed.; Sinauer Associates: Sunderland, MA, USA, 2016. [Google Scholar]
- DeHaan, R.L. Development of form in the embryonic heart. An experimental approach. Circulation 1967, 35, 821–833. [Google Scholar]
- Plein, A.; Fantin, A.; Ruhrberg, C. Neural crest cells in cardiovascular development. Curr. Top. Dev. Biol. 2015, 111, 183–200. [Google Scholar] [CrossRef]
- Keller, B.B. Embryonic cardiovascular function, coupling and maturation: A species view. In Development of Cardiovascular Systems; Burggren, W.W., Keller, B.B., Eds.; University Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Burggren, W.W.; Santin, J.F.; Antich, M.R. Cardio-respiratory development in bird embryos: New insights from a venerable animal model. Rev. Bras. De Zootec. 2016, 45, 709–728. [Google Scholar]
- Midgett, M.; Thornburg, K.L.; Rugonyi, S. Blood Flow Patterns Underlie Developmental Heart Defects. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H632–H642. [Google Scholar] [CrossRef]
- Roest, P.A.M.; van Iperen, L.; Vis, S.; Wisse, L.J.; Poelmann, R.E.; Steegers-Theunissen, R.P.M.; Molin, D.G.M.; Eriksson, U.J.; Gittenberger-De Groot, A.C. Exposure of neural crest cells to elevated glucose leads to congenital heart defects, an effect that can be prevented by N-acetylcysteine. Birth Defects Res. Part. A Clin. Mol. Teratol. 2006, 79, 231–235. [Google Scholar] [CrossRef]
- Midgett, M.; Rugonyi, S. Congenital heart malformations induced by hemodynamic altering surgical interventions. Front. Physiol. 2014, 5, 287. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Zhang, H.; Tang, W.W.C.; Irie, N.; Withey, S.; Klisch, D.; Sybirna, A.; Dietmann, S.; Contreras, D.A.; Webb, R.; et al. Principles of early human development and germ cell program from conserved model systems. Nature 2017, 546, 416–420. [Google Scholar] [CrossRef]
- New, D. A new technique for the cultivation of the chick embryo in vitro. J. Embryol. Exp. Morphol. 1955, 3, 320–331. [Google Scholar]
- Chapman, S.C.; Collignon, J.; Schoenwolf, G.C.; Lumsden, A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev. Dyn. 2001, 220, 284–289. [Google Scholar] [CrossRef]
- Arguello, C.; de la Cruz, M.V.; Gomez, C.S. Experimental study of the formation of the heart tube in the chick embryo. J. Embryol. Exp. Morphol. 1975, 33, 1–11. [Google Scholar]
- de la Cruz, M.V.; Sanchez Gomez, C.; Arteaga, M.M.; Arguello, C. Experimental study of the development of the truncus and the conus in the chick embryo. J. Anat. 1977, 123, 661–686. [Google Scholar]
- De La Cruz, M.V.; Sánchez-Gómez, C.; Palomino, M.A. The primitive cardiac regions in the straight tube heart (Stage 9) and their anatomical expression in the mature heart: An experimental study in the chick embryo. J. Anat. 1989, 165, 121–131. [Google Scholar]
- Sato, Y.; Poynter, G.; Huss, D.; Filla, M.B.; Czirok, A.; Rongish, B.J.; Little, C.D.; Fraser, S.E.; Lansford, R. Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PLoS ONE 2010, 5, e12674. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrova, A.; Czirok, A.; Szabo, A.; Filla, M.B.; Hossain, M.J.; Whelan, P.F.; Lansford, R.; Rongish, B.J. Convective tissue movements play a major role in avian endocardial morphogenesis. Dev. Biol. 2012, 363, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrova, A.; Czirok, A.; Kosa, E.; Galkin, O.; Cheuvront, T.J.; Rongish, B.J. The endoderm and myocardium join forces to drive early heart tube assembly. Dev. Biol. 2015, 404, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrova, A.; Filla, M.; Kosa, E.; Little, C.; Petersen, A.; Rongish, B. Altered VEGF signaling leads to defects in heart tube elongation and omphalomesenteric vein fusion in quail embryos. Anat. Rec. 2019, 302, 175–185. [Google Scholar]
- Rosenquist, G.C.; DeHaan, R.L. Migration of precardiac cells in the chick embryo: A radiographic study. Contrib. Embryol. 1966, 263, 112–121. [Google Scholar]
- Rosenquist, G.C. Aortic arches in the chick embryo: Origin of the cells as determined by radioautographic mapping. Anat. Rec. 1970, 168, 351–359. [Google Scholar] [CrossRef]
- Rosenquist, G.C. The origin of the trabeculated ventricular septum in the chick embryo as determined by radioautographic mapping. Anat. Rec. 1970, 168, 187–193. [Google Scholar] [CrossRef]
- Rosenquist, G.C. Location and movements of cardiogenic cells in the chick embryo: The heart-forming portion of the primitive streak. Dev. Biol. 1970, 22, 461–475. [Google Scholar] [CrossRef]
- Kuratani, S.C.; Kirby, M.L. Migration and distribution of circumpharyngeal crest cells in the chick embryo. Formation of the circumpharyngeal ridge and E/C8+ crest cells in the vertebrate head region. Anat. Rec. 1992, 234, 263–280. [Google Scholar] [CrossRef]
- Ward, C.; Stadt, H.; Hutson, M.; Kirby, M.L. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Dev. Biol. 2005, 284, 72–83. [Google Scholar] [CrossRef] [Green Version]
- van Wijk, B.; van den Berg, G.; Abu-Issa, R.; Barnett, P.; van der Velden, S.; Schmidt, M.; Ruijter, J.M.; Kirby, M.L.; Moorman, A.F.; van den Hoff, M.J. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ. Res. 2009, 105, 431–441. [Google Scholar] [CrossRef]
- DeHaan, R.L. Organization of the cardiogenic plate in the early chick embryo. Acta Embryol. Morphol. Exp. 1963, 6, 26–38. [Google Scholar]
- Rawles, M.E. The heart-forming areas of the early chick blastoderm. Physiol. Zool. 1943, 16, 22–42. [Google Scholar]
