Validating the Paradigm That Biomechanical Forces Regulate Embryonic Cardiovascular Morphogenesis and Are Fundamental in the Etiology of Congenital Heart Disease
Abstract
:1. Introduction
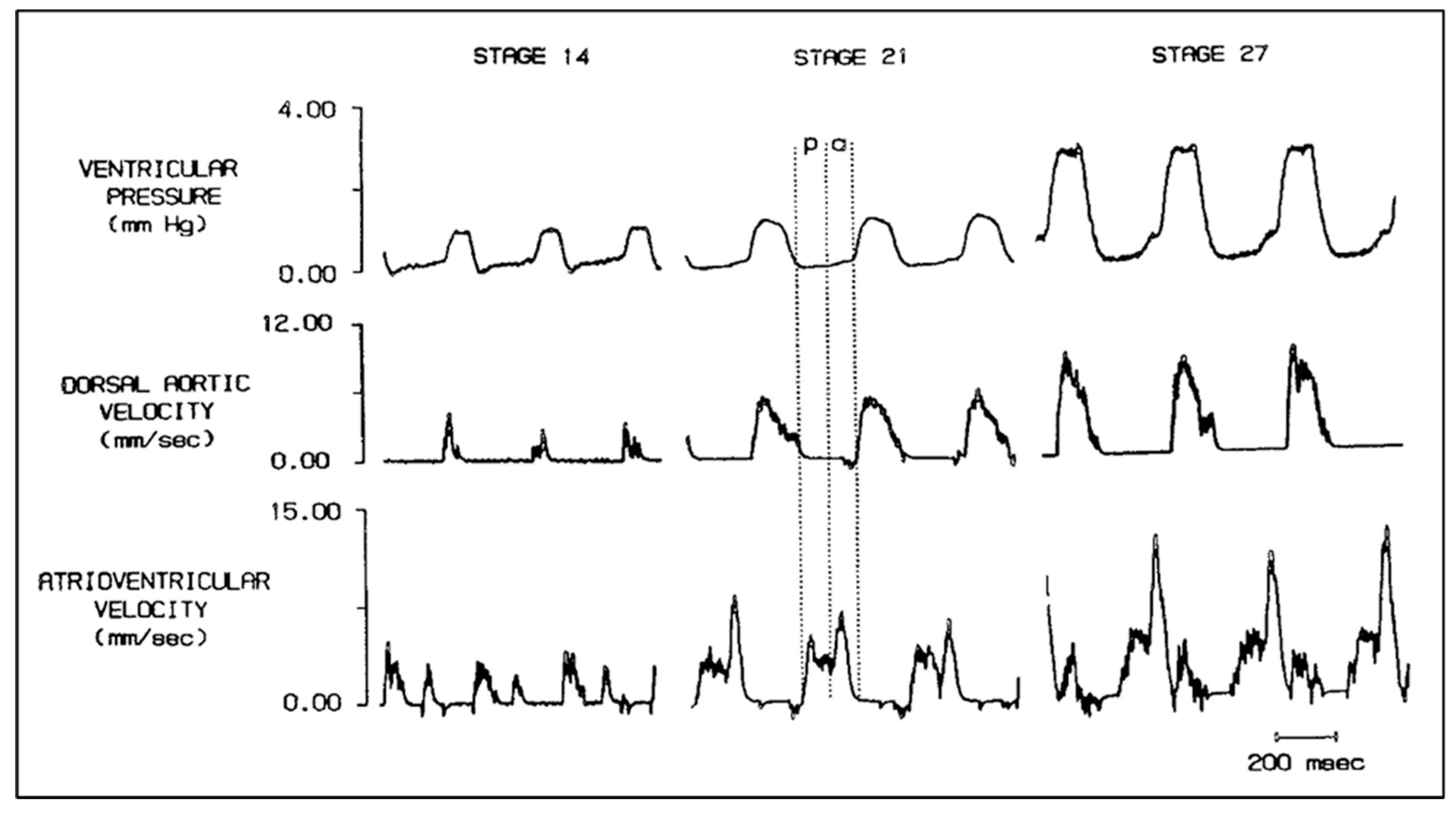
2. Quantifying Embryonic CV Structure–Function Relationships Using Servo-Null Pressure and Pulsed-Doppler Velocity Recordings
3. Applying Adult Ventricular Biomechanics Paradigms to the Rapidly Developing Embryonic Chick CV System
4. Initial Observations on Embryonic Ventricular and Atrial Pressure–Area Relations
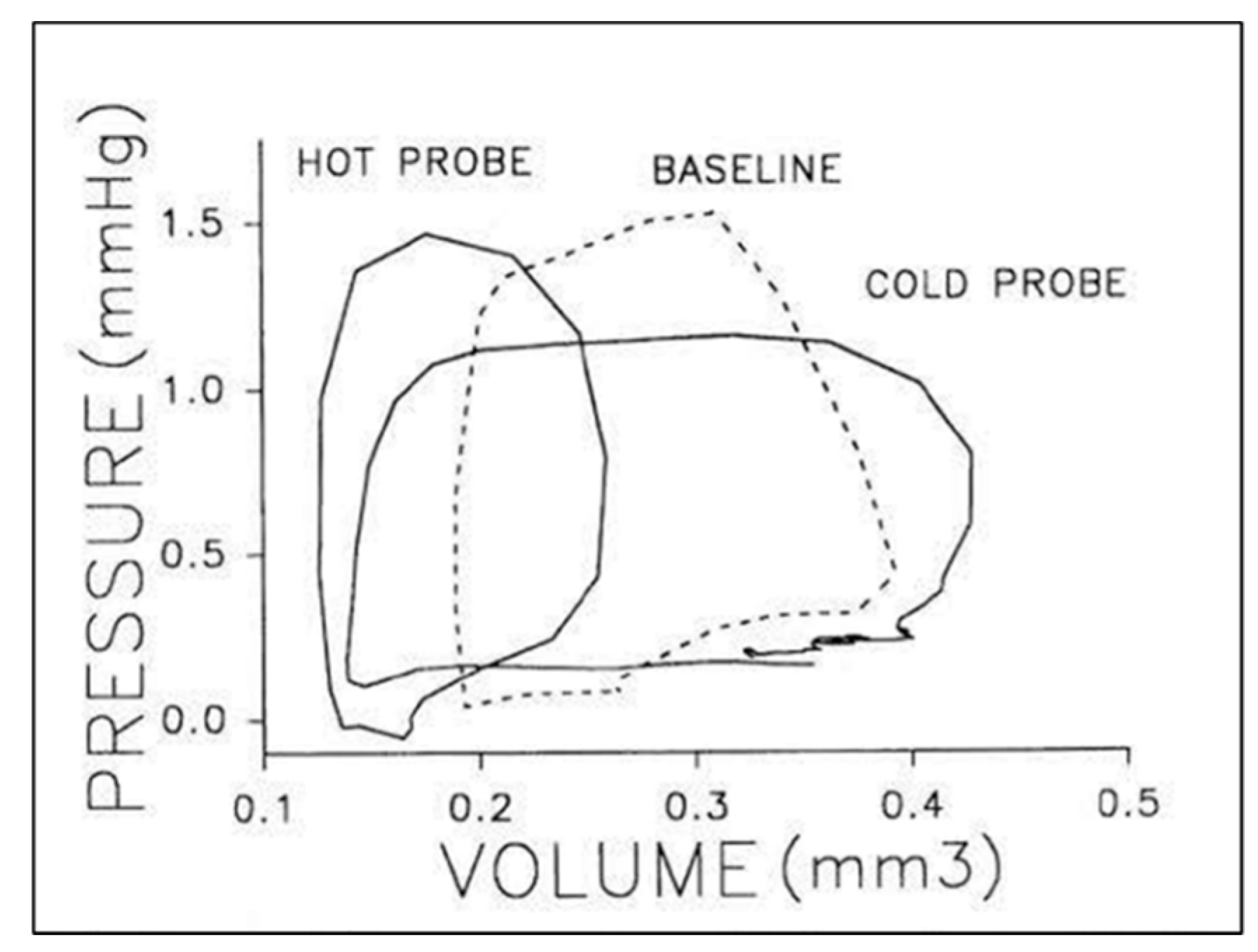
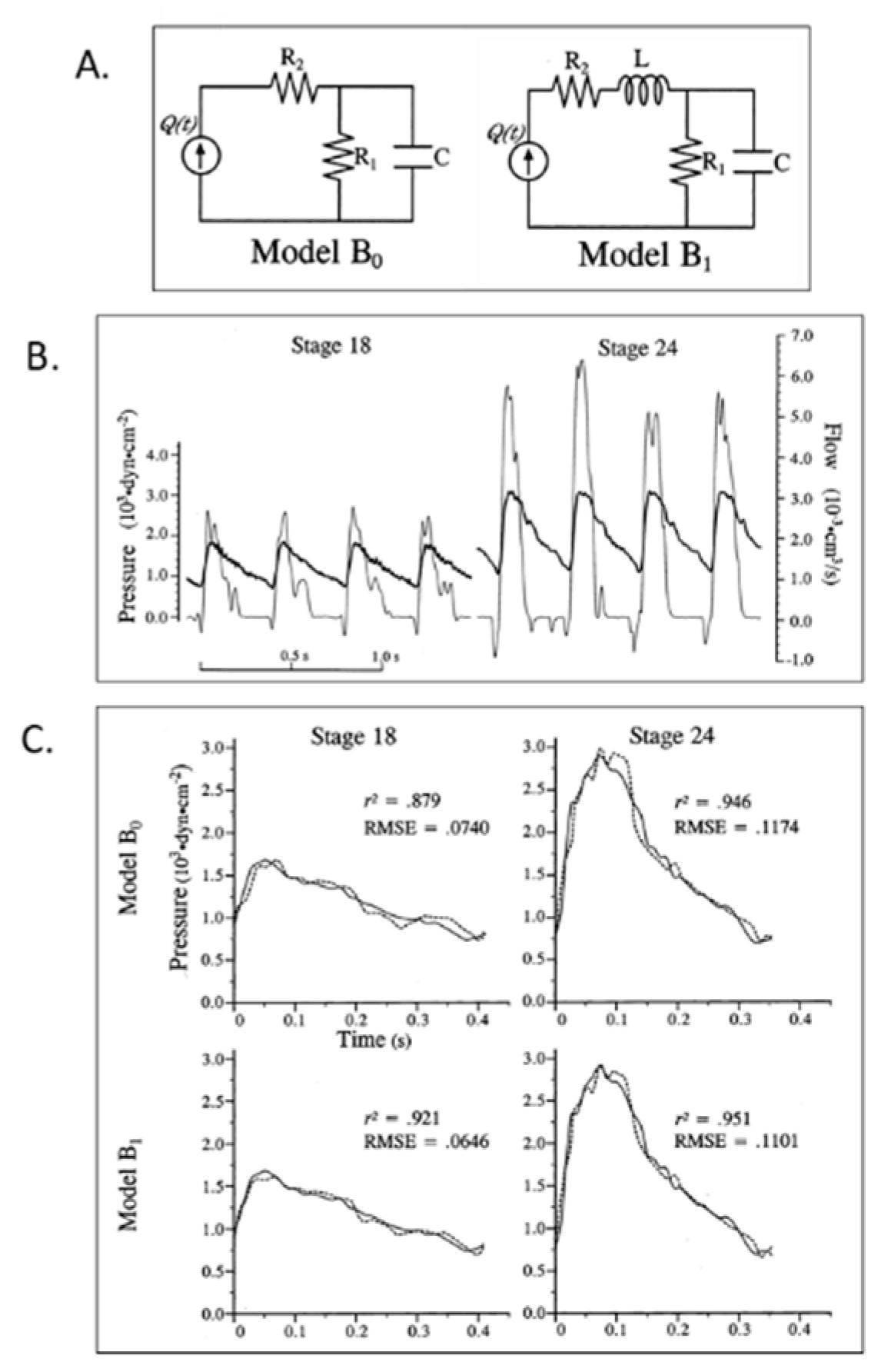
5. Embryonic Hemodynamic and Hydraulic Ventricular–Vascular Coupling
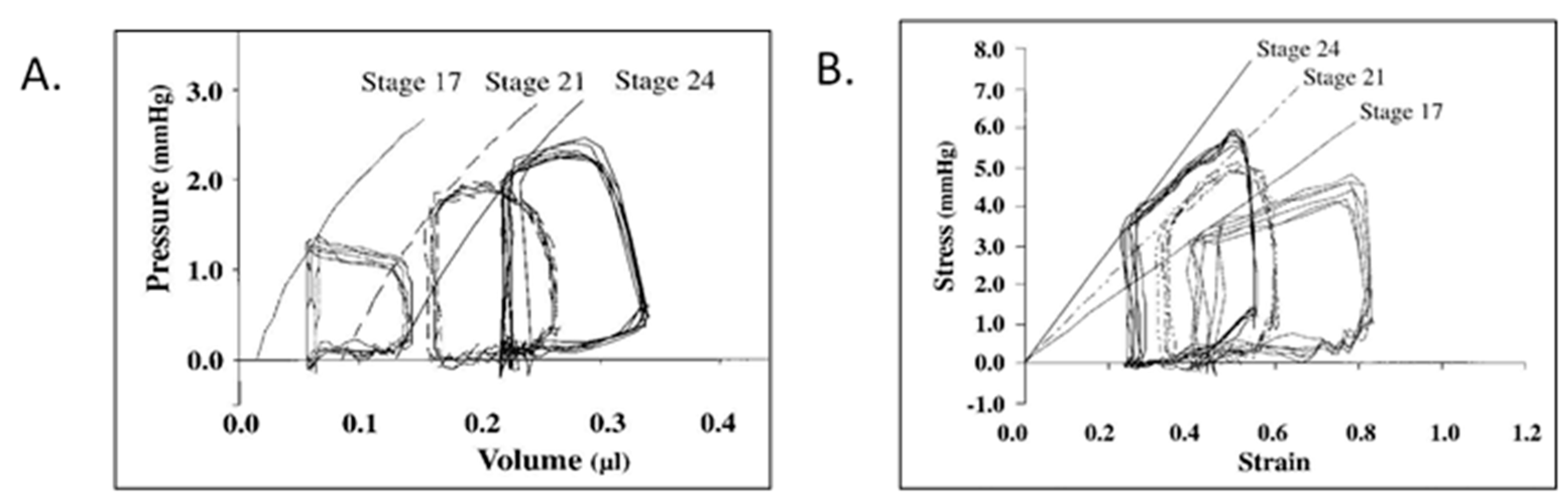
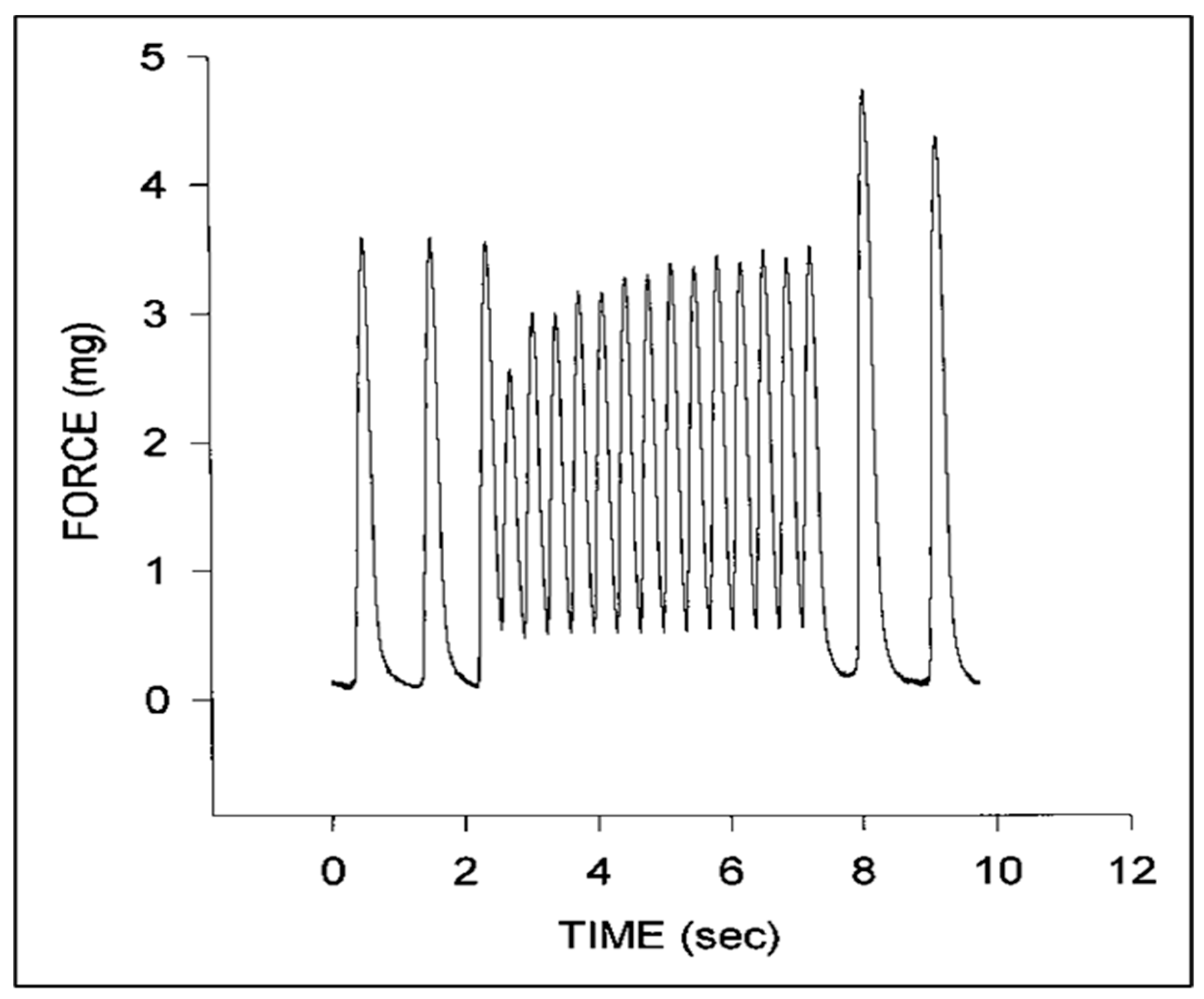
6. Embryonic Ventricular and Vascular Biomechanics
7. Chronic Interventional Models Investigate the Relationships between Embryonic Hemodynamics and Morphogenesis
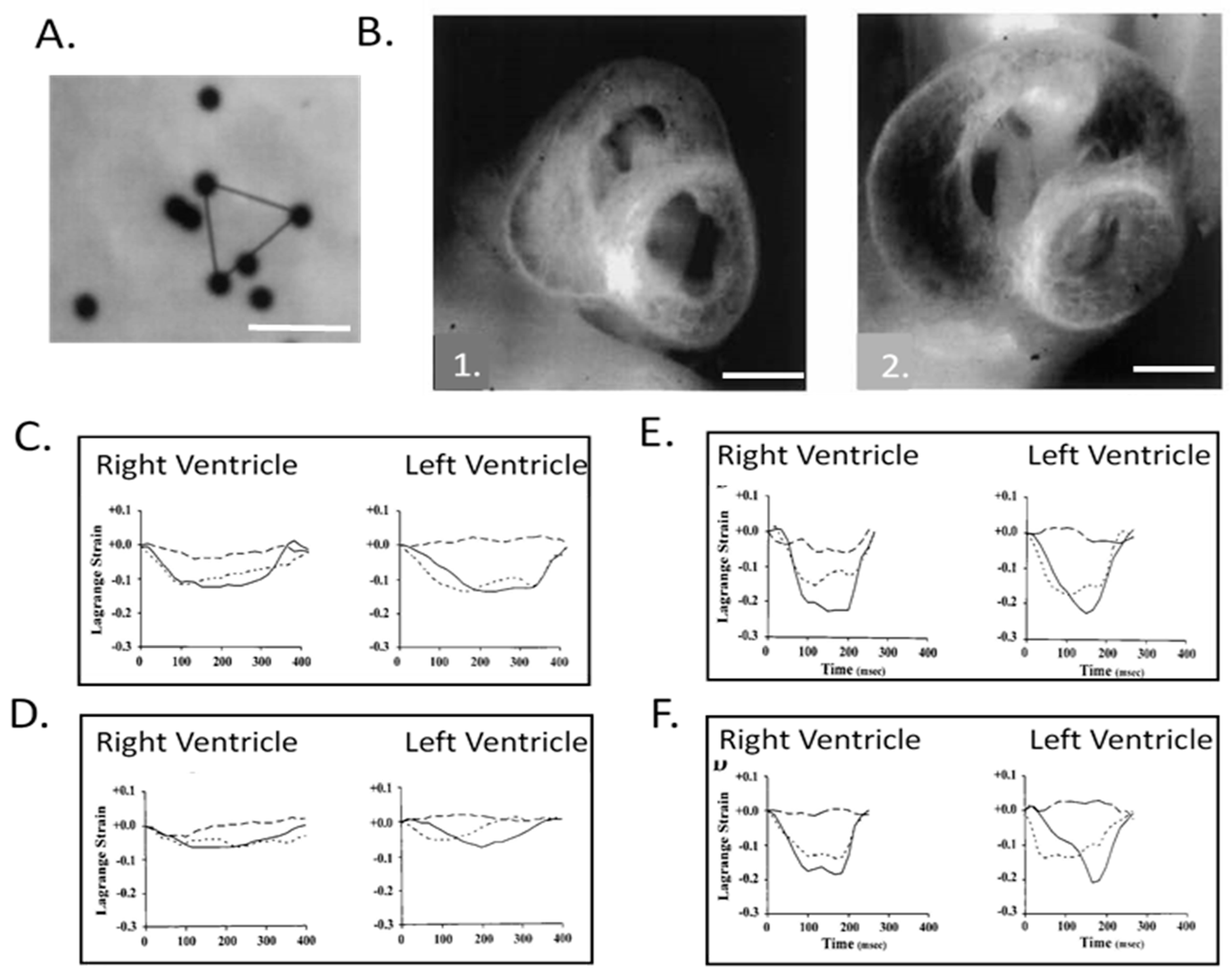
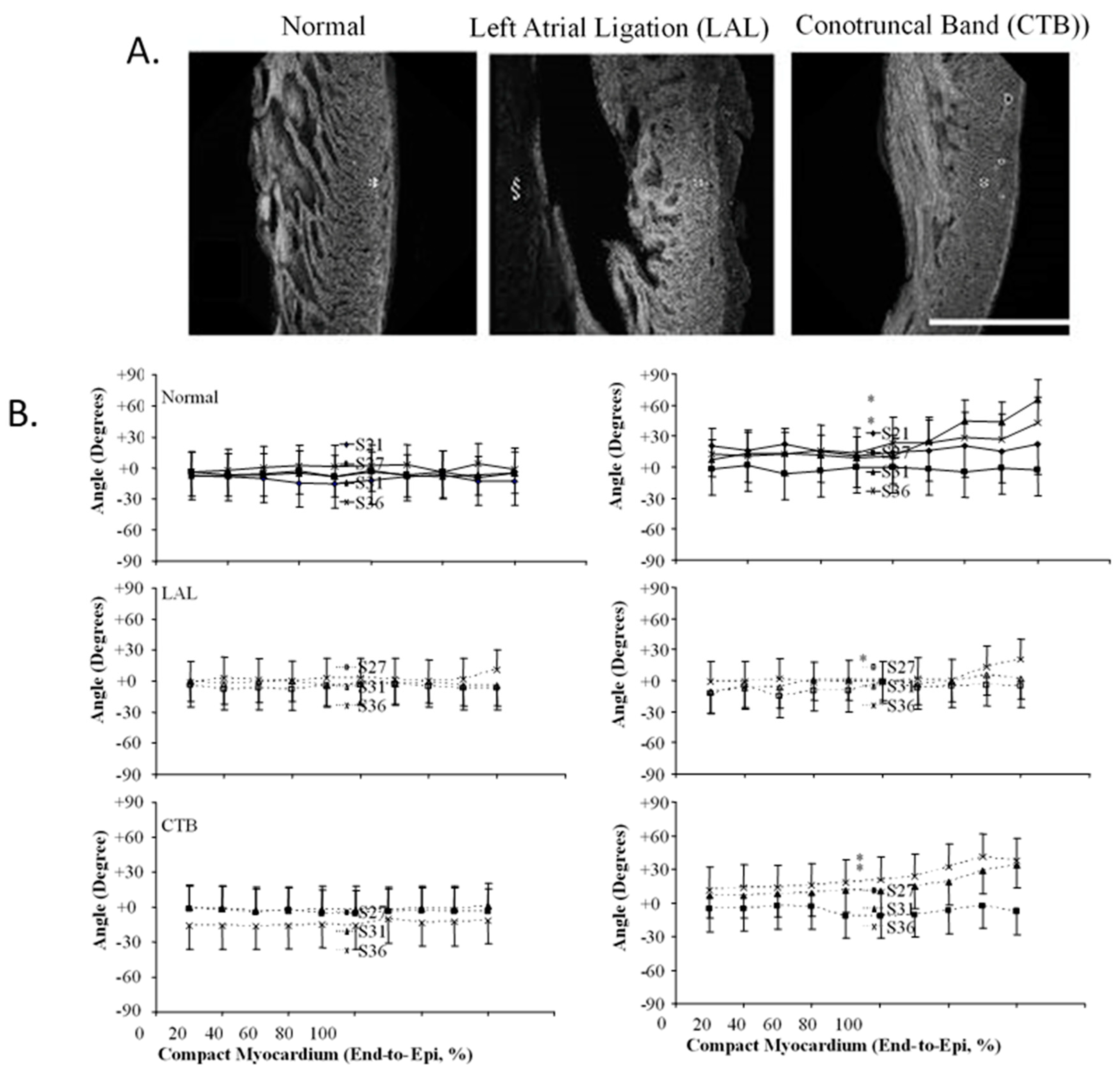
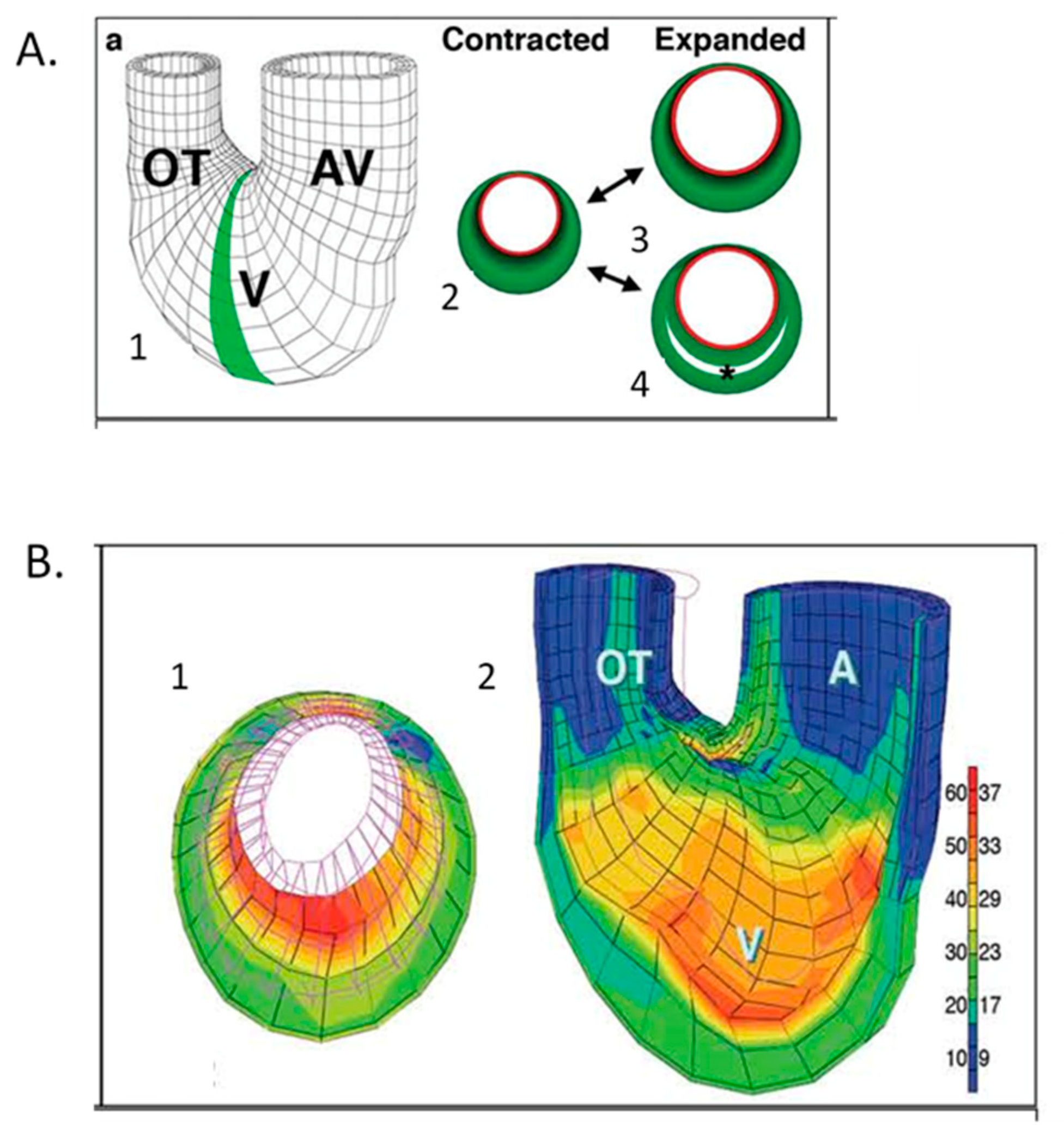
8. Expanding Developmental Cardiovascular Biomechanics Paradigms in Model Systems
9. Future Horizons
Funding
Acknowledgments
Conflicts of Interest
References
- Patten, B.M. The Early Embryology of The Chick; P. Blakiston’s Son & Co: Philadelphia, PA, USA, 1920; p. 228. [Google Scholar]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Romanoff, A.L. The Avian Embryo; Structural and Functional Development; Macmillan: New York, NY, USA, 1960. [Google Scholar]
- Manasek, F.J. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J. Morphol. 1968, 125, 329–365. [Google Scholar] [CrossRef]
- Sissman, N.J. Developmental landmarks in cardiac morphogenesis: Comparative chronology. Am. J. Cardiol. 1970, 25, 141–148. [Google Scholar] [CrossRef]
- Pexieder, T. Mechanisms of Cardiac Morphogenesis and Teratogenesis; Raven Press: New York, NY, USA, 1981. [Google Scholar]
- Sedmera, D.; Pexieder, T.; Hu, N.; Clark, E.B. Developmental changes in the myocardial architecture of the chick. Anat. Rec. 1997, 248, 421–432. [Google Scholar] [CrossRef]
- Sedmera, D.; Pexieder, T.; Vuillemin, M.; Thompson, R.P.; Anderson, R.H. Developmental patterning of the myocardium. Anat. Rec. 2000, 258, 319–337. [Google Scholar] [CrossRef]
- Szepsenwol, J. Electrical excitability and spontaneous activity in explants of skeletal and heart muscle of chick embryos. Anat. Rec. 1947, 98, 67–85. [Google Scholar] [CrossRef]
- Dufourm, J.J.; Posternak, O.J.M. Chronotropic effects of acetylcholine on the chick embryo heart. Helv. Physiol. Pharmacol. Acta (French) 1960, 18, 563–580. [Google Scholar]
- DeHaan, R.L. Development of pacemaker tissue in the embryonic heart. Ann. NY Acad. Sci. 1965, 127, 7–18. [Google Scholar] [CrossRef]
- Barry, A. The functional significance of the cardiac jelly in the tubular heart of the chick embryo. Anat. Rec. 1948, 102, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Patten, B.M.; Kramer, T.C.; Barry, A. Valvular action in the embryonic chick heart by localized apposition of endocardial masses. Anat. Rec. 1948, 102, 299–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, A.F.W. The heart output of the chick embryo. J. R. Microsc. Soc. 1949, 69, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Jaffee, O.C. Hemodynamic factors in the development of the chick embryo heart. Anat. Rec. 1965, 151, 69–75. [Google Scholar] [CrossRef]
- Jaffee, O.C. Hemodynamics and cardiogenesis: The effects of physiologic factors on cardiac development. Birth Defects Orig. Artic. Ser. 1978, 14, 393–404. [Google Scholar] [PubMed]
- Boucek, R.J.; Murphy, W.P., Jr.; Paff, G.H. Electrical and mechanical properties of chick embryo heart chambers. Circ. Res. 1959, 7, 787–793. [Google Scholar] [CrossRef] [Green Version]
- De La Cruz, M.V.; Campillo, S.C.; Munoz Armas, U.S.; Pascual, C.A.; Zamundo, B.R. Congenital heart disease and other malformations produced by Influenza A virus and allantoic fluid in the chick embryo. Circ. Res. 1963, 13, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paff, G.H.; Boucek, R.J.; Klopfenstein, H.S. Experimental heart-block in the chick embryo. Anat. Rec. 1964, 149, 217–223. [Google Scholar] [CrossRef]
- Van Mierop, L.H.; Bertuch, C.J., Jr. Development of arterial blood pressure in the chick embryo. Am. J. Physiol. 1967, 212, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Faber, J.J. Mechanical function of the septating embryonic heart. Am. J. Physiol. 1968, 214, 475–481. [Google Scholar] [CrossRef]
- Faber, J.J.; Green, T.J.; Thornburg, K.L. Embryonic stroke volume and cardiac output in the chick. Dev. Biol. 1974, 41, 14–21. [Google Scholar] [CrossRef]
- Manasek, F.J.; Monroe, R.G. Early cardiac morphogenesis is independent of function. Dev. Biol. 1972, 27, 584–588. [Google Scholar] [CrossRef]
- Hill, L.; Azuma, Y. Blood pressure in the two-three-day chick embryo. J. Physiol. 1927, 62, 27–28. [Google Scholar]
- Hughes, A.F.W. The blood pressure of the chick embryo during development. J. Exp. Biol. 1942, 19, 232–237. [Google Scholar]
- Paff, G.H.; Boucek, R.J.; Gutten, G.S. Ventricular blood pressure and competency of valves in the early embryonic chick heart. Anat. Rec. 1965, 151, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Rychter, Z.; Lemez, L. Changes in localization in aortic arches of laminar blood streams of main venous trunks to heart after exclusion of vitelline vessels on second day of incubation. Fed. Proc. Transl. Suppl. 1965, 24, 815–820. [Google Scholar]
- Dbalý, J.; Rychter, Z. The vascular system of the chick embryo. XVII. The development of branching of the coronary arteries in the chick embryos with experimentally induced left-half heart hypoplasy. Folia. Morphol. (Praha) 1967, 15, 358–368. [Google Scholar] [PubMed]
- Gessner, I.H. Cardiovascular anomalies resulting from manipulating the truncoconal region of the early chick embryo heart. Birth Defects Orig. Art. Ser. 1978, 14, 405–422. [Google Scholar] [PubMed]
- Gourdie, R.G.; Kubalak, S.; Mikawa, T. Conducting the embryonic heart: Orchestrating development of specialized cardiac tissues. Trends Cardiovasc. Med. 1999, 9, 18–26. [Google Scholar] [CrossRef]
- Monsoro-Burq, A.H.; Levin, M. Avian models and the study of invariant asymmetry: How the chicken and the egg taught us to tell right from left. Int. J. Dev. Biol. 2018, 62, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Sylva, M.; van den Hoff, M.J.; Moorman, A.F. Development of the human heart. Am. J. Med. Genet. A. 2014, 164, 1347–1371. [Google Scholar] [CrossRef]
- Tazawa, H.; Akiyama, R.; Moriya, K. Development of cardiac rhythm in birds. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002, 123, 675–689. [Google Scholar] [CrossRef]
- Courchaine, K.; Rykiel, G.; Rugonyi, S. Influence of blood flow on cardiac development. Prog. Biophys. Mol. Biol. 2018, 137, 95–110. [Google Scholar] [CrossRef]
- Xavier-Neto, J.; Sousa Costa, Â.M.; Figueira, A.C.; Caiaffa, C.D.; Amaral, F.N.; Peres, L.M.; da Silva, B.S.; Santos, L.N.; Moise, A.R.; Castillo, H.A. Signaling through retinoic acid receptors in cardiac development: Doing the right things at the right times. Biochim. Biophys. Acta 2015, 1849, 94–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meilhac, S.M.; Buckingham, M.E. The deployment of cell lineages that form the mammalian heart. Nat. Rev. Cardiol. 2018, 15, 705–724. [Google Scholar] [CrossRef] [PubMed]
- MacGrogan, D.; Münch, J.; de la Pompa, J.L. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat. Rev. Cardiol. 2018, 15, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Lescroart, F.; Zaffran, S. Hox and Tale transcription factors in heart development and disease. Int. J. Dev. Biol. 2018, 62, 837–846. [Google Scholar] [CrossRef]
- Duncan, A.R.; Khokha, M.K. Xenopus as a model organism for birth defects-Congenital heart disease and heterotaxy. Semin. Cell Dev. Biol. 2016, 51, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Warren, K.S.; Fishman, M.C. “Physiological genomics”: Mutant screens in zebrafish. Am. J. Physiol. 1998, 275, 1–7. [Google Scholar] [CrossRef]
- Espinosa, M.G.; Taber, L.A.; Wagenseil, J.E. Reduced embryonic blood flow impacts extracellular matrix deposition in the maturing aorta. Dev. Dyn. 2018, 247, 914–923. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, V.K.; Godby, R.C.; Liu, G.R.; Smith, J.M.; Hiratzka, L.F.; Narmoneva, D.A.; Hinton, R.B. Review of molecular and mechanical interactions in the aortic valve and aorta: Implications for the shared pathogenesis of aortic valve disease and aortopathy. J. Cardiovasc. Transl. Res. 2014, 7, 823–846. [Google Scholar] [CrossRef]