- Gregg, C.L.; Butcher, J.T. Quantitative in vivo imaging of embryonic development: Opportunities and challenges. Differentiation 2012, 84, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Butcher, J.T.; Sedmera, D.; Guldberg, R.E.; Markwald, R.R. Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Dev. Dyn. 2007, 236, 802–809. [Google Scholar]
- Lucitti, J.L.; Visconti, R.; Novak, J.; Keller, B.B. Increased arterial load alters aortic structural and functional properties during embryogenesis. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1919–H1926. [Google Scholar]
- Kowalski, W.J.; Dur, O.; Wang, Y.; Patrick, M.J.; Tinney, J.P.; Keller, B.B.; Pekkan, K. Critical Transitions in Early Embryonic Aortic Arch Patterning and Hemodynamics. PLoS ONE 2013, 8, e60271. [Google Scholar] [CrossRef] [Green Version]
- International Chicken Genome Sequencing Consortium. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 2004, 432, 695–716. [Google Scholar]
- Wallis, J.W.; Aerts, J.; Groenen, M.A.; Crooijmans, R.P.; Layman, D.; Graves, T.A.; Scheer, D.E.; Kremitzki, C.; Fedele, M.J.; Mudd, N.K.; et al. A physical map of the chicken genome. Nature 2004, 432, 761–764. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, Y.; Hou, Z.; Fan, G.; Pi, J.; Sun, S.; Chen, J.; Liu, H.; Du, X.; Shen, J.; et al. Population genomic data reveal genes related to important traits of quail. GigaScience 2018, 7, 1–16. [Google Scholar] [CrossRef]
- Kayang, B.B.; Fillon, V.; Inoue-Murayama, M.; Miwa, M.; Leroux, S.; Feve, K.; Monvoisin, J.L.; Pitel, F.; Vignoles, M.; Mouilhayrat, C.; et al. Integrated maps in quail (Coturnix japonica) confirm the high degree of synteny conservation with chicken (Gallus gallus) despite 35 million years of divergence. BMC Genom. 2006, 7, 101. [Google Scholar] [CrossRef] [Green Version]
- Kawahara-Miki, R.; Sano, S.; Nunome, M.; Shimmura, T.; Kuwayama, T.; Takahashi, S.; Kawashima, T.; Matsuda, Y.; Yoshimura, T.; Kono, T. Next-generation sequencing reveals genomic features in the Japanese quail. Genomics 2013, 101, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, L.; Pedersen, D.; Ching, K.H.; Yi, H.; Collarini, E.J.; Izquierdo, S.; van de Lavoir, M.C.; Leighton, P.A. Germline Gene Editing in Chickens by Efficient CRISPR-Mediated Homologous Recombination in Primordial Germ Cells. PLoS ONE 2016, 11, e0154303. [Google Scholar] [CrossRef]
- Idoko-Akoh, A.; Taylor, L.; Sang, H.M.; McGrew, M.J. High fidelity CRISPR/Cas9 increases precise monoallelic and biallelic editing events in primordial germ cells. Sci. Rep. 2018, 8, 15126. [Google Scholar] [CrossRef] [Green Version]
- Veron, N.; Qu, Z.; Kipen, P.A.; Hirst, C.E.; Marcelle, C. CRISPR mediated somatic cell genome engineering in the chicken. Dev. Biol. 2015, 407, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Taylor, L.; Carlson, D.F.; Nandi, S.; Sherman, A.; Fahrenkrug, S.C.; McGrew, M.J. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development 2017, 144, 928–934. [Google Scholar] [CrossRef] [Green Version]
- Mende, M.; Christophorou, N.A.; Streit, A. Specific and effective gene knock-down in early chick embryos using morpholinos but not pRFPRNAi vectors. Mech. Dev. 2008, 125, 947–962. [Google Scholar] [CrossRef] [Green Version]
- Andermatt, I.; Wilson, N.; Stoeckli, E.T. In ovo electroporation of miRNA-based-plasmids to investigate gene function in the developing neural tube. Methods Mol. Biol. 2014, 1101, 353–368. [Google Scholar] [CrossRef]
- Rao, M.; Baraban, J.H.; Rajaii, F.; Sockanathan, S. In vivo comparative study of RNAi methodologies by in ovo electroporation in the chick embryo. Dev. Dyn. 2004, 231, 592–600. [Google Scholar] [CrossRef]
- Das, R.M.; Van Hateren, N.J.; Howell, G.R.; Farrell, E.R.; Bangs, F.K.; Porteous, V.C.; Manning, E.M.; McGrew, M.J.; Ohyama, K.; Sacco, M.A.; et al. A robust system for RNA interference in the chicken using a modified microRNA operon. Dev. Biol. 2006, 294, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Scott, B.B.; Lois, C. Generation of tissue-specific transgenic birds with lentiviral vectors. Proc. Natl. Acad. Sci. USA 2005, 102, 16443–16447. [Google Scholar]
- Seidl, A.H.; Sanchez, J.T.; Schecterson, L.; Tabor, K.M.; Wang, Y.; Kashima, D.T.; Poynter, G.; Huss, D.; Fraser, S.E.; Lansford, R.; et al. Transgenic quail as a model for research in the avian nervous system: A comparative study of the auditory brainstem. J. Comp. Neurol. 2013, 521, 5–23. [Google Scholar] [CrossRef]
- Huss, D.; Benazeraf, B.; Wallingford, A.; Filla, M.; Yang, J.; Fraser, S.E.; Lansford, R. A transgenic quail model that enables dynamic imaging of amniote embryogenesis. Development 2015, 142, 2850–2859. [Google Scholar] [CrossRef] [Green Version]
- Moreau, C.; Caldarelli, P.; Rocancourt, D.; Roussel, J.; Denans, N.; Pourquie, O.; Gros, J. Timed Collinear Activation of Hox Genes during Gastrulation Controls the Avian Forelimb Position. Curr. Biol. 2019, 29, 35–50 e34. [Google Scholar] [CrossRef] [Green Version]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar]
- Nagahara, H.; Ma, Y.; Takenaka, Y.; Kageyama, R.; Yoshikawa, K. Spatiotemporal pattern in somitogenesis: A non-Turing scenario with wave propagation. Phys. Rev. E 2009, 80, 021906. [Google Scholar] [CrossRef]
- Santillán, M.; Mackey, M.C. A Proposed Mechanism for the Interaction of the Segmentation Clock and the Determination Front in Somitogenesis. PLoS ONE 2008, 3, e1561. [Google Scholar] [CrossRef]
- DeRuiter, C. Somites: Formation and Role in Developing the Body Plan. Embryo Project Encyclopedia. Available online: https://embryo.asu.edu/pages/somites-formation-and-role-developing-body-plan (accessed on 20 February 2020).