- Karunamuni, G.H.; Ma, P.; Gu, S.; Rollins, A.M.; Jenkins, M.W.; Watanabe, M. Connecting teratogen-induced congenital heart defects to neural crest cells and their effect on cardiac function. Birth Defects Res. C. Embryo Today. 2014, 102, 227–250. [Google Scholar] [CrossRef] [Green Version]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics--2013 update: A report from the American Heart Association. Circulation 2013, 127, 6–245. [Google Scholar] [CrossRef]
- DeHaan, R.L. Development of form in the embryonic heart. An experimental approach. Circulation 1967, 35, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, J.I. Natural history of congenital heart disease. Problems in its assessment with special reference to ventricular septal defects. Circulation 1968, 37, 97–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neill, C.A. Genetic aspects of congenital heart disease. In Heart Disease in Infants, Children, and Adolescents; Moss, A.J., Adams, F.H., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1968; pp. 36–46. [Google Scholar]
- Clark, E.B. Mechanisms in the pathogenesis of congenital heart defects. In The Genetics of Cardiovascular Disease; Pierpont, M.E., Moller, J., Eds.; Martinuus-Nijoff: Boston, MA, USA, 1986; pp. 3–11. [Google Scholar]
- Clark, E.B. Pathogenetic mechanisms of congenital cardiovascular malformations revisited. Semin. Perinatol. 1996, 20, 465–472. [Google Scholar] [CrossRef]
- Ferencz, C. Congenital heart disease: An epidemiologic and teratologic challenge. In Perspectives in Pediatric Cardiology; Vol. 4; Epidemiology of Congenital Heart Disease: The Baltimore-Washington Infant Study 1981–1989; Futura Publishing: Mount Kisco, NY, USA, 1993; pp. 1–16. [Google Scholar]
- Clark, E.B. Cardiac embryology. Its relevance to congenital heart disease. Am. J. Dis. Child. 1986, 140, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Bartelings, M.M.; Deruiter, M.C.; Poelmann, R.E. Basics of cardiac development for the understanding of congenital heart malformations. Pediatr. Res. 2005, 57, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, E.B.; Hu, N. Developmental hemodynamic changes in the chick embryo from stage 18 to 27. Circ. Res. 1982, 51, 810–815. [Google Scholar]
- Clark, E.B.; Hu, N.; Dummett, J.L.; Vandekieft, G.K.; Olson, C.; Tomanek, R. Ventricular function and morphology in chick embryo from stages 18 to 29. Am. J. Physiol. 1986, 250, 407–413. [Google Scholar]
- Hu, N.; Clark, E.B. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ. Res. 1989, 65, 1665–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, N.; Connuck, D.M.; Keller, B.B.; Clark, E.B. Diastolic filling characteristics in the stage 12 to 27 chick embryo ventricle. Pediatr. Res. 1991, 29, 334–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekhuizen, M.L.; Mast, F.; Struijk, P.C.; van der Bie, W.; Mulder, P.G.; Gittenberger-de Groot, A.C.; Wladimiroff, J.W. Hemodynamic parameters of stage 20 to stage 35 chick embryo. Pediatr. Res. 1993, 34, 44–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursem, N.T.; Struijk, P.C.; Poelmann, R.E.; Gittenberger-De Groot, A.C.; Wladimiroff, J.W. Dorsal aortic flow velocity in chick embryos of stage 16 to 28. Ultrasound Med. Bio. 2001, 27, 919–924. [Google Scholar] [CrossRef]
- Burggren, W.W.; Keller, B.B. (Eds.) Development of Cardiovascular Systems: Molecules to Organisms; Cambridge University Press: New York, NY, USA, 1998. [Google Scholar]
- Keller, B.B.; Liu, L.J.; Tinney, J.P.; Tobita, K. Cardiovascular developmental insights from embryos. Ann. Ny Acad. Sci. 2007, 100, 1399–1401. [Google Scholar] [CrossRef] [PubMed]
- Pekkan, K.; Keller, B.B. Fetal and embryonic hemodynamics: Developmental fetal cardiovascular biomechanics in the 21st century: Another tipping point. Cardiovasc. Eng. Technol. 2013, 4, 231–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalski, W.J.; Pekkan, K.; Tinney, J.P.; Keller, B.B. Investigating developmental cardiovascular biomechanics and the origins of congenital heart defects. Front. Biophys. 2014, 5, 4–8. [Google Scholar]
- Voorhees, A.P.; Han, H.C. Biomechanics of Cardiac Function. Compr. Physiol. 2015, 5, 1623–1644. [Google Scholar]
- Poelmann, R.E.; Gittenberger-de Groot, A.C. Hemodynamics in Cardiac Development. J. Cardiovasc. Dev. Dis. 2018, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Wispé, J.; Hu, N.; Clark, E.B. Effect of environmental hypothermia on dorsal aortic blood flow in the chick embryo, stages 18 to 24. Pediatr. Res. 1983, 17, 945–948. [Google Scholar]
- Nakazawa, M.; Clark, E.B.; Hu, N.; Wispé, J. Effect of environmental hypothermia on vitelline artery blood pressure and vascular resistance in the stage 18, 21, and 24 chick embryo. Pediatr. Res. 1985, 19, 651–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunnigan, A.; Hu, N.; Benson, D.W., Jr.; Clark, E.B. Effect of heart rate increase on dorsal aortic flow in the stage 24 chick embryo. Pediatr. Res. 1987, 22, 442–444. [Google Scholar] [CrossRef] [Green Version]
- Benson, D.W., Jr.; Hughes, S.F.; Hu, N.; Clark, E.B. Effect of heart rate increase on dorsal aortic flow before and after volume loading in the stage 24 chick embryo. Pediatr. Res. 1989, 26, 438–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedmera, D.; Wessels, A.; Trusk, T.C.; Thompson, R.P.; Hewett, K.W.; Gourdie, R.G. Changes in activation sequence of embryonic chick atria correlate with developing myocardial architecture. Am. J. Physiol. 2006, 291, 1646–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagman, A.J.; Hu, N.; Clark, E.B. Effect of changes in circulating blood volume on cardiac output and arterial and ventricular blood pressure in the stage 18, 24, and 29 chick embryo. Circ. Res. 1990, 67, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Ostádal, B.; Rychterová, V.; Rychter, Z. Isoproterenol-induced necrotic lesions of embryonic heart tissue. Recent Adv. Stud. Cardiac Struct. Metab. 1975, 6, 453–459. [Google Scholar]
- Hawkins, J.A.; Hu, N.; Clark, E.B. Effect of caffeine on cardiovascular function in the stage 24 chick embryo. Dev. Pharm. 1984, 7, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.B.; Hu, N.; Turner, D.R.; Litter, J.E.; Hansen, J. Effect of chronic verapamil treatment on ventricular function and growth in chick embryos. Am. J. Physiol. 1991, 261, 166–171. [Google Scholar] [CrossRef]
- Nakazawa, M.; Morishima, M.; Tomita, H.; Tomita, S.M.; Kajio, F. Hemodynamics and ventricular function in the day-12 rat embryo: Basic characteristics and the responses to cardiovascular drugs. Pediatr. Res. 1995, 37, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Hansen, A.L.; Clark, E.B.; Keller, B.B. Effect of atrial natriuretic peptide on diastolic filling in the stage 21 chick embryo. Pediatr. Res. 1995, 37, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Sedmera, D.; Pexieder, T.; Hu, N.; Clark, E.B. A quantitative study of the ventricular myoarchitecture in the stage 21-29 chick embryo following decreased loading. Eur. J. Morphol. 1998, 36, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Tenthorey, D.; De Ribaupierre, Y.; Kucera, P.; Raddatz, E. Effects of verapamil and ryanodine on activity of the embryonic chick heart during anoxia and reoxygenation. J. Cardiovasc. Pharm. 1998, 31, 195–202. [Google Scholar] [CrossRef]
- Wojtczak, J.A. The hemodynamic effects of halothane and isoflurane in chick embryo. Anesth. Analg. 2000, 90, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
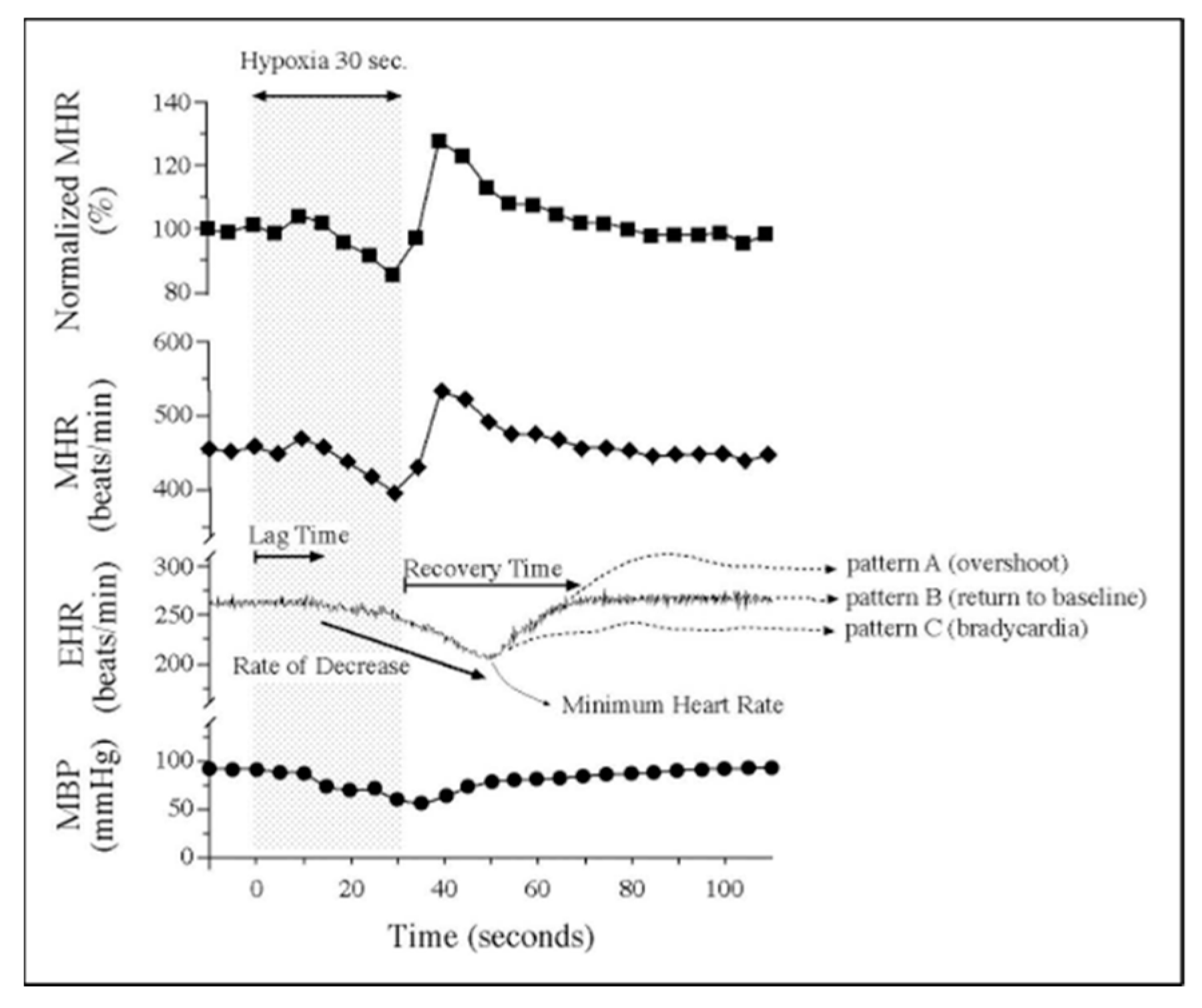
- Sharma, S.K.; Lucitti, J.L.; Nordman, C.; Tinney, J.P.; Tobita, K.; Keller, B.B. Impact of hypoxia on early chick embryo growth and cardiovascular function. Pediatr. Res. 2006, 59, 116–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, E.B.; Hu, N.; Dooley, J.B. The effect of isoproterenol on cardiovascular function in the stage 24 chick embryo. Teratology 1985, 31, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Pappano, A.J. Ontogenetic development of autonomic neuroeffector transmission and transmitter reactivity in embryonic and fetal hearts. Pharm. Rev. 1977, 29, 3–33. [Google Scholar] [PubMed]
- Suga, H.; Sagawa, K.; Shoukas, A.A. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ. Res. 1973, 32, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagawa, K.; Suga, H.; Shoukas, A.A.; Bakalar, K.M. End-systolic pressure/volume ratio: A new index of ventricular contractility. Am. J. Cardiol. 1977, 40, 748–753. [Google Scholar] [CrossRef]
- Khalafbeigui, F.; Suga, H.; Sagawa, K. Left ventricular systolic pressure-volume area correlates with oxygen consumption. Am. J Physiol. 1979, 237, 566–569. [Google Scholar] [CrossRef]
- Kass, D.A.; Yamazaki, T.; Burkhoff, D.; Maughan, W.L.; Sagawa, K. Determination of left ventricular end-systolic pressure-volume relationships by the conductance (volume) catheter technique. Circulation 1986, 73, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Kass, D.A.; Maughan, W.L.; Guo, Z.M.; Kono, A.; Sunagawa, K.; Sagawa, K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: Experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987, 76, 1422–1436. [Google Scholar] [CrossRef] [Green Version]
- Maughan, W.L.; Sunagawa, K.; Burkhoff, D.; Sagawa, K. Effect of arterial impedance changes on the end-systolic pressure-volume relation. Circ. Res. 1984, 54, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Sunagawa, K.; Sagawa, K.; Maughan, W.L. Ventricular interaction with the loading system. Ann. Biomed. Eng. 1984, 12, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, K.; Maughan, W.L.; Sagawa, K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ. Res. 1985, 56, 586–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kass, D.A.; Beyar, R.; Lankford, E.; Heard, M.; Maughan, W.L.; Sagawa, K. Influence of contractile state on curvilinearity of in situ end-systolic pressure-volume relations. Circulation 1989, 79, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Ruckman, R.N.; Rosenquist, G.C.; Rademaker, D.A.; Morse, D.E.; Getson, P.R. The effect of graded hypoxia on the embryonic chick heart. Teratology 1985, 32, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.B.; Hu, N.; Clark, E.B. Correlation of ventricular area, perimeter, and conotruncal diameter with mass and function in the stage 12 to 24 chick embryo. Circ. Res. 1990, 66, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, B.B.; Hu, N.; Serrino, P.J.; Clark, E.B. Ventricular pressure-area loop characteristics in the stage 16 to 24 chick embryo. Circ. Res. 1991, 68, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, B.B.; Hu, N.; Tinney, J.P. Embryonic ventricular diastolic and systolic pressure-volume relation. Cardiol. Young 1994, 4, 19–27. [Google Scholar] [CrossRef]
- Yoshigi, M.; Keller, B.B. Pressure-volume relationships of the embryonic heart analyzed by custom-developed IMAQ image analysis system [Japanese]. InterLab. 1999, 4, 58–59. [Google Scholar]
- Tobita, K.; Keller, B.B. End-systolic myocardial stiffness is a load independent index of contractility in the stage 24 chick embryonic heart. Am. J. Physiol. 1999, 276, 2101–2108. [Google Scholar] [CrossRef]
- Tobita, K.; Keller, B.B. Maturation of end-systolic stress-strain relations in chick embryo myocardium. Am. J. Physiol. 2000, 279, 216–224. [Google Scholar]
- Keller, B.B.; Yoshigi, M.; Tinney, J.P. Ventricular-vascular uncoupling by acute conotruncal occlusion in the stage 21 chick embryo. Am. J. Physiol. 1997, 273, 2861–2866. [Google Scholar] [CrossRef]
- Hu, N.; Keller, B.B. Relationship of simultaneous atrial and ventricular pressures in the stage 16 to 27 chick embryo. Am. J. Physiol. 1995, 269, 1359–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.A.; Hu, N.; Clark, E.B.; Keller, B.B. Analysis of dynamic atrial dimension and function during early cardiac development in the chick embryo. Ped. Res. 1992, 32, 333–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, F.J.; Hughes, S.F.; Cuneo, B.; Benson, D.W., Jr. The effect of cardiac cycle length on ventricular end-diastolic pressure and maximum time derivative of pressure in the stage 24 chick embryo. Pediatr. Res. 1991, 29, 338–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuneo, B.; Hughes, S.; Benson, D.W., Jr. Heart rate perturbation in chick embryos: A comparison of two methods. Am. J. Physiol. 1991, 260, 1864–1869. [Google Scholar] [CrossRef]
- Cuneo, B.; Hughes, S.; Benson, D.W., Jr. Heart rate perturbation in the stage 17-27 chick embryo: Effect on stroke volume and aortic flow. Am. J. Physiol. 1993, 264, 755–759. [Google Scholar] [CrossRef]
- Casillas, C.B.; Tinney, J.P.; Keller, B.B. Influence of acute alterations in cycle length on ventricular function in the chick embryo. Am. J. Physiol. 1994, 267, 905–911. [Google Scholar] [CrossRef]
- Yin, F.C.P. Ventricular/Vascular Coupling: Clinical, Physiological and Engineering Aspects; Springer-Verlag: New York, NY, USA, 1987; pp. 1–19. [Google Scholar]
- Burkhoff, D.; Alexander, J., Jr.; Schipke, J. Assessment of Windkessel as a model of aortic input impedance. Am. J. Physiol. 1988, 255, 742–753. [Google Scholar] [CrossRef]
- Zahka, K.G.; Hu, N.; Brin, K.P.; Yin, F.C.; Clark, E.B. Aortic impedance and hydraulic power in the chick embryo from stages 18 to 29. Circ. Res. 1989, 64, 1091–1095. [Google Scholar]
- Yoshigi, M.; Hu, N.; Keller, B.B. Dorsal aortic impedance in the stage 24 chick embryo following acute changes in circulating blood volume. Am. J. Physiol. 1996, 270, 1597–1606. [Google Scholar] [CrossRef] [Green Version]
- Yoshigi, M.; Keller, B.B. Linearity of pulsatile pressure–flow relations in the embryonic chick vascular system. Circ. Res. 1996, 79, 864–870. [Google Scholar] [CrossRef]
- Yoshigi, M.; Keller, B.B. Characterization of the embryonic aortic impedance with lumped parameter models. Am. J. Physiol. 1997, 273, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yoshigi, M.; Ettel, J.M.; Keller, B.B. Developmental changes in flow wave propagation velocity in the embryonic chick vascular system. Am. J. Physiol. 1997, 273, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Yoshigi, M.; Knott, G.; Keller, B.B. Lumped parameter estimation for the embryonic chick vascular system: A time domain approach using MLAB. Computer Programs Biomed. J. 2000, 63, 29–41. [Google Scholar] [CrossRef]
- Shi, L.; Goenezen, S.; Haller, S.; Hinds, M.T.; Thornburg, K.L.; Rugonyi, S. Alterations in pulse wave propagation reflect the degree of outflow tract banding in HH18 chicken embryos. Am. J. Physiol. 2013, 305, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Bowers, P.N.; Tinney, J.P.; Keller, B.B. Nitroprusside selectively reduces ventricular preload in the stage 21 chick embryo. Cardiovasc. Res. 1996, 31, 132–138. [Google Scholar] [CrossRef]
- Miller, C.E.; Vanni, M.A.; Taber, L.A.; Keller, B.B. Passive stress-strain measurements in the stage 16 and stage 18 embryonic chick heart. J. Biomech. Eng. 1997, 119, 445–451. [Google Scholar] [CrossRef]
- Miller, C.E.; Vanni, M.A.; Keller, B.B. Characterization of passive embryonic myocardium by quasi-linear viscoelasticity theory. J. Biomech. 1997, 30, 985–988. [Google Scholar] [CrossRef]
- Ling, P.; Taber, L.A.; Humphrey, J.D. Approach to quantify the mechanical behavior of the intact embryonic chick heart. Ann. Biomed. Eng. 2002, 30, 636–645. [Google Scholar] [CrossRef]
- Barry, W.H.; Pitzen, R.; Protas, K.; Harrison, D.C. Inotropic effects of different calcium ion concentration on the embryonic chick ventricle. Comparison of single cultured cells and intact muscle strips. Circ. Res. 1975, 36, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, G.T.; Chen, F.; Klitzner, T.S. Ca2+ channel kinetics in acutely isolated fetal, neonatal, and adult rabbit cardiac myocytes. Circ. Res. 1993, 72, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Tsyvian, P.B.; Routkevich, S.M.; Protsenko, Y.L.; Tsyvian, E.P.; Miller, C.E.; Keller, B.B. The role of the intra- and extracellular sources in “Force-Frequency” relations in the chick embryonic myocardium. Russ. J. Physiol. 1998, 84, 1402–1411. [Google Scholar]
- Tsyvian, P.B.; Markhasin, V.S.; Solovyeva, O.E.; Rutkevitch, S.M.; Protsenko, L.L.; Artemeva, O.G.; Schroder, E.A.; Keller, B.B. Contraction-relaxation dynamics and mechanical restitution in the developing myocardium of the chick embryo. Ross. Fiziol. Zh. Im. I. M. Sechenova. 2001, 87, 901–910. [Google Scholar]
- Solovyeva, O.E.; Markhasin, V.S.; Tsyvian, P.B.; Keller, B.B. Experimental and theoretical investigation of force-interval relationships in the developing chick myocardium. Biofizika 1999, 44, 337–349. [Google Scholar]