- Martinsen, B.J. Reference guide to the stages of chick heart embryology. Dev. Dyn. 2005, 233, 1217–1237. [Google Scholar]
- Tomanek, R.J. Developmental Progression of the Coronary Vasculature in Human Embryos and Fetuses. Anat. Rec. (Hoboken) 2016, 299, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Savolainen, S.M.; Foley, J.F.; Elmore, S.A. Histology atlas of the developing mouse heart with emphasis on E11.5 to E18.5. Toxicol. Pathol. 2009, 37, 395–414. [Google Scholar] [CrossRef] [Green Version]
- Padgett, C.A.; Ivey, W.D. Coturnix quail as a laboratory research animal. Science 1959, 129, 267–268. [Google Scholar]
- Padgett, C.S.; Ivey, W.D. The normal embryology of the Coturnix quail. Anat. Rec. 1960, 137, 1–11. [Google Scholar]
- Zacchei, A.M. Archivio italiano di anatomia e di embriologia: Lo sviluppo embrionale della quaglia giapponese. Arch. Ital. Anat. Embriol. 1961, 66, 36–62. [Google Scholar]
- Ainsworth, S.J.; Stanley, R.L.; Evans, D.J. Developmental stages of the Japanese quail. J. Anat. 2010, 216, 3–15. [Google Scholar] [CrossRef]
- Ruffins, S.W.; Martin, M.; Keough, L.; Truong, S.; Fraser, S.E.; Jacobs, R.E.; Lansford, R. Digital Three-Dimensional Atlas of Quail Development Using High-Resolution MRI. Sci. J. 2007, 2, 47–59. [Google Scholar]
- Le Douarin, N.; Barq, G. Use of Japanese quail cells as “biological markers” in experimental embryology. Comptes Rendus Hebd. Seances L’academie Sci. Ser. D Sci. Nat. 1969, 269, 1543–1546. [Google Scholar]
- Le Douarin, N.; Kalcheim, C. The Neural Crest, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Le Douarin, N. A biological cell labeling technique and its use in expermental embryology. Dev. Biol. 1973, 30, 217–222. [Google Scholar]
- Kirby, M.L.; Gale, T.F.; Stewart, D.E. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983, 220, 1059–1061. [Google Scholar]
- Kirby, M.L.; Waldo, K.L. Neural Crest and Cardiovascular Patterning. Circ. Res. 1995, 77, 211–215. [Google Scholar]
- Garcia-Martinez, V.; Schoenwolf, G.C. Primitive-streak origin of the cardiovascular system in avian embryos. Dev. Biol. 1993, 159, 706–719. [Google Scholar] [CrossRef]
- Cui, C.; Cheuvront, T.J.; Lansford, R.D.; Moreno-Rodriguez, R.A.; Schultheiss, T.M.; Rongish, B.J. Dynamic positional fate map of the primary heart-forming region. Dev. Biol. 2009, 332, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Linask, K.K.; Lash, J.W. Precardiac cell migration: Fibronectin localization at mesoderm-endoderm interface during directional movement. Dev. Biol. 1986, 114, 87–101. [Google Scholar]
- Schultheiss, T.M.; Xydas, S.; Lassar, A.B. Induction of avian cardiac myogenesis by anterior endoderm. Development 1995, 121, 4203–4214. [Google Scholar]
- Rawles, M.E. A study in the localization of organ-forming areas in the chick blastoderm of the head-process stage. J. Exp. Zool. 1936, 72, 271–315. [Google Scholar]
- Abu-Issa, R.; Kirby, M.L. Heart field: From mesoderm to heart tube. Annu. Rev. Cell Dev. Biol. 2007, 23, 45–68. [Google Scholar] [CrossRef]
- Hosseini, H.S.; Garcia, K.E.; Taber, L.A. A new hypothesis for foregut and heart tube formation based on differential growth and actomyosin contraction. Development 2017, 144, 2381–2391. [Google Scholar] [CrossRef] [Green Version]
- Stalsberg, H. The origin of heart asymmetry: Right and left contributions to the early chick embryo heart. Dev. Biol. 1969, 19, 109–127. [Google Scholar]
- Abu-Issa, R.; Kirby, M.L. Patterning of the heart field in the chick. Dev. Biol. 2008, 319, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Linask, K.K.; Lash, J.W. Morphoregulatory mechanisms underlying early heart development: Precardiac stages to the looping, tubular heart. In Living Morphogenesis of the Heart; de la Cruz, M.V., Markwald, R.R., Eds.; Birkhäuser: Boston, MA, USA, 1998; pp. 1–42. [Google Scholar]
- Harvey, R.P. Patterning the vertebrate heart. Nat. Rev. Genet. 2002, 3, 544–556. [Google Scholar] [CrossRef]
- Lockhart, M.; Wirrig, E.; Phelps, A.; Wessels, A. Extracellular matrix and heart development. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Drake, C.J.; Davis, L.A.; Walters, L.; Little, C.D. Avian vasculogenesis and the distribution of collagens I, IV, laminin, and fibronectin in the heart primordia. J. Exp. Zool. 1990, 255, 309–322. [Google Scholar]
- Linask, K.K.; Lash, J.W. A role for fibronectin in the migration of avian precardiac cells. II. Rotation of the heart-forming region during different stages and its effects. Dev. Biol. 1988, 129, 324–329. [Google Scholar]
- George, E.L.; Baldwin, H.S.; Hynes, R.O. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood 1997, 90, 3073–3081. [Google Scholar]
- Tsuda, T.; Philp, N.; Zile, M.H.; Linask, K.K. Left-right asymmetric localization of flectin in the extracellular matrix during heart looping. Dev. Biol. 1996, 173, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Linask, K.K.; Han, M.; Cai, D.H.; Brauer, P.R.; Maisastry, S.M. Cardiac morphogenesis: Matrix metalloproteinase coordination of cellular mechanisms underlying heart tube formation and directionality of looping. Dev. Dyn. 2005, 233, 739–753. [Google Scholar] [CrossRef]
- Shi, Y.; Varner, V.D.; Taber, L.A. Why is cytoskeletal contraction required for cardiac fusion before but not after looping begins? Phys. Biol. 2015, 12, 016012. [Google Scholar] [CrossRef] [Green Version]
- Varner, V.D.; Taber, L.A. Not just inductive: A crucial mechanical role for the endoderm during heart tube assembly. Development 2012, 139, 1680–1690. [Google Scholar]
- Mikawa, T. Cardiac Lineages. In Heart Development; Harvey, R.P., Rosenthal, N., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 19–33. [Google Scholar]
- Cohen-Gould, L.; Mikawa, T. The fate diversity of mesodermal cells within the heart field during chicken early embryogenesis. Dev. Biol. 1996, 177, 265–273. [Google Scholar]