- Schroder, E.A.; Satin, J.; Routkevitch, S.; Tsyvian, P.; Keller, B.B. T-type and L-Type Calcium Currents Modulate Force in Embryonic Chick Myocardium. In Cardiac Development; Ostadal, B., Nagano, M., Dhalla, N.S., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2002; pp. 113–132. [Google Scholar]
- Schroder, E.A.; Tobita, K.; Tinney, J.P.; Foldes, J.K.; Keller, B.B. Microtubule involvement in the adaptation to altered mechanical load in developing chick myocardium. Circ. Res. 2002, 91, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Omens, J.H.; Fung, Y.C. Residual strain in rat left ventricle. Circ. Res. 1990, 66, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Hunter, P.J.; Nielsen, P.M.; Smaill, B.H.; LeGrice, I.J.; Hunter, I.W. An anatomical heart model with applications to myocardial activation and ventricular mechanics. Crit. Rev. Biomed. Eng. 1992, 20, 403–426. [Google Scholar]
- McCulloch, A.; Waldman, L.; Rogers, J.; Guccione, J. Large-scale finite element analysis of the beating heart. Crit. Rev. Biomed. Eng. 1992, 20, 427–449. [Google Scholar]
- Taber, L.A.; Keller, B.B.; Clark, E.B. Cardiac mechanics in the stage 16 chick embryo. J. Biomech. Eng. 1992, 114, 427–434. [Google Scholar] [CrossRef]
- Taber, L.A.; Hu, N.; Pexieder, T.; Clark, E.B.; Keller, B.B. Residual strain in the embryonic ventricle of the stage 16 to 24 chick embryo heart. Circ. Res. 1993, 72, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Taber, L.A.; Clark, E.B. A nonliner poroelastic model for the trabecular embryonic heart. J. Biomech Eng. 1994, 116, 213–223. [Google Scholar] [CrossRef]
- Taber, L.A. Mechanical aspects of cardiac development. Prog. Biophys. Mol. Biol. 1998, 69, 237–255. [Google Scholar] [CrossRef]
- Taber, L.A.; Sun, H.; Cartmell, J.S.; Clark, E.B.; Keller, B.B. Epicardial strains in the stage 16–24 embryonic chick ventricle. Circ. Res. 1994, 75, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Costa, K.D.; May-Newman, K.; Farr, D.; O’Dell, W.G.; McCulloch, A.D.; Omens, J.H. Three-dimensional residual strain in midanterior canine left ventricle. Am. J. Physiol. 1997, 273, 1968–1976. [Google Scholar] [CrossRef]
- Tobita, K.; Keller, B.B. Ventricular epicardial deformation patterns during normal development and during the development of left heart hypoplasia in the chick embryo. Am. J. Physiol. 2000, 279, 959–969. [Google Scholar]
- Tobita, K.; Garrison, J.B.; Liu, L.J.; Tinney, J.P.; Keller, B.B. Three dimensional myofiber architecture of the embryonic left ventricle during normal and development of altered mechanical loads. Anat. Rec. 2005, 283, 193–201. [Google Scholar] [CrossRef]
- Damon, B.J.; Rémond, M.C.; Bigelow, M.R.; Trusk, T.C.; Xie, W.; Perucchio, R.; Sedmera, D.; Denslow, S.; Thompson, R.P. Patterns of muscular strain in the embryonic heart wall. Dev. Dyn. 2009, 238, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.R.; Effmann, E.L.; Johnson, G.A. MR microscopy of chick embryo vasculature. J. Magn. Reson. Imaging. 1992, 2, 237–240. [Google Scholar] [CrossRef]
- Smith, B.R. Magnetic resonance microscopy in cardiac development. Microsc.. Res. Tech. 2001, 52, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Yelbuz, T.M.; Choma, M.A.; Thrane, L.; Kirby, M.L.; Izatt, J.A. Optical coherence tomography: A new high-resolution imaging technology to study cardiac development in chick embryos. Circulation 2002, 106, 2771–2774. [Google Scholar] [CrossRef] [Green Version]
- Manner, J.; Thrane, L.; Norozi, K.; Yelbuz, T.M. High-resolution in vivo imaging of the cross-sectional deformations of contracting embryonic heart loops using optical coherence tomography. Dev. Dyn. 2008, 237, 953–961. [Google Scholar] [CrossRef]
- Larina, I.V.; Sudheendran, N.; Ghosn, M.; Jiang, J.; Cable, A.; Larin, K.V.; Dickinson, M.E. Live imaging of blood flow in mammalian embryos using Doppler swept-source optical coherence tomography. J. Biomed. Opt. 2008, 13. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lakomy, D.S.; Garcia, M.D.; Lopez, A.L., 3rd; Larin, K.V.; Larina, I.V. Four-dimensional live imaging of hemodynamics in mammalian embryonic heart with Doppler optical coherence tomography. J. Biophotonics. 2016, 9, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Butcher, J.T.; Sedmera, D.; Guldberg, R.E.; Markwald, R.R. Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Dev. Dyn. 2007, 236, 802–809. [Google Scholar] [CrossRef]
- Männer, J. Cardiac looping in the chick embryo: A morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat. Rec. 2000, 259, 248–262. [Google Scholar] [CrossRef]
- Miller, C.E.; Wong, C.L.; Sedmera, D. Pressure overload alters stress-strain properties of the developing chick heart. Am. J. Physiol. 2003, 285, 1849–1856. [Google Scholar] [CrossRef] [Green Version]
- Groenendijk, B.C.; Hierck, B.P.; Vrolijk, J.; Baiker, M.; Pourquie, M.J.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ. Res. 2005, 96, 1291–1298. [Google Scholar] [CrossRef] [Green Version]
- Vennemann, P.; Kiger, K.T.; Lindken, R.; Groenendijk, B.C.; Stekelenburg-de Vos, S.; ten Hagen, T.L.; Ursem, N.T.; Poelmann, R.E.; Westerweel, J.; Hierck, B.P. In vivo micro particle image velocimetry measurements of blood-plasma in the embryonic avian heart. J. Biomech. 2006, 39, 1191–1200. [Google Scholar] [CrossRef]
- McQuinn, T.C.; Bratoeva, M.; Dealmeida, A.; Remond, M.; Thompson, R.P.; Sedmera, D. High-frequency ultrasonographic imaging of avian cardiovascular development. Dev. Dyn. 2007, 236, 3503–3513. [Google Scholar] [CrossRef]
- Oosterbaan, A.M.; Ursem, N.T.; Struijk, P.C.; Bosch, J.G.; van der Steen, A.F.; Steegers, E.A. Doppler flow velocity waveforms in the embryonic chicken heart at developmental stages corresponding to 5-8 weeks of human gestation. Ultrasound Obs. Gynecol. 2009, 33, 638–644. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Peterson, L.; Gu, S.; Gargesha, M.; Wilson, D.L.; Watanabe, M.; Rollins, A.M. Measuring hemodynamics in the developing heart tube with four-dimensional gated Doppler optical coherence tomography. J. Biomed. Opt. 2010, 15. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Shekhar, A.; McQuinn, T.C.; Butcher, J.T. Hemodynamic patterning of the avian atrioventricular valve. Dev. Dyn. 2011, 240, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Peterson, L.M.; Jenkins, M.W.; Gu, S.; Barwick, L.; Watanabe, M.; Rollins, A.M. 4D shear stress maps of the developing heart using Doppler optical coherence tomography. Biomed. Opt. Express. 2012, 3, 3022–3032. [Google Scholar] [CrossRef] [Green Version]
- Buskohl, P.R.; Jenkins, J.T.; Butcher, J.T. Computational simulation of hemodynamic-driven growth and remodeling of embryonic atrioventricular valves. Biomech. Model. Mechanobiol. 2012, 11, 1205–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buskohl, P.R.; Gould, R.A.; Butcher, J.T. Quantification of embryonic atrioventricular valve biomechanics during morphogenesis. J. Biomech. 2012, 45, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Karunamuni, G.H.; Gu, S.; Ford, M.R.; Peterson, L.M.; Ma, P.; Wang, Y.T.; Rollins, A.M.; Jenkins, M.W.; Watanabe, M. Capturing structure and function in an embryonic heart with biophotonic tools. Front. Physiol. 2013, 5, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Liu, A.; Yin, X.; Troyer, A.; Thornburg, K.; Wang, R.K.; Rugonyi, S. Measurement of absolute blood flow velocity in outflow tract of HH18 chicken embryo based on 4D reconstruction using spectral domain optical coherence tomography. Biomed. Opt. Express. 2010, 1, 798–811. [Google Scholar] [CrossRef]
- Poelma, C.; Van der Heiden, K.; Hierck, B.P.; Poelmann, R.E.; Westerweel, J. Measurements of the wall shear stress distribution in the outflow tract of an embryonic chicken heart. J. R. Soc. Interface. 2010, 7, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yin, X.; Shi, L.; Rugonyi, S.; Wang, R.K. In vivo functional imaging of blood flow and wall strain rate in outflow tract of embryonic chick heart using ultrafast spectral domain optical coherence tomography. J. Biomed. Opt. 2012, 17, 96006–96010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Yin, X.; Shi, L.; Li, P.; Thornburg, K.L.; Wang, R.; Rugonyi, S. Biomechanics of the chick embryonic heart outflow tract at HH18 using 4D optical coherence tomography imaging and computational modeling. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.; Tan, G.X.Y.; Foo, T.J.; Phan-Thien, N.; Yap, C.H. Organ dynamics and fluid dynamics of the HH25 chick embryonic cardiac ventricle as revealed by a novel 4D high-frequency Ultrasound imaging technique and computational flow simulations. Ann. Biomed. Eng. 2017, 45, 2309–2323. [Google Scholar] [CrossRef]