- Coffin, J.D.; Poole, T.J. Endothelial cell origin and migration in embryonic heart and cranial blood vessel development. Anat. Rec. 1991, 231, 383–395. [Google Scholar] [CrossRef]
- Noden, D.M. Origins and patterning of avian outflow tract endocardium. Development 1991, 111, 867–876. [Google Scholar]
- Flamme, I.; Frolich, T.; Risau, W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J. Cell Physiol. 1997, 173, 206–210. [Google Scholar] [CrossRef]
- Ishii, Y.; Langberg, J.; Rosborough, K.; Mikawa, T. Endothelial cell lineages of the heart. Cell Tissue Res. 2009, 335, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Milgrom-Hoffman, M.; Harrelson, Z.; Ferrara, N.; Zelzer, E.; Evans, S.M.; Tzahor, E. The heart endocardium is derived from vascular endothelial progenitors. Development 2011, 138, 4777–4787. [Google Scholar] [CrossRef] [Green Version]
- Masino, A.M.; Gallardo, T.D.; Wilcox, C.A.; Olson, E.N.; Williams, R.S.; Garry, D.J. Transcriptional regulation of cardiac progenitor cell populations. Circ. Res. 2004, 95, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Bu, L.; Jiang, X.; Martin-Puig, S.; Caron, L.; Zhu, S.; Shao, Y.; Roberts, D.J.; Huang, P.L.; Domian, I.J.; Chien, K.R. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 2009, 460, 113–117. [Google Scholar] [CrossRef]
- Kattman, S.J.; Huber, T.L.; Keller, G.M. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev. Cell 2006, 11, 723–732. [Google Scholar]
- Motoike, T.; Markham, D.W.; Rossant, J.; Sato, T.N. Evidence for novel fate of Flk1+ progenitor: Contribution to muscle lineage. Genesis 2003, 35, 153–159. [Google Scholar] [CrossRef]
- Lescroart, F.; Chabab, S.; Lin, X.; Rulands, S.; Paulissen, C.; Rodolosse, A.; Auer, H.; Achouri, Y.; Dubois, C.; Bondue, A.; et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat. Cell Biol. 2014, 16, 829–840. [Google Scholar] [CrossRef]
- Ruckert, J. Entwickelung der extraembryonalen Gefasse de Vogel. In Entwickelungslehre Der Wirbeltiere; Hertwig, O., Ed.; Verlag von Gustav Fischer: Jena, Germany, 1906; Volume 1, pp. 1203–1244. [Google Scholar]
- Sabin, F.R. Studies on the origin of the blood vessels and of red blood corpusles as seen in the living blastoderm of chick during the second day of incubation. Contrib. Embryol Carneg Inst. 1920, 9, 215–262. [Google Scholar]
- Murray, P.D.F. The development in vitro of the blood of the early chick embryo. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 1932, 111, 497–521. [Google Scholar] [CrossRef]
- Tam, P.P.; Beddington, R.S. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development 1987, 99, 109–126. [Google Scholar]
- Lawson, K.A.; Pedersen, R.A. Clonal analysis of cell fate during gastrulation and early neurulation in the mouse. Ciba Found. Symp. 1992, 165, 3–21. [Google Scholar]
- Ueno, H.; Weissman, I.L. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev. Cell 2006, 11, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Ferkowicz, M.J.; Yoder, M.C. Blood island formation: Longstanding observations and modern interpretations. Exp. Hematol. 2005, 33, 1041–1047. [Google Scholar] [CrossRef]
- Sheng, G. Primitive and definitive erythropoiesis in the yolk sac: A bird’s eye view. Int. J. Dev. Biol. 2010, 54, 1033–1043. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, F.; Nagai, H.; Shin, M.; Sheng, G. Negative regulation of primitive hematopoiesis by the FGF signaling pathway. Blood 2006, 108, 3335–3343. [Google Scholar] [CrossRef] [Green Version]
- Hnilica, L.S. The specificity of histones in chicken erythrocytes. Experientia 1964, 20, 13–14. [Google Scholar] [CrossRef]
- Neelin, J.M.; Callahan, P.X.; Lamb, D.C.; Murray, K. The Histones of Chicken Erythrocyte Nuclei. Can. J. Biochem. 1964, 42, 1743–1752. [Google Scholar] [CrossRef]
- Edwards, L.J.; Hnilica, L.S. The specificity of histones in nucleated erythrocytes. Experientia 1968, 24, 228–229. [Google Scholar] [CrossRef]
- Williams, A.F. DNA synthesis in purified populations of avian erythroid cells. J. Cell Sci. 1972, 10, 27–46. [Google Scholar]
- Rodnan, G.P.; Ebaugh, F.G., Jr.; Fox, M.R. The life span of the red blood cell and the red blood cell volume in the chicken, pigeon and duck as estimated by the use of Na2Cr51O4, with observations on red cell turnover rate in the mammal, bird and reptile. Blood 1957, 12, 355–366. [Google Scholar]
- Altland, P.D.; Brace, K.C. Life span of duck and chicken erythrocyte as determined with C14. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1956, 92, 615–617. [Google Scholar] [CrossRef]
- Nirmalan, G.P.; Robinson, G.A. The survival time of erythrocytes (DF 32 P label) in the Japanese quail. Poult. Sci. 1973, 52, 355–359. [Google Scholar] [CrossRef]
- Rohme, D. Evidence for a relationship between longevity of mammalian species and life spans of normal fibroblasts in vitro and erythrocytes in vivo. Proc. Natl. Acad. Sci. USA 1981, 78, 5009–5013. [Google Scholar] [CrossRef] [Green Version]
- Beutler, E. Williams Hematology, 7th ed.; McGraw-Hill Book Co: New York, NY, USA, 2005. [Google Scholar]
- Bressan, M.; Liu, G.; Mikawa, T. Early mesodermal cues assign avian cardiac pacemaker fate potential in a tertiary heart field. Science 2013, 340, 744–748. [Google Scholar] [CrossRef] [Green Version]
- Van Mierop, L.H. Location of pacemaker in chick embryo heart at the time of initiation of heartbeat. Am. J. Physiol. 1967, 212, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Hirota, A.; Fujii, S.; Kamino, K. Optical monitoring of spontaneous electrical activity of 8-somite embryonic chick heart. Jpn. J. Physiol. 1979, 29, 635–639. [Google Scholar]
- Kamino, K.; Hirota, A.; Fujii, S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature 1981, 290, 595–597. [Google Scholar]