- Wang, Y.; Dur, O.; Patrick, M.J.; Tinney, J.P.; Tobita, K.; Keller, B.B.; Pekkan, K. Aortic arch morphogenesis and flow modeling in the chick embryo. Ann. Biomed. Eng. 2009, 37, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
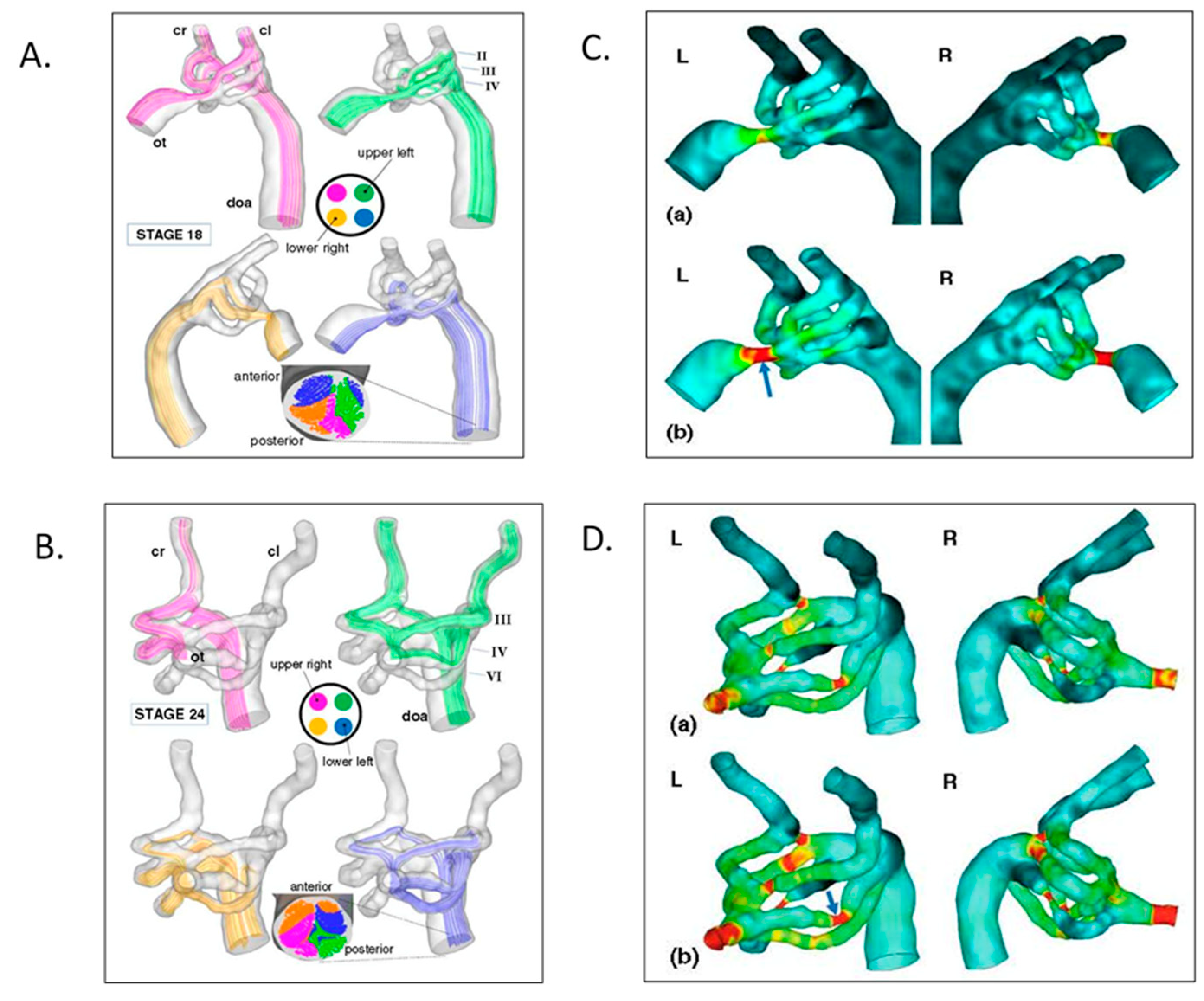
- Kowalski, W.J.; Teslovich, N.C.; Dur, O.; Keller, B.B.; Pekkan, K. Computational hemodynamic optimization predicts dominant aortic arch selection is driven by embryonic outflow tract orientation in the chick embryo. Biomech. Model. Mechanobiol. 2012, 11, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W.J.; Dur, O.; Wang, Y.; Patrick, M.J.; Tinney, J.P.; Keller, B.B.; Pekkan, K. Critical transitions in early embryonic aortic arch patterning and hemodynamics. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, W.J.; Teslovich, N.K.; Menon, P.G.; Tinney, J.P.; Keller, B.B.; Pekkan, K. Left atrial ligation alters intracardiac flow patterns and the biomechanical landscape in the chick embryo. Dev. Dyn. S 2014, 243, 652–662. [Google Scholar] [CrossRef]
- Goktas, S.; Uslu, F.E.; Kowalski, W.J.; Ermek, E.; Keller, B.B.; Pekkan, K. Time-series interactions of gene expression, vascular growth and hemodynamics during early embryonic arterial development. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Karakaya, C.; Goktas, S.; Celik, M.; Kowalski, W.J.; Keller, B.B.; Pekkan, K. Asymmetry in mechanosensitive gene expression during aortic arch morphogenesis. Sci. Rep. 2018, 8, 16948. [Google Scholar] [CrossRef]
- Benslimane, F.M.; Alser, M.; Zakaria, Z.Z.; Sharma, A.; Abdelrahman, H.A.; Yalcin, H.C. Adaptation of a mice Doppler echocardiography platform to measure cardiac flow velocities for embryonic chicken and adult zebrafish. Front. Bioeng. Biotechnol. 2019, 7, 96. [Google Scholar] [CrossRef]
- Tobita, K.; Schroder, E.A.; Tinney, J.P.; Garrison, J.B.; Keller, B.B. Regional passive ventricular pressure-strain relations during development of altered loads in the chick embryo. Am. J. Physiol. 2002, 282, 2386–2396. [Google Scholar]
- Buffinton, C.M.; Faas, D.; Sedmera, D. Stress and strain adaptation in load-dependent remodeling of the embryonic left ventricle. Biomech. Model. Mechanobiol. 2013, 12, 1037–1051. [Google Scholar] [CrossRef] [Green Version]
- Chivukula, V.K.; Goenezen, S.; Liu, A.; Rugonyi, S. Effect of outflow tract banding on embryonic cardiac hemodynamics. J. Cardiovasc. Dev. Dis. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, S.E.; Menon, P.G.; Kowalski, W.J.; Shekhar, A.; Yalcin, H.C.; Nishimura, N.; Schaffer, C.B.; Butcher, J.T.; Pekkan, K. Growth and hemodynamics after early embryonic aortic arch occlusion. Biomech. Model. Mechanobiol. 2015, 14, 735–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midgett, M.; Chivukula, V.K.; Dorn, C.; Wallace, S.; Rugonyi, S. Blood flow through the embryonic heart outflow tract during cardiac looping in HH13-HH18 chicken embryos. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef]
- Midgett, M.; López, C.S.; David, L.; Maloyan, A.; Rugonyi, S. Increased hemodynamic load in early embryonic stages alters myofibril and mitochondrial organization in the myocardium. Front. Physiol. 2017, 8, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennie, M.Y.; Stovall, S.; Carson, J.P.; Danilchik, M.; Thornburg, K.L.; Rugonyi, S. Hemodynamics modify collagen deposition in the early embryonic chicken heart outflow tract. J. Cardiovasc. Dev. Dis. 2017, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Celik, M.; Goktas, S.; Karakaya, C.; Cakiroglu, A.I.; Karahuseyinoglu, S.; Lashkarinia, S.S.; Ermek, E.; Pekkan, K. Microstructure of early embryonic aortic arch and its reversibility following mechanically-altered hemodynamic load release. Am. J. Physiol. 2020. [Google Scholar] [CrossRef]
- Pang, K.L.; Parnall, M.; Loughna, S. Effect of altered haemodynamics on the developing mitral valve in chick embryonic heart. J. Mol. Cell. Cardiol. 2017, 108, 114–126. [Google Scholar] [CrossRef]
- Menon, V.; Eberth, J.F.; Goodwin, R.L.; Potts, J.D. Altered hemodynamics in the embryonic heart affects outflow valve development. J. Cardiovasc. Dev. Dis. 2015, 2, 108–124. [Google Scholar] [CrossRef]
- Biechler, S.V.; Junor, L.; Evans, A.N.; Eberth, J.F.; Price, R.L.; Potts, J.D.; Yost, M.J.; Goodwin, R.L. The impact of flow-induced forces on the morphogenesis of the outflow tract. Front. Physiol. 2014, 5, 225. [Google Scholar] [CrossRef]
- Perdios, C.; Parnall, M.; Pang, K.L.; Loughna, S. Altered haemodynamics causes aberrations in the epicardium. J. Anat. 2019, 234, 800–814. [Google Scholar] [CrossRef]
- Harh, J.Y.; Paul, M.H.; Gallen, W.J.; Friedberg, D.Z.; Kaplan, S. Experimental production of hypoplastic left heart syndrome in the chick embryo. Am. J. Cardiol. 1973, 31, 51–56. [Google Scholar] [CrossRef]
- Sedmera, D.; Pexieder, T.; Rychterova, V.; Hu, N.; Clark, E.B. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat. Rec. 1999, 254, 238–252. [Google Scholar] [CrossRef]
- Sedmera, D.; Hu, N.; Weiss, K.M.; Keller, B.B.; Denslow, S.; Thompson, R.P. Cellular changes in experimental left heart hypoplasia. Anat. Rec. 2002, 267, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Christensen, D.A.; Agrawal, A.K.; Beaumont, C.; Clark, E.B.; Hawkins, J.A. Dependence of aortic arch morphogenesis on intracardiac blood flow in the left atrial ligated chick embryo. Anat. Rec. (Hoboken) 2009, 292, 652–660. [Google Scholar] [CrossRef]
- McCaffrey, F.M.; Sherman, F.S. Prenatal diagnosis of severe aortic stenosis. Pediatr. Cardiol. 1997, 18, 276–281. [Google Scholar] [CrossRef]
- Mäkikallio, K.; McElhinney, D.B.; Levine, J.C.; Marx, G.R.; Colan, S.D.; Marshall, A.C.; Lock, J.E.; Marcus, E.N.; Tworetzky, W. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: Patient selection for fetal intervention. Circulation. 2006, 113, 1401–1405. [Google Scholar] [CrossRef] [Green Version]
- Sedmera, D.; Harris, B.S.; Grant, E.; Zhang, N.; Jourdan, J.; Kurkova, D.; Gourdie, R.G. Cardiac expression patterns of endothelin-converting enzyme (ECE): Implications for conduction system development. Dev. Dyn. 2008, 237, 1746–5317. [Google Scholar] [CrossRef] [Green Version]
- Pesevski, Z.; Kvasilova, A.; Stopkova, T.; Nanka, O.; Drobna Krejci, E.; Buffinton, C.; Kockova, R.; Eckhardt, A.; Sedmera, D. Endocardial fibroelastosis is secondary to hemodynamic alterations in the chick embryonic model of hypoplastic left heart syndrome. Dev. Dyn. 2018, 247, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Van der Heiden, K.; Groenendijk, B.C.; Hierck, B.P.; Hogers, B.; Koerten, H.K.; Mommaas, A.M.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev. Dyn. 2006, 235, 19–28. [Google Scholar] [CrossRef]
- Ricci, M.; Xu, Y.; Hammond, H.L.; Willoughby, D.A.; Nathanson, L.; Rodriguez, M.M.; Vatta, M.; Lipshultz, S.E.; Lincoln, J. Myocardial alternative RNA splicing and gene expression profiling in early stage hypoplastic left heart syndrome. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Sucharov, C.C.; Sucharov, J.; Karimpour-Fard, A.; Nunley, K.; Stauffer, B.L.; Miyamoto, S.D. Micro-RNA expression in hypoplastic left heart syndrome. J. Card. Fail. 2015, 21, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, K.; Al-Ahdab, M.K.; Ilbawi, M.N.; Hassaniya, N.; Gupta, M. Transcription repression and blocks in cell cycle progression in hypoplastic left heart syndrome. Am. J. Physiol. 2008, 294, 2268–2275. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.; Lindroth, J.R.; Wong, Y.M.M. Regional differences in DNA-synthetic activity in the preseptation myocardium of the chick. In Developmental Cardiology: Morphogenesis and Function; Clark, E.B., Takao, A., Eds.; Futura Publishing Co: Mount Kisco, NY, USA, 1990; pp. 219–234. [Google Scholar]
- Thompson, R.P.; Kanai, T.; Germroth, P.G.; Gourdie, R.G.; Chan-Thomas, P.; Barton, P.J.R.; Mikawa, T.; Anderson, R.H. Organization and function of early specialized myocardium. In Developmental Mechanisms of Heart Disease; Clark, E.B., Markwald, R.R., Takao, A., Eds.; Futura Publishing Co: Mount Kisco, NY, USA, 1995; pp. 269–280. [Google Scholar]
- Gourdie, R.G.; Mima, T.; Thompson, R.P.; Mikawa. T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development 1995, 121, 1423–1431. [Google Scholar]
- Reckova, M.; Rosengarten, C.; deAlmeida, A.; Stanley, C.P.; Wessels, A.; Gourdie, R.G.; Thompson, R.P.; Sedmera, D. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ. Res. 2003, 93, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.E.; Hurtado, R.; Hewett, K.W.; Shulimovich, M.; Poma, C.P.; Reckova, M.; Justus, C.; Pennisi, D.J.; Tobita, K.; Sedmera, D.; et al. Hemodynamic-dependent patterning of endothelin converting enzyme 1 expression and differentiation of impulse-conducting Purkinje fibers in the embryonic heart. Development 2004, 131, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Hogers, B.; Deruiter, M.C.; Baasten, A.M.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Intracardiac blood flow patterns related to the yolk sac circulation of the chick embryo. Circ. Res. 1995, 76, 871–877. [Google Scholar] [CrossRef]
- Hogers, B.; Deruiter, M.C.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryo lethal. Cardiovasc. Res. 1999, 41, 87–99. [Google Scholar] [CrossRef]
- Hogers, B.; van der Weerd, L.; Olofsen, H.; van der Graaf, L.M.; DeRuiter, M.C.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Non-invasive tracking of avian development in vivo by MRI. NMR Biomed. 2009, 22, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Broekhuizen, M.L.; Bouman, H.G.; Mast, F.; Mulder, P.G.; Gittenberger-De Groot, A.C.; Wladimiroff, J.W. Hemodynamic changes in HH stage 34 chick embryos after treatment with all-trans-retinoic acid. Pediatr. Res. 1995, 38, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekhuizen, M.L.; Hogers, B.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; Wladimiroff, J.W. Altered hemodynamics in chick embryos after extraembryonic venous obstruction. Ultrasound. Obstet. Gynecol. 1999, 13, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Stekelenburg-De Vos, S.; Ursem, N.T.; Hop, W.C.; Wladimiroff, J.W.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Acutely altered hemodynamics following venous obstruction in the early chick embryo. J. Exp. Biol. 2003, 206, 1051–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stekelenburg-De Vos, S.; Steendijk, P.; Ursem, N.T.; Wladimiroff, J.W.; Delfos, R.; Poelmann, R.E. Systolic and dias2olic ventricular function assessed by pressure-volume loops in the stage 21 venous clipped chick embryo. Pediatr. Res. 2005, 57, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stekelenburg-de Vos, S.; Steendijk, P.; Ursem, N.T.; Wladimiroff, J.W.; Poelmann, R.E. Systolic and diastolic ventricular function in the normal and extra-embryonic venous clipped chicken embryo of stage 24: A pressure-volume loop assessment. Ultrasound. Obstet. Gynecol. 2007, 30, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ursem, N.T.; Stekelenburg-de Vos, S.; Wladimiroff, J.W.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; Hu, N.; Clark, E.B. Ventricular diastolic filling characteristics in stage-24 chick embryos after extra-embryonic venous obstruction. J. Exp. Biol. 2004, 207, 1487–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenendijk, B.C.; Hierck, B.P.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev. Dyn. 2004, 230, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, B.C.; Van der Heiden, K.; Hierck, B.P.; Poelmann, R.E. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology (Bethesda). 2007, 22, 380–389. [Google Scholar] [CrossRef]
- Groenendijk, B.C.; Stekelenburg-de Vos, S.; Vennemann, P.; Wladimiroff, J.W.; Nieuwstadt, F.T.; Lindken, R.; Westerweel, J.; Hierck, B.P.; Ursem, N.T.; Poelmann, R.E. The endothelin-1 pathway and the development of cardiovascular defects in the hemodynamically challenged chicken embryo. J. Vasc. Res. 2008, 45, 54–68. [Google Scholar] [CrossRef]
- Kurihara, Y.; Kurihara, H.; Oda, H.; Maemura, K.; Nagai, R.; Ishikawa, T.; Yazaki, Y. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J. Clin. Investig. 1995, 96, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.C.; Zhao, Y.D.; Courtman, D.W.; Stewart, D.J. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000, 101, 2345–2348. [Google Scholar] [CrossRef] [Green Version]
- Gessner, I.H. Spectrum of congenital cardiac anomalies produced in chick embryos by mechanical interference with cardiogenesis. Circ. Res. 1966, 18, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Clark, E.B.; Rosenquist, G.C. Spectrum of cardiovascular anomalies following cardiac loop constriction in the chick embryo. Birth Defects Orig. Artic. Ser. 1978, 14, 431–442. [Google Scholar] [PubMed]
- Clark, E.B.; Hu, N.; Rosenquist, G.C. Effect of conotruncal constriction on aortic-mitral valve continuity in the stage 18, 21 and 24 chick embryo. Am. J. Cardiol. 1984, 53, 324–327. [Google Scholar] [CrossRef]
- Clark, E.B.; Hu, N.; Frommelt, P.; Vandekieft, G.K.; Dummett, J.L.; Tomanek, R.J. Effect of increased pressure on ventricular growth in stage 21 chick embryos. Am. J. Physiol. 1989, 257, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelmann, G.L.; Campbell, S.E.; Rakusan, K. Immediate postnatal rat heart development modified by abdominal aortic banding: analysis of gene expression. Mol. Cell Biochem. 1996, 163–164, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Anversa, P.; Ricci, R.; Olivetti, G. Quantitative structural analysis of the myocardium during physiologic growth and induced cardiac hypertrophy: A review. J. Am. Coll. Cardiol. 1986, 7, 1140–1149. [Google Scholar] [CrossRef] [Green Version]
- Sedmera, D.; Thompson, R.P. Myocyte proliferation in the developing heart. Dev. Dyn. 2011, 240, 1322–1334. [Google Scholar] [CrossRef] [Green Version]
- Parnall, M.; Perdios, C.; Pang, K.L.; Rochette, S.; Loughna, S. Characterization of the developing heart in a pressure overloaded model utilizing RNA sequencing to direct functional analysis. J. Anat. 2020, 236, 549–563. [Google Scholar] [CrossRef]
- Menon, V.; Eberth, J.F.; Junor, L.; Potts, A.J.; Belhaj, M.; Dipette, D.J.; Jenkins, M.W.; Potts, J.D. Removing vessel constriction on the embryonic heart results in changes in valve gene expression, morphology, and hemodynamics. Dev. Dyn. 2018, 247, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Lucitti, J.L.; Tobita, K.; Keller, B.B. Arterial hemodynamics and mechanical properties after circulatory intervention in the chick embryo. J. Exp. Biol. 2005, 208, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Lucitti, J.L.; Visconti, R.; Novak, J.; Keller, B.B. Increased arterial load alters aortic structural and functional properties during embryogenesis. Am. J. Physiol. 2006, 291, 1919–1926. [Google Scholar] [CrossRef]
- Kirby, M.; Stewart, D. Adrenergic innervation of the developing chick heart: Neural crest ablations to produce sympathetically aneural hearts. Am. J. Anat. 1984, 171, 295–305. [Google Scholar] [CrossRef]
- Kirby, M.L.; Stewart, D.E. Neural crest origin of cardiac ganglion cells in the chick embryo: Identification and extirpation. Dev. Biol. 1983, 97, 433–443. [Google Scholar] [CrossRef]
- Kirby, M.L.; Turnage, K.L., 3rd; Hays, B.M. Characterization of conotruncal malformations following ablation of “cardiac” neural crest. Anat. Rec. 1985, 213, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hutson, M.R.; Kirby, M.L. Neural crest and cardiovascular development: A 20-year perspective. Birth Defects Res. C. Embryo Today. 2003, 69, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.E.; Kirby, M.L.; Sulik, K.K. Hemodynamic changes in chick embryos precede heart defects after cardiac neural crest ablation. Circ. Res. 1986, 59, 545–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, H.; Connuck, D.M.; Leatherbury, L.; Kirby, M.L. Relation of early hemodynamic changes to final cardiac phenotype and survival after neural crest ablation in chick embryos. Circulation 1991, 84, 1289–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leatherbury, L.; Connuck, D.M.; Gauldin, H.E.; Kirby, M.L. Hemodynamic changes and compensatory mechanisms during early cardiogenesis after neural crest ablation in chick embryos. Pediatr. Res. 1991, 30, 509–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, S.J.; Godt, R.E.; Hatcher, C.J.; Leatherbury, L.; Zolotouchnikov, V.V.; Brotto, M.A.; Copp, A.J.; Kirby, M.L.; Creazzo, T.L. Neural crest is involved in development of abnormal myocardial function. J. Mol. Cell. Cardiol. 1997, 29, 2675–2685. [Google Scholar] [CrossRef]
- Nosek, T.M.; Fogaça, R.T.; Hatcher, C.J.; Brotto, M.A.; Godt, R.E. Effect of cardiac neural crest ablation on contractile force and calcium uptake and release in chick heart. Am. J. Physiol. 1997, 273, 1464–1471. [Google Scholar] [CrossRef]