- Satin, J.; Fujii, S.; DeHaan, R.L. Development of cardiac beat rate in early chick embryos is regulated by regional cues. Dev. Biol. 1988, 129, 103–113. [Google Scholar] [CrossRef]
- Pearson, J.T.; Tsudzuki, M.; Nakane, Y.; Akiyama, R.; Tazawa, H. Development of heart rate in the precocial king quail Coturnix chinensis. J. Exp. Biol. 1998, 201, 931–941. [Google Scholar]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef]
- Kelly, R.G.; Buckingham, M.E.; Moorman, A.F. Heart fields and cardiac morphogenesis. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.G.; Brown, N.A.; Buckingham, M.E. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. Cell 2001, 1, 435–440. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, G.; Abu-Issa, R.; de Boer, B.A.; Hutson, M.R.; de Boer, P.A.; Soufan, A.T.; Ruijter, J.M.; Kirby, M.L.; van den Hoff, M.J.; Moorman, A.F. A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ. Res. 2009, 104, 179–188. [Google Scholar] [CrossRef]
- Waldo, K.L.; Kumiski, D.H.; Wallis, K.T.; Stadt, H.A.; Hutson, M.R.; Platt, D.H.; Kirby, M.L. Conotruncal myocardium arises from a secondary heart field. Development 2001, 128, 3179–3188. [Google Scholar]
- Waldo, K.L.; Hutson, M.R.; Ward, C.C.; Zdanowicz, M.; Stadt, H.A.; Kumiski, D.; Abu-Issa, R.; Kirby, M.L. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev. Biol. 2005, 281, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Meilhac, S.M.; Lescroart, F.; Blanpain, C.; Buckingham, M.E. Cardiac cell lineages that form the heart. Cold Spring Harb. Perspect. Med. 2014, 4, a013888. [Google Scholar] [CrossRef] [Green Version]
- DeHaan, R.L. Development of pacemaker tissue in the embryonic heart. Ann. N. Y. Acad. Sci. 1965, 127, 7–18. [Google Scholar]
- Moorman, A.F.M.; Christoffels, V.M. Cardiac Chamber Formation: Development, Genes, and Evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef]
- Paff, G.H.; Boucek, R.J.; Harrell, T.C. Observations on the development of the electrocardiogram. Anat. Rec. 1968, 160, 575–582. [Google Scholar] [CrossRef]
- Taber, L.A.; Voronov, D.A.; Ramasubramanian, A. The role of mechanical forces in the torsional component of cardiac looping. Ann. N. Y. Acad. Sci. 2010, 1188, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Le Garrec, J.F.; Dominguez, J.N.; Desgrange, A.; Ivanovitch, K.D.; Raphael, E.; Bangham, J.A.; Torres, M.; Coen, E.; Mohun, T.J.; Meilhac, S.M. A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics. eLife 2017, 6, e28951. [Google Scholar] [CrossRef] [Green Version]
- Chuck, E.T.; Freeman, D.M.; Watanabe, M.; Rosenbaum, D.S. Changing activation sequence in the embryonic chick heart. Implications for the development of the His-Purkinje system. Circ. Res. 1997, 81, 470–476. [Google Scholar] [CrossRef]
- Gourdie, R.G.; Harris, B.S.; Bond, J.; Justus, C.; Hewett, K.W.; O’Brien, T.X.; Thompson, R.P.; Sedmera, D. Development of the cardiac pacemaking and conduction system. Birth Defects Res. C Embryo Today 2003, 69, 46–57. [Google Scholar]
- Rentschler, S.; Vaidya, D.M.; Tamaddon, H.; Degenhardt, K.; Sassoon, D.; Morley, G.E.; Jalife, J.; Fishman, G.I. Visualization and functional characterization of the developing murine cardiac conduction system. Development 2001, 128, 1785–1792. [Google Scholar]
- Gourdie, R.G.; Mima, T.; Thompson, R.P.; Mikawa, T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development 1995, 121, 1423–1431. [Google Scholar]
- Patten, B.M. The formation of the cardiac loop in the chick. Am. J. Anat. 1922, 30, 373–397. [Google Scholar]
- Manner, J. Cardiac looping in the chick embryo: A morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat. Rec. 2000, 259, 248–262. [Google Scholar]
- Taber, L.A. Biophysical mechanisms of cardiac looping. Int. J. Dev. Biol. 2006, 50, 323–332. [Google Scholar]
- Manner, J. On the form problem of embryonic heart loops, its geometrical solutions, and a new biophysical concept of cardiac looping. Ann. Anat. 2013, 195, 312–323. [Google Scholar] [CrossRef]
- Mercola, M.; Levin, M. Left-right asymmetry determination in vertebrates. Annu. Rev. Cell Dev. Biol. 2001, 17, 779–805. [Google Scholar] [CrossRef] [Green Version]
- Franco, D.; Campione, M. The role of Pitx2 during cardiac development. Linking left-right signaling and congenital heart diseases. Trends Cardiovasc. Med. 2003, 13, 157–163. [Google Scholar]
- Ramsdell, A.F. Left-right asymmetry and congenital cardiac defects: Getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 2005, 288, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Ocana, O.H.; Coskun, H.; Minguillon, C.; Murawala, P.; Tanaka, E.M.; Galceran, J.; Munoz-Chapuli, R.; Nieto, M.A. A right-handed signalling pathway drives heart looping in vertebrates. Nature 2017, 549, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Rago, L.; Castroviejo, N.; Fazilaty, H.; Garcia-Asencio, F.; Ocana, O.H.; Galceran, J.; Nieto, M.A. MicroRNAs Establish the Right-Handed Dominance of the Heart Laterality Pathway in Vertebrates. Dev. Cell 2019, 51, 446–459. [Google Scholar] [CrossRef]
- Jenkins, M.; Watanabe, M.; Rollins, A. Longitudinal imaging of heart development with optical coherence tomography. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1166–1175. [Google Scholar]
- Rugonyi, S.; Shaut, C.; Liu, A.; Thornburg, K.; Wang, R.K. Changes in wall motion and blood flow in the outflow tract of chick embryonic hearts observed with optical coherence tomography after outflow tract banding and vitelline-vein ligation. Phys. Med. Biol. 2008, 53, 5077–5091. [Google Scholar]
- Ford, S.M.; McPheeters, M.T.; Wang, Y.T.; Ma, P.; Gu, S.; Strainic, J.; Snyder, C.; Rollins, A.M.; Watanabe, M.; Jenkins, M.W. Increased regurgitant flow causes endocardial cushion defects in an avian embryonic model of congenital heart disease. Congenit. Heart Dis. 2017, 12, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Männer, J.; Männer, T.M.; Yelbuz, T.M. Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos. J. Cardiovasc. Dev. Dis. 2019, 6, 12. [Google Scholar]
- Garita, B.; Jenkins, M.W.; Han, M.; Zhou, C.; VanAuker, M.; Rollins, A.M.; Watanabe, M.; Fujimoto, J.G.; Linask, K.K. Blood flow dynamics of one cardiac cycle and relationship to mechanotransduction and trabeculation during heart looping. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H879–H891. [Google Scholar]
- Taber, L.; Zhang, J.; Perucchio, R. Computational model for the transition from peristaltic to pulsatile flow in the embryonic heart tube. J. Biomech. Eng. 2007, 129, 441–449. [Google Scholar]
- Midgett, M.; Chivukula, V.K.; Dorn, C.; Wallace, S.; Rugonyi, S. Blood flow through the embryonic heart outflow tract during cardiac looping in HH13–HH18 chicken embryos. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef]
- Markwald, R.R.; Fitzharris, T.P.; Manasek, F.J. Structural development of endocardial cushions. Am. J. Anat. 1976, 148, 85–120. [Google Scholar]
- Camenisch, T.D.; Runyan, R.B.; Markwald, R.R. Chapter 6.1—Molecular Regulation of Cushion Morphogenesis. In Heart Development and Regeneration; Academic Press: Cambridge, MA, USA, 2010; pp. 363–387. [Google Scholar]
- Runyan, R.B.; Markwald, R.R. Invasion of mesenchyme into three-dimensional collagen gels: A regional and temporal analysis of interaction in embryonic heart tissue. Dev. Biol. 1983, 95, 108–114. [Google Scholar]
- Potts, J.D.; Runyan, R.B. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor β. Dev. Biol. 1989, 134, 392–401. [Google Scholar] [CrossRef]
- Runyan, R.B.; Potts, J.D.; Sharma, R.V.; Loeber, C.P.; Chiang, J.J.; Bhalla, R.C. Signal transduction of a tissue interaction during embryonic heart development. Cell Regul. 1990, 1, 301–313. [Google Scholar]
- Runyan, R.B.; Potts, J.D.; Weeks, D.L.; Sharma, R.V.; Loeber, C.L.; Chiang, J.J.; Bhalla, R.C. Tissue Interaction and Signal Transduction in the Atrioventricular Canal of the Embryonic Hearta. Ann. N. Y. Acad. Sci. 1990, 588, 442–443. [Google Scholar] [CrossRef]
- Goodwin, R.L.; Nesbitt, T.; Price, R.L.; Wells, J.C.; Yost, M.J.; Potts, J.D. A three-dimensional model system of valvulogenesis. Dev. Dyn. 2005, 233, 122–129. [Google Scholar]
- Garside, V.C.; Chang, A.C.; Karsan, A.; Hoodless, P.A. Co-ordinating Notch, BMP, and TGFβ Signalling During Heart Valve Development. Cell. Mol. Life Sci. 2013, 70, 2899–2917. [Google Scholar] [CrossRef]
- Tan, H.; Biechler, S.; Junor, L.; Yost, M.J.; Dean, D.; Li, J.; Potts, J.D.; Goodwin, R.L. Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Dev. Biol. 2013, 374, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Biechler, S.V.; Junor, L.; Evans, A.N.; Eberth, J.F.; Price, R.L.; Potts, J.D.; Yost, M.J.; Goodwin, R.L. The impact of flow-induced forces on the morphogenesis of the outflow tract. Front. Physiol. 2014, 5, 225. [Google Scholar] [CrossRef]
- Menon, V.; Eberth, J.; Goodwin, R.; Potts, J. Altered Hemodynamics in the Embryonic Heart Affects Outflow Valve Development. J. Cardiovasc. Dev. Dis. 2015, 2, 108. [Google Scholar]
- Menon, V.; Eberth, J.F.; Junor, L.; Potts, A.J.; Belhaj, M.; Dipette, D.J.; Jenkins, M.W.; Potts, J.D. Removing vessel constriction on the embryonic heart results in changes in valve gene expression, morphology, and hemodynamics. Dev. Dyn. 2018, 247, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, J.D. Stress, strain, and mechanotransduction in cells. J. Biomech. Eng. 2001, 123, 638–641. [Google Scholar]
- Taber, L.A.; Humphrey, J.D. Stress-modulated growth, residual stress, and vascular heterogeneity. J. Biomech. Eng. 2001, 123, 528–535. [Google Scholar]
- Humphrey, J.D.; Rajagopal, K.R. A constrained mixture model for arterial adaptations to a sustained step change in blood flow. Biomech. Modeling Mechanobiol. 2003, 2, 109–126. [Google Scholar]
- Karunamuni, G.; Gu, S.; Doughman, Y.Q.; Peterson, L.M.; Mai, K.; McHale, Q.; Jenkins, M.W.; Linask, K.K.; Rollins, A.M.; Watanabe, M. Ethanol exposure alters early cardiac function in the looping heart: A mechanism for congenital heart defects? Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H414–H421. [Google Scholar] [CrossRef] [Green Version]
- Makwana, O.; King, N.M.P.; Ahles, L.; Selmin, O.; Granzier, H.L.; Runyan, R.B. Exposure to low dose trichloroethylene alters sheer stress gene expression and function in the developing chick heart. Cardiovasc. Toxicol. 2010, 10, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Scott-Dreschel, D.E.; Rugonyi, S.; Marks, D.L.; Thornburg, K.L.; Hinds, M.T. Hyperglycemia slows embryonic growth and suppresses cell cycle via Cyclin D1 and P21. Diabetes 2013, 62, 234–242. [Google Scholar]
- Lawson, T.; Scott-Drechsel, D.; Chivukula, V.; Rugonyi, S.; Thornburg, K.; Hinds, M. Hyperglycemia Alters the Structure and Hemodynamics of the Developing Embryonic Heart. J. Cardiovasc. Dev. Dis. 2018, 5, 13. [Google Scholar]
- Midgett, M.; Goenezen, S.; Rugonyi, S. Blood flow dynamics reflect degree of outflow tract banding in Hamburger-Hamilton stage 18 chicken embryos. J. R. Soc. Interface 2014, 11, 20140643. [Google Scholar]
- Jensen, B.; Wang, T.; Moorman, A.F.M. Evolution and Development of the Atrial Septum. Anat. Rec. 2019, 302, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Burns, T.; Yang, Y.; Hiriart, E.; Wessels, A. The Dorsal Mesenchymal Protrusion and the Pathogenesis of Atrioventricular Septal Defects. J. Cardiovasc. Dev. Dis. 2016, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Morse, D.; Rogers, C.; McCann, P. Atrial septation in the chick and rat: A review. J. Submicrosc. Cytol. Pathol. 1984, 16, 259–272. [Google Scholar]