- Creazzo, T.L.; Wang, Q.; Godt, R.E. Colocalization of dihydropyridine and ryanodine receptors in developing heart with a neural crest-associated defect. Exp. Clin. Cardiol. 2001, 6, 11–16. [Google Scholar]
- Keller, B.B.; MacLennan, M.J.; Tinney, J.P.; Yoshigi, M. In vivo assessment of embryonic cardiovascular dimensions and function in day 10.5 To 14.5 mouse embryos. Circ. Res. 1996, 79, 247–255. [Google Scholar] [CrossRef]
- Furukawa, S.; MacLennan, M.J.; Keller, B.B. Hemodynamic response to anesthesia in pregnant and nonpregnant ICR mice. Lab. Animal Sci. 1998, 48, 357–363. [Google Scholar]
- Furukawa, S.; Tinney, J.P.; Tobita, K.; Keller, B.B. Hemodynamic vulnerability to acute hypoxia in day 10.5-16.5 murine embryos. J. Obstet. Gynecol. Res. 2007, 33, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, O.; Christopher, D.A.; Foster, F.S.; Turnbull, D.H. 40-MHZ echocardiography scanner for cardiovascular assessment of mouse embryos. Ultrasound. Med. Biol. 1998, 24, 1407–1417. [Google Scholar] [CrossRef]
- Foster, F.S.; Zhang, M.Y.; Zhou, Y.Q.; Liu, G.; Mehi, J.; Cherin, E.; Harasiewicz, K.A.; Starkoski, B.G.; Zan, L.; Knapik, D.A.; et al. A new ultrasound instrument for in vivo microimaging of mice. Ultrasound. Med. Biol. 2002, 28, 1165–1172. [Google Scholar] [CrossRef]
- Huang, C.; Sheikh, F.; Hollander, M.; Cai, C.; Becker, D.; Chu, P.H.; Evans, S.; and Chen, J. Embryonic atrial function is essential for mouse embryogenesis, cardiac morphogenesis and angiogenesis. Development 2003, 130, 6111–6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiwata, T.; Nakazawa, M.; Pu, W.T.; Tevosian, S.G.; Izumo, S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ. Res. 2003, 93, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.Q.; Foster, F.S.; Parkes, R.; Adamson, S.L. Developmental changes in left and right ventricular diastolic filling patterns in mice. Am. J. Physiol. 2003, 285, 1563–1575. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.A.; Baron, M.H.; Fraser, S.E.; Dickinson, M.E. Measuring hemodynamic changes during mammalian development. Am. J. Physiol. 2004, 287, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Slevin, J.C.; Byers, L.; Gertsenstein, M.; Qu, D.; Mu, J.; Sunn, N.; Kingdom, J.C.; Rossant, J.; Adamson, S.L. High resolution ultrasound-guided microinjection for interventional studies of early embryonic and placental development in vivo in mice. BMC Dev. Biol. 2006, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Hoog, T.G.; Fredrickson, S.J.; Hsu, C.W.; Senger, S.M.; Dickinson, M.E.; Udan, R.S. The effects of reduced hemodynamic loading on morphogenesis of the mouse embryonic heart. Dev. Biol. 2018, 442, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.H.; Linask, K.K.; Khowsathit, P.; Huhta, J.C. Doppler echocardiography of normal and abnormal embryonic mouse heart. Pediatr. Res. 1996, 40, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
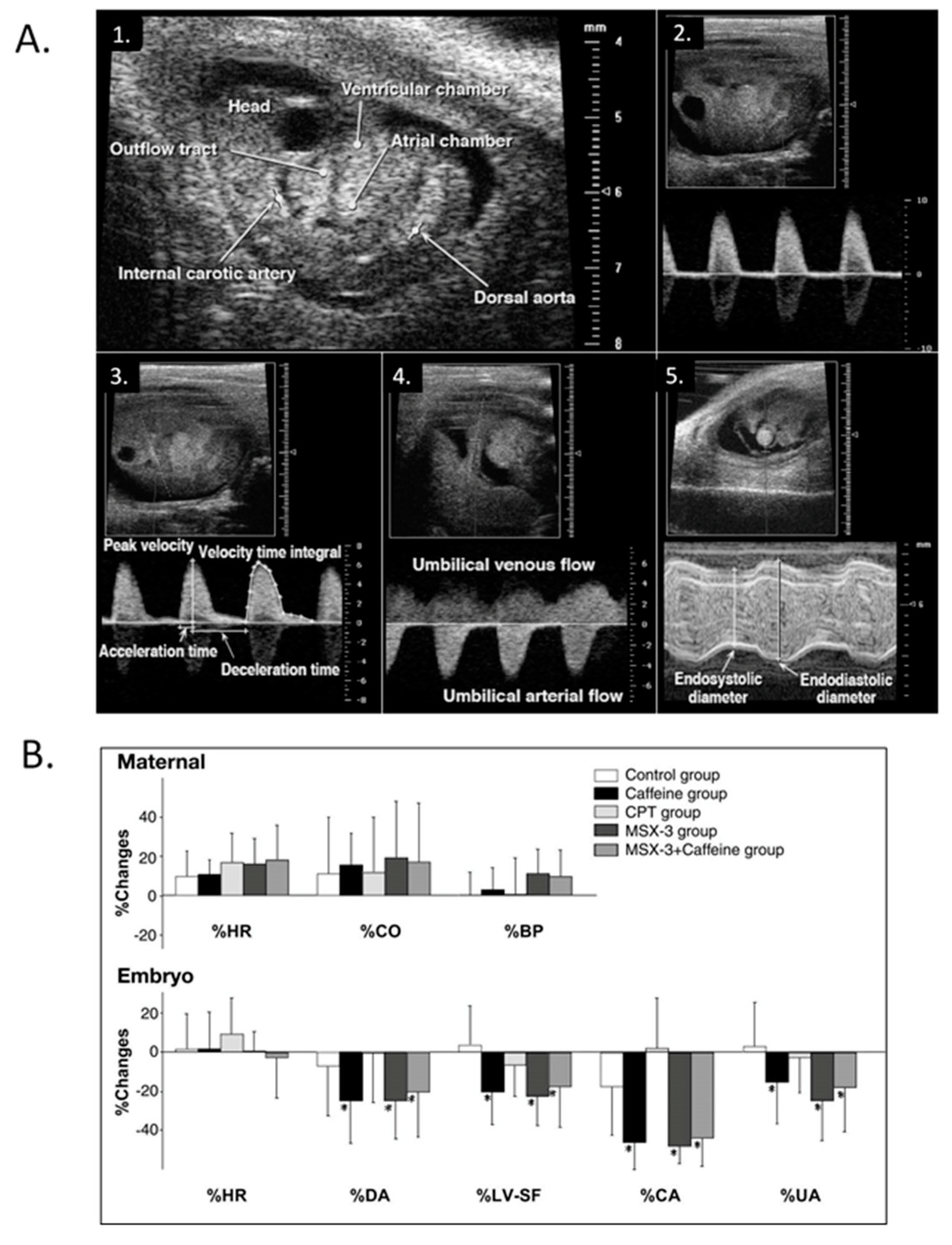
- Momoi, N.; Tinney, J.P.; Keller, B.B.; Tobita, K. Maternal hypoxia and caffeine exposure depress fetal cardiovascular function during primary organogenesis. J. Obstet. Gynecol. Res. 2012, 38, 1343–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momoi, N.; Tinney, J.P.; Liu, L.J.; Elshershari, H.; Hoffmann, P.J.; Ralphe, J.C.; Keller, B.B.; Tobita, K. Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth. Am. J. Physiol. 2008, 294, 2248–2256. [Google Scholar] [CrossRef]
- Ursem, N.T.C.; Brinkman, H.J.F.; Struijk, P.C.; Hop, W.C.J.; Kempski, M.H.; Keller, B.B.; Wladimiroff, J.W. Umbilical artery waveform analysis based on maximum, mean, and mode velocity in early human pregnancy. Ultrasound Med. Biol. 1998, 24, 1–7. [Google Scholar] [CrossRef]
- Ursem, N.T.C.; Clark, E.B.; Keller, B.B.; Hop, W.C.J.; Wladimiroff, J.W. Assessment of fetal heart rate and velocity variability by Doppler velocimetry of the descending aorta at 10-20 weeks of gestation. Ultrasound Obstet. Gynecol. 1999, 14, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Phoon, C.K.; Aristizabal, O.; Turnbull, D.H. 40 MHz Doppler characterization of umbilical and dorsal aortic blood flow in the early mouse embryo. Ultrasound Med. Biol. 2000, 26, 1275–1283. [Google Scholar] [CrossRef]
- Phoon, C.K.; Aristizábal, O.; Turnbull, D.H. Spatial velocity profile in mouse embryonic aorta and Doppler-derived volumetric flow: A preliminary model. Am. J. Physiol. 2002, 283, 908–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phoon, C.K.; Ji, R.P.; Aristizábal, O.; Worrad, D.M.; Zhou, B.; Baldwin, H.S.; Turnbull, D.H. Embryonic heart failure in NFATc1-/- mice: Novel mechanistic insights from in utero ultrasound biomicroscopy. Circ. Res. 2004, 95, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struijk, P.C.; Ursem, N.T.C.; Mathews, J.; Clark, E.B.; Keller, B.B.; Wladimiroff, J.W. Power spectrum analysis of heart rate and blood flow velocity variability in the umbilical artery and uterine artery in early pregnancy. A comparative study. Ultrasound Obstet. Gynecol. 2001, 17, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Foster, F.S.; Qu, D.W.; Zhang, M.; Harasiewicz, K.A.; Adamson, S.L. Applications for multifrequency ultrasound biomicroscopy in mice from implantation to adulthood. Physiol. Genom. 2002, 10, 113–126. [Google Scholar] [CrossRef] [Green Version]
- MacLennan, M.J.; Keller, B.B. Murine embryonic umbilical arterial velocity during development and following acutely increased heart rate. Ultrasound Med. Biol. 1999, 25, 361–370. [Google Scholar] [CrossRef]
- Yashiro, K.; Shiratori, H.; Hamada, H. Hemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 2007, 450, 285–288. [Google Scholar] [CrossRef]
- Shen, Y.; Leatherbury, L.; Rosenthal, J.; Yu, Q.; Pappas, M.A.; Wessels, A.; Lucas, J.; Siegfried, B.; Chatterjee, B.; Svenson, K.; et al. Cardiovascular phenotyping of fetal mice by noninvasive high-frequency ultrasound facilitates recovery of ENU-induced mutations causing congenital cardiac and extracardiac defects. Physiol. Genom. 2005, 24, 24,23–36. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Tobita, K.; Francis, R.J.; Lo, C.W. Imaging techniques for visualizing and phenotyping congenital heart defects in murine models. Birth Defects Res. C. Embryo Today. 2013, 99, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Francis, R.; Kim, A.J.; Ramirez, R.; Chen, G.; Subramanian, R.; Anderton, S.; Kim, Y.; Wong, L.; Morgan, J.; et al. Interrogating congenital heart defects with noninvasive fetal echocardiography in a mouse forward genetic screen. Circ. Cardiovasc. Imaging. 2014, 7, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kim, A.J.; Reynolds, W.; Wu, Y.; Lo, C.W. Phenotyping cardiac and structural birth defects in fetal and newborn mice. Birth Defects Res. 2017, 109, 778–790. [Google Scholar] [CrossRef]
- Cleary, J.O.; Price, A.N.; Thomas, D.L.; Scambler, P.J.; Kyriakopoulou, V.; McCue, K.; Schneider, J.E.; Ordidge, R.J.; Lythgoe, M.F. Cardiac phenotyping in ex vivo murine embryos using microMRI. NMR Biomed. 2009, 22, 857–866. [Google Scholar] [CrossRef]
- Stainier, D.Y.; Fishman, M.C. The zebrafish as a model system to study cardiovascular development. Trends Cardiovasc. Med. 1994, 4, 207–212. [Google Scholar] [CrossRef]
- Hu, N.; Sedmera, D.; Yost, H.J.; Clark, E.B. Structure and function of the developing zebrafish heart. Anat. Rec. 2000, 260, 148–157. [Google Scholar] [CrossRef]
- Hove, J.R.; Köster, R.W.; Forouhar, A.S.; Acevedo-Bolton, G.; Fraser, S.E.; Gharib, M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003, 421, 172–177. [Google Scholar] [CrossRef]
- Bartman, T.; Walsh, E.C.; Wen, K.K.; McKane, M.; Ren, J.; Alexander, J.; Rubenstein, P.A.; Stainier, D.Y. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004, 2, 129. [Google Scholar] [CrossRef] [Green Version]
- Forouhar, A.S.; Liebling, M.; Hickerson, A.; Nasiraei-Moghaddam, A.; Tsai, H.J.; Hove, J.R.; Fraser, S.E.; Dickinson, M.E.; Gharib, M. The embryonic vertebrate heart tube is a dynamic suction pump. Science 2006, 312, 751–753. [Google Scholar] [CrossRef] [Green Version]
- Liebling, M.; Forouhar, A.S.; Wolleschensky, R.; Zimmermann, B.; Ankerhold, R.; Fraser, S.E.; Gharib, M.; Dickinson, M.E. Rapid three-dimensional imaging and analysis of the beating embryonic heart reveals functional changes during development. Dev. Dyn. 2006, 235, 2940–2948. [Google Scholar] [CrossRef]