- Dyer, L.A.; Kirby, M.L. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev. Biol. 2009, 330, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Dyer, L.A.; Kirby, M.L. The role of secondary heart field in cardiac development. Dev. Biol. 2009, 336, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, S.; Piacentino, M.L.; Vieceli, F.M.; Bronner, M.E. Optimization of CRISPR/Cas9 genome editing for loss-of-function in the early chick embryo. Dev. Biol. 2017, 432, 86–97. [Google Scholar] [CrossRef]
- Williams, R.M.; Senanayake, U.; Artibani, M.; Taylor, G.; Wells, D.; Ahmed, A.A.; Sauka-Spengler, T. Genome and epigenome engineering CRISPR toolkit for in vivo modulation of cis-regulatory interactions and gene expression in the chicken embryo. Development 2018, 145. [Google Scholar] [CrossRef] [Green Version]
- Abu-Bonsrah, K.D.; Zhang, D.; Newgreen, D.F. CRISPR/Cas9 Targets Chicken Embryonic Somatic Cells In Vitro and In Vivo and generates Phenotypic Abnormalities. Sci. Rep. 2016, 6, 34524. [Google Scholar] [CrossRef] [Green Version]
- Goenezen, S.; Rennie, M.; Rugonyi, S. Biomechanics of Early cardiac Development. Biomech. Modeling Mechanobiol. 2012, 11, 1187–1204. [Google Scholar]
- Reese, D.E.; Mikawa, T.; Bader, D.M. Development of the Coronary Vessel System. Circ. Res. 2002, 91, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Bernanke, D.H.; Velkey, J.M. Development of the coronary blood supply: Changing concepts and current ideas. Anat. Rec. 2002, 269, 198–208. [Google Scholar] [CrossRef]
- Groot, A.C.G.-d.; Peeters, M.-P.F.M.V.; Mentink, M.M.T.; Gourdie, R.G.; Poelmann, R.E. Epicardium-Derived Cells Contribute a Novel Population to the Myocardial Wall and the Atrioventricular Cushions. Circ. Res. 1998, 82, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Männer, J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 1999, 255, 212–226. [Google Scholar] [CrossRef]
- Paradis, A.N.; Gay, M.S.; Zhang, L. Binucleation of cardiomyocytes: The transition from a proliferative to a terminally differentiated state. Drug Discov. Today 2014, 19, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Jeter, J.R.; Cameron, I.L. Cell proliferation patterns during cytodifferentiation in embryonic chick tissues: Liver, heart and erythrocytes. J. Embryol. Exp. Morphol. 1971, 25, 405–422. [Google Scholar]
- Li, F.; McNelis, M.R.; Lustig, K.; Gerdes, A.M. Hyperplasia and hypertrophy of chicken cardiac myocytes during posthatching development. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1997, 273, R518–R526. [Google Scholar] [CrossRef]
- Dzialowski, E.M. Comparative physiology of the ductus arteriosus among vertebrates. Semin. Perinatol. 2018, 42, 203–211. [Google Scholar] [CrossRef]
- Vostarek, F.; Svatunkova, J.; Sedmera, D. Acute temperature effects on function of the chick embryonic heart. Acta Physiol. 2016, 217, 276–286. [Google Scholar] [CrossRef]
- Kockova, R.; Svatunkova, J.; Novotny, J.; Hejnova, L.; Ostadal, B.; Sedmera, D. Heart rate changes mediate the embryotoxic effect of antiarrhythmic drugs in the chick embryo. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H895–H902. [Google Scholar] [CrossRef] [Green Version]
- Liebling, M.; Forouhar, A.S.; Gharib, M.; Fraser, S.E.; Dickinson, M.E. Four-dimensional cardiac imaging in living embryos via postacquisition synchronization of nongated slice sequences. J. Biomed. Opt. 2005, 10, 054001. [Google Scholar] [CrossRef] [Green Version]
- Ohn, J.; Tsai, H.J.; Liebling, M. Joint dynamic imaging of morphogenesis and function in the developing heart. Organogenesis 2009, 5, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Ohn, J.; Yang, J.; Fraser, S.E.; Lansford, R.; Liebling, M. High-speed multicolor microscopy of repeating dynamic processes. Genesis 2011, 49, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, V.; Truong, T.V.; Trinh le, A.; Holland, D.B.; Liebling, M.; Fraser, S.E. Dynamic structure and protein expression of the live embryonic heart captured by 2-photon light sheet microscopy and retrospective registration. Biomed. Opt. Express 2015, 6, 2056–2066. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Wang, R.K.; Thornburg, K.; Rugonyi, S. Efficient post-acquisition synchronization of 4D non-gated cardiac images obtained from optical coherence tomography: Application to 4D reconstruction of the chick embryonic heart. J. Biomed. Opt. 2009, 14, 044020. [Google Scholar]
- Forouhar, A.S.; Liebling, M.; Hickerson, A.; Nasiraei-Moghaddam, A.; Tsai, H.J.; Hove, J.R.; Fraser, S.E.; Dickinson, M.E.; Gharib, M. The embryonic vertebrate heart tube is a dynamic suction pump. Science 2006, 312, 751–753. [Google Scholar] [CrossRef] [Green Version]
- Vermot, J.; Forouhar, A.S.; Liebling, M.; Wu, D.; Plummer, D.; Gharib, M.; Fraser, S.E. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol. 2009, 7, e1000246. [Google Scholar] [CrossRef] [Green Version]
- Goktas, S.; Uslu, F.E.; Kowalski, W.J.; Ermek, E.; Keller, B.B.; Pekkan, K. Time-Series Interactions of Gene Expression, Vascular Growth and Hemodynamics during Early Embryonic Arterial Development. PLoS ONE 2016, 11, e0161611. [Google Scholar] [CrossRef]
- Varner, V.D.; Voronov, D.A.; Taber, L.A. Mechanics of head fold formation: Investigating tissue-level forces during early development. Development 2010, 137, 3801–3811. [Google Scholar] [CrossRef] [Green Version]
- Filas, B.A.; Efimov, I.R.; Taber, L.A. Optical coherence tomography as a tool for measuring morphogenetic deformation of the looping heart. Anat. Rec. 2007, 290, 1057–1068. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Latacha, K.S.; Benjamin, J.M.; Voronov, D.A.; Ravi, A.; Taber, L.A. Computational model for early cardiac looping. Ann. Biomed. Eng. 2006, 34, 1655–1669. [Google Scholar]
- Tobita, K.; Keller, B.B. Maturation of end-systolic stress-strain relations in chick embryonic myocardium. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H216–H224. [Google Scholar]