- Denvir, M.A.; Tucker, C.S.; Mullins, J.J. Systolic and diastolic ventricular function in zebrafish embryos: Influence of norepenephrine, MS-222 and temperature. BMC Biotechnol. 2008, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.T.; Pomerantsev, E.V.; Mably, J.D.; MacRae, C.A. High-resolution cardiovascular function confirms functional orthology of myocardial contractility pathways in zebrafish. Physiol. Genom. 2010, 42, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Patrick, M.J.; Corti, P.; Kowalski, W.J.; Roman, B.L.; Pekkan, K. Analysis of early embryonic great-vessel microcirculation in zebrafish using high-speed confocal μPIV. Biorheology 2011, 48, 305–321. [Google Scholar] [CrossRef]
- Anton, H.; Harlepp, S.; Ramspacher, C.; Wu, D.; Monduc, F.; Bhat, S.; Liebling, M.; Paoletti, C.; Charvin, G.; Freund, J.B.; et al. Pulse propagation by a capacitive mechanism drives embryonic blood flow. Development 2013, 140, 4426–4434. [Google Scholar] [CrossRef] [Green Version]
- Jamison, R.A.; Samarage, C.R.; Bryson-Richardson, R.J.; Fouras, A. In vivo wall shear measurements within the developing zebrafish heart. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Kalogirou, S.; Malissovas, N.; Moro, E.; Argenton, F.; Stainier, D.Y.; Beis, D. Intracardiac flow dynamics regulate atrioventricular valve morphogenesis. Cardiovasc. Res. 2014, 104, 49–60. [Google Scholar] [CrossRef]
- Boselli, F.; Vermot, J. Live imaging and modeling for shear stress quantification in the embryonic zebrafish heart. Methods 2016, 9, 129–134. [Google Scholar] [CrossRef]
- Boselli, F.; Steed, E.; Freund, J.B.; Vermot, J. Anisotropic shear stress patterns predict the orientation of convergent tissue movements in the embryonic heart. Development 2017, 144, 4322–4327. [Google Scholar] [CrossRef] [Green Version]
- Bark, D.L., Jr.; Johnson, B.; Garrity, D.; Dasi, L.P. Valveless pumping mechanics of the embryonic heart during cardiac looping: Pressure and flow through micro-PIV. J. Biomech. 2017, 50, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, H.C.; Amindari, A.; Butcher, J.T.; Althani, A.; Yacoub, M. Heart function and hemodynamic analysis for zebrafish embryos. Dev. Dyn. 2017, 246, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Battista, N.A.; Douglas, D.R.; Lane, A.N.; Samsa, L.A.; Liu, J.; Miller, L.A. Vortex Dynamics in Trabeculated Embryonic Ventricles. J. Cardiovasc. Dev. Dis. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Sidhwani, P.; Yelon, D. Fluid forces shape the embryonic heart: Insights from zebrafish. Curr. Top. Dev. Biol. 2019, 132, 395–416. [Google Scholar]
- Salman, H.E.; Yalcin, H.C. Advanced blood flow assessment in Zebrafish via experimental digital particle image velocimetry and computational fluid dynamics modelling. Micron 2020, 130. [Google Scholar] [CrossRef]
- Dietrich, A.C.; Lombardo, V.A.; Veerkamp, J.; Priller, F.; Abdelilah-Seyfried, S. Blood flow and Bmp signaling control endocardial chamber morphogenesis. Dev. Cell 2014, 30, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Goddard, L.M.; Duchemin, A.L.; Ramalingan, H.; Wu, B.; Chen, M.; Bamezai, S.; Yang, J.; Li, L.; Morley, M.P.; Wang, T.; et al. Hemodynamic forces sculpt developing heart valves through a KLF2-WNT9B paracrine signaling axis. Dev. Cell 2017, 43, 274–289. [Google Scholar]
- Taylor, J.M.; Nelson, C.J.; Bruton, F.A.; Baghbadrani, A.K.; Buckley, C.; Tucker, C.S.; Rossi, A.G.; Mullins, J.J.; Denvir, M.A. Adaptive prospective optical gating enables day-long 3D time-lapse imaging of the beating embryonic zebrafish heart. Nat. Commun. 2019, 10, 5073. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Yost, H.J. Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Dev. Biol. 2003, 257, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Banjo, T.; Grajcarek, J.; Yoshino, D.; Osada, H.; Miyasaka, K.Y.; Kida, Y.S.; Ueki, Y.; Nagayama, K.; Kawakami, K.; Matsumoto, T.; et al. Haemodynamically dependent valvulogenesis of zebrafish heart is mediated by flow-dependent expression of miR-21. Nat. Commun. 2013, 4, 1978. [Google Scholar] [CrossRef] [Green Version]
- Cordes, K.R.; Srivastava, D. MicroRNA regulation of cardiovascular development. Circ. Res. 2009, 104, 724–732. [Google Scholar] [CrossRef]
- Ursem, N.T.C.; Clark, E.B.; Keller, B.B.; Wladimiroff, J.W. Fetal heart rate and umbilical artery velocity variability in pregnancies complicated by insulin-dependent diabetes mellitus. Ultrasound Obstet. Gynecol. 1999, 13, 312–316. [Google Scholar] [CrossRef]
- Ursem, N.T.C.; Clark, E.B.; Keller, B.B.; Hop, W.C.J.; Wladimiroff, J.W. Do heart rate and velocity variability derived from umbilical artery velocity waveforms change prior to clinical pregnancy induced hypertension? Ultrasound Obstet. Gynecol. 1999, 14, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Acharya, G.; Gui, Y.; Cnota, W.; Huhta, J.; Wloch, A. Human embryonic cardiovascular function. Acta. Obstet. Gynecol. Scand. 2016, 95, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.P.; Bruneau, B.G. Epigenetics and cardiovascular development. Annu. Rev. Physiol. 2012, 74, 41–68. [Google Scholar] [CrossRef]
- Srivastava, D. Genetic regulation of cardiogenesis and congenital heart disease. Annu. Rev. Pathol. 2006, 1, 199–249. [Google Scholar] [CrossRef]
- Pierpont, M.E.; Basson, C.T.; Benson, D.W.J.; Gelb, B.D.; Giglia, T.M.; Goldmuntz, E.; McGee, G.; Sable, C.A.; Srivastava, D.; Webb, C.L. American Heart Association Congenital Cardiac Defects Committee; Council on Cardiovascular Disease in the Young. Genetic basis for congenital heart defects: Current knowledge: A scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: Endorsed by the American Academy of Pediatrics. Circulation 2007, 115, 3015–3038. [Google Scholar]
- Pierpont, M.E.; Brueckner, M.; Chung, W.K.; Garg, V.; Lacro, R.V.; McGuire, A.L.; Mital, S.; Priest, J.R.; Pu, W.T.; Roberts, A.; et al. Genetic basis for congenital heart disease: Revisited: A scientific statement from the american heart association. Circulation 2018, 138, 653–7011. [Google Scholar] [CrossRef]
- Fahed, A.C.; Gelb, B.D.; Seidman, J.G.; Seidman, C.E. Genetics of congenital heart disease: The glass half empty. Circ. Res. 2013, 112, 707–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelb, B.D.; Chung, W.K. Complex genetics and the etiology of human congenital heart disease. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef]
- Edwards, J.J.; Gelb, B.D. Genetics of congenital heart disease. Curr. Opin. Cardiol. 2016, 31, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.; Brueckner, M. Genetics and genomics of congenital heart disease. Circ. Res. 2017, 120, 923–940. [Google Scholar] [CrossRef]
- Drews, J.D.; Pepper, V.K.; Best, C.A.; Szafron, J.M.; Cheatham, J.P.; Yates, A.R.; Hor, K.N.; Zbinden, J.C.; Chang, Y.C.; Mirhaidari, G.J.M.; et al. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Domae, K.; Yoshikawa, Y.; Fukushima, S.; Nakamura, T.; Saito, A.; Sakata, Y.; Hamada, S.; Toda, K.; Pak, K.; et al. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J. Am. Heart. Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Bittle, G.J.; Morales, D.; Deatrick, K.B.; Parchment, N.; Saha, P.; Mishra, R.; Sharma, S.; Pietris, N.; Vasilenko, A.; Bor, C.; et al. Stem cell therapy for hypoplastic left heart syndrome: Mechanism, clinical application, and future directions. Circ Res. 2018, 123, 288–300. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, B.B.; Kowalski, W.J.; Tinney, J.P.; Tobita, K.; Hu, N. Validating the Paradigm That Biomechanical Forces Regulate Embryonic Cardiovascular Morphogenesis and Are Fundamental in the Etiology of Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2020, 7, 23. https://doi.org/10.3390/jcdd7020023
Keller BB, Kowalski WJ, Tinney JP, Tobita K, Hu N. Validating the Paradigm That Biomechanical Forces Regulate Embryonic Cardiovascular Morphogenesis and Are Fundamental in the Etiology of Congenital Heart Disease. Journal of Cardiovascular Development and Disease. 2020; 7(2):23. https://doi.org/10.3390/jcdd7020023
Chicago/Turabian StyleKeller, Bradley B., William J. Kowalski, Joseph P. Tinney, Kimimasa Tobita, and Norman Hu. 2020. "Validating the Paradigm That Biomechanical Forces Regulate Embryonic Cardiovascular Morphogenesis and Are Fundamental in the Etiology of Congenital Heart Disease" Journal of Cardiovascular Development and Disease 7, no. 2: 23. https://doi.org/10.3390/jcdd7020023