- Tobita, K.; Schroder, E.; Tinney, J.; Garrison, J.; Keller, B.B. Regional passive ventricular stress-strain relations during development of altered loads in chick embryo. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2386–H2396. [Google Scholar]
- Kowalski, W.J.; Pekkan, K.; Tinney, J.P.; Keller, B.B. Investigating developmental cardiovascular biomechanics and the origins of congenital heart defects. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Sedmera, D.; Pexieder, T.; Hu, N.; Clark, E.B. Developmental changes in the myocardial architecture of the chick. Anat. Rec. 1997, 248, 421–432. [Google Scholar]
- McQuinn, T.C.; Bratoeva, M.; deAlmeida, A.; Remond, M.; Thompson, R.P.; Sedmera, D. High-frequency ultrasonographic imaging of avian cardiovascular development. Dev. Dyn. 2007, 236, 3503–3513. [Google Scholar]
- Fujimoto, J.G. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat. Biotechnol. 2003, 21, 1361–1367. [Google Scholar]
- Jenkins, M.W.; Adler, D.C.; Gargesha, M.; Huber, R.; Rothenberg, F.; Belding, J.; Watanabe, M.; Wilson, D.L.; Fujimoto, J.G.; Rollins, A.M. Ultrahigh-speed optical coherence tomography imaging and visualization of the embryonic avian heart using a buffered fourier domain mode locked laser. Opt. Express 2007, 15, 6251–6267. [Google Scholar]
- Manner, J.; Thrane, L.; Norozi, K.; Yelbuz, T.M. High-resolution in vivo imaging of the cross-sectional deformations of contracting embryonic heart loops using optical coherence tomography. Dev. Dyn. 2008, 237, 953–961. [Google Scholar]
- Larina, I.V.; Ivers, S.; Syed, S.; Dickinson, M.E.; Larin, K.V. Hemodynamic measurements from individual blood cells in early mammalian embryos with Doppler swept source OCT. Opt. Lett. 2009, 34, 986–988. [Google Scholar]
- Larina, I.V.; Larin, K.V.; Justice, M.J.; Dickinson, M.E. Optical Coherence Tomography for live imaging of mammalian development. Curr. Opin. Genet. Dev. 2011, 21, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.M.; Rothenberg, F.G.; Shepherd, N.; Izatt, J.A. In vivo spectral domain optical coherence tomography volumetric imaging and spectral Doppler velocimetry of early stage embryonic chicken heart development. J. Opt. Soc. Am. A 2008, 25, 3134–3143. [Google Scholar]





| Event | Avian Hamburger-Hamilton Stages | Human Post-Ovulatory Days | Mouse Days Post-Coitum |
|---|---|---|---|
| Formation of heart tube | HH9 | ~22D | E8 |
| Heartbeat onset | HH10 | ~22D | E8.5 |
| Tubular heart looping | HH10-HH24 | 22D–30D | E8–E10 |
| Valve formation | HH24-HH34 | 37D–47D | E12–E17 |
| Coronary system formation | HH18-HH26 | 33D–16 weeks | E10.5–E12.5 |
| Atrial septation | HH16-HH34 | 41D–44D | E10.0–E14.5 |
| Ventricular septation | HH19-HH34 | 37D–44D | E11.5–E13.5 |
| Outflow tract septation | HH25-HH34 | 30D–47D | E11.5–E13.5 |
| Fully formed heart | HH34 | 16 weeks | E15.5 |
| Heart Similarities | Heart Differences |
|---|---|
| Hearts have to support resting and aerobic activities | Birds have higher metabolic needs. Resting heart rate faster in avian than humans and mice |
| Four-chambered heart | Chambers are larger, more muscular, and smoother in avian than mammals; heart size relative to body mass is larger in birds |
| Four heart valves. Semilunar valves (pulmonary and aortic valves) are tricuspid in both avian and humans | Right AV valve is a single spiral flap in birds, fibrous tricuspid valve in humans. Left AV is tricuspid in avian, but bicuspid in humans (mitral valve) |
| Coronary system runs through the surface of the heart, in the epicardium, and branches into the myocardium | The proepicardial organ that gives rise to the epicardium is continuous in birds, forming a sheet; but consists of groups of epithelial cells in mice that eventually form a continuous sheet |
| Transport of blood includes pulmonary and systemic circulations, with separation of oxygenated and deoxygenated blood | Right aortic arch develops in avian; left aortic arch in mammals |
| Foramen ovale closes after hatching or birth | Atrial septum formed by the septum primum and dorsal mesenchymal protrusion in birds; in mammals, in addition, there is a septum secundum that also contributes to atrial septation |
| Ductus arteriosi close after hatching or birth | Paired ductus arteriosus in avians (left and right ductus arteriosus); single ductus arteriosus in mammals |
| Cardiomyocytes, the heart muscle cells, proliferate during developmental stages in both avian and mammals increasing the heart size | Shortly after birth, mammal cardiomyocytes binucleate and stop proliferating. Further heart growth is due to volume increase. Avian cardiomyocytes continue to proliferate after hatching, and binucleate at slower rates than mammals. Binucleated avian cardiomyocytes can proliferate. Cardiac growth is due to both proliferation and volume increase |
| Red blood cells are present in both avian and mammals to supply oxygen to organs | Red blood cells are nucleated in avian; not nucleated in mammals, but with a larger time span than in avians |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lansford, R.; Rugonyi, S. Follow Me! A Tale of Avian Heart Development with Comparisons to Mammal Heart Development. J. Cardiovasc. Dev. Dis. 2020, 7, 8. https://doi.org/10.3390/jcdd7010008
Lansford R, Rugonyi S. Follow Me! A Tale of Avian Heart Development with Comparisons to Mammal Heart Development. Journal of Cardiovascular Development and Disease. 2020; 7(1):8. https://doi.org/10.3390/jcdd7010008
Chicago/Turabian StyleLansford, Rusty, and Sandra Rugonyi. 2020. "Follow Me! A Tale of Avian Heart Development with Comparisons to Mammal Heart Development" Journal of Cardiovascular Development and Disease 7, no. 1: 8. https://doi.org/10.3390/jcdd7010008





