Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds
Abstract
:1. Introduction
2. Antifungal Peptides
2.1. Natural Peptides
2.1.1. Bacterial and Fungal Peptides
2.1.2. Plant Peptides
2.1.3. Insect Peptides
2.1.4. Amphibian Peptides
2.1.5. Avian and Mammalian Antifungal Peptides
2.2. Interactions of Natural Antifungal Peptides with Other Antifungal Drugs
2.3. Natural Antifungal Peptides in New Delivery Systems
2.4. Synthetic Peptides and Peptide Derivatives
2.5. Dendrimeric Peptide Mimics
2.5.1. Dendrimers as Carriers of Bioactive Substances and Solubility Enhancers
2.5.2. Inherent Activity of Dendrimeric Peptide Mimics and Their Antifungal Formulations
3. Conclusions
Acknowledgments
Authors contribution
Conflicts of Interest
References
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.Y.; Tay, S.T.; Le, C.F.; Lee, V.S.; Sabri, N.H.; Velayuthan, R.D.; Hassan, H.; Sekaran, S.D. Activity of novel synthetic peptides against Candida albicans. Sci. Rep. 2015, 5, 9657. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Pluim, T.; Halasa, N.; Phillips, S.E.; Fleming, G. The morbidity and mortality of patients with fungal infections before and during extracorporeal membrane oxygenation support. Pediatr. Crit. Care Med. 2012, 13, e288–e293. [Google Scholar] [CrossRef] [PubMed]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. [Google Scholar] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Pappas, P.G.; Wingard, J.R. Invasive fungal pathogens: Current epidemiological trends. Clin. Infect. Dis. 2006, 43, S3–S14. [Google Scholar] [CrossRef]
- Leroy, O.; Gangneux, J.P.; Montravers, P.; Mira, J.P.; Gouin, F.; Sollet, J.P.; Carlet, J.; Reynes, J.; Rosenheim, M.; Regnier, B.; et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in France (2005–2006). Crit. Care Med. 2009, 37, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Niu, S.; Zhang, C.; Xu, X.; Qin, M.; Huang, S.; Zhang, L. Epidemiology, antifungal susceptibilities, and risk factors for invasive candidiasis from 2011 to 2013 in a teaching hospital in southwest China. J. Microbiol. Immunol. Infect. 2017, 50, 97–103. [Google Scholar] [CrossRef]
- Mikulska, M.; Bassetti, M.; Ratto, S.; Viscoli, C. Invasive candidiasis in non-hematological patients. Miditerr. J. Hematol. Infect. Dis. 2011, 3, e2011007. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Bille, J.; Marchetti, O. Diagnosis of invasive candidiasis in the ICU. Ann. Intensive Care 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Paswan, A.K.; Rajum, D.C.; Singh, D.K.; Dubey, R.K.; Mishra, P.K. An observational study of the risk factors and incidence of invasive fungal infections in ICU patients. Aneasthesia Pain Intensive Care 2013, 17, 136–140. [Google Scholar]
- Healey, K.R.; Zhao, Y.; Perez, W.B.; Lockhart, S.R.; Sobel, J.D.; Farmakiotis, D.; Kontoyiannis, D.P.; Sanglard, D.; Taj-Aldeen, S.J.; Alexander, B.D.; et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat. Commun. 2016, 7, 11128. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Tabernero, L.; Denning, D.W.; Anderson, M.J. Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 4377–4386. [Google Scholar] [CrossRef] [PubMed]
- Kołaczkowska, A.; Kołaczkowski, M. Drug resistance mechanisms and their regulation in non- albicans Candida species. J. Antimicrob. Chemother. 2016, 71, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Skiada, A. Recent advances in antifungal chemotherapy. Int. J. Antimicrob. Agents 2007, 30, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Sanguinetti, M.; Sanglard, D.; La Sorda, M.; Boccia, S.; Romano, L.; Morace, G.; Fadda, G. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol. Microbiol. 2003, 47, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D. Emerging threats in antifungal-resistant fungal pathogens. Front. Med. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.P.; Patel, J.; Magobo, R.E.; Naicker, S.; Wadula, J.; Whitelaw, A.; Coovadia, Y.; Kularatne, R.; Govind, C.; Lockhart, S.R.; et al. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: Results from laboratory-based sentinel surveillance in South Africa. J. Antimicrob. Chemother. 2016, 71, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Rosa, E.A. Editorial: Antifungal drug discovery: New theories and new therapies. Front. Microbiol. 2016, 7, 728. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Castanheira, M.; Lockhart, S.R.; Ahlguist, A.M.; Messer, S.A.; Jones, R.N. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J. Clin. Microbiol. 2012, 50, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E. Current concepts in antifungal pharmacology. Mayo Clin. Proc. 2011, 86, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D. Echinocandins for Prevention and Treatment of Invasive Fungal Infections. In Antifungal Therapy; Ghannoum, M.A., Perfect, J.R., Eds.; Informa Healthcare USA Inc.: New York, NY, USA, 2010; pp. 219–242. ISBN 978-084938787-6. [Google Scholar]
- Chen, S.C.; Slavin, M.A.; Sorrell, T.C. Echinocandin antifungal drugs in fungal infections a comparison. Drugs 2011, 71, 11–41. [Google Scholar] [CrossRef] [PubMed]
- Kohler, S.; Wheat, L.J.; Connolly, P.; Schnizlein-Bick, C.; Durkin, M.; Smedema, M.; Goldberg, J.; Brizendine, E. Comparison of the echinocandin caspofungin with amphotericin B for treatment of histoplasmosis following pulmonary challenge in a murine model. Antimicrob. Agents Chemother. 2000, 44, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 1998, 36, 2950–2956. [Google Scholar] [PubMed]
- Stringer, J.R.; Beard, C.B.; Miller, F.R.; Wakefield, A.E. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg. Infect. Dis. 2002, 8, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Lundgren, J.D.; Masur, H.; Walzer, P.D.; Hanson, D.L.; Frederick, T.; Huang, L.; Beard, C.B.; Kaplan, J.E. Current epidemiology of Pneumocystis pneumonia. Emerg. Infect. Dis. 2004, 10, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Wheat, J.; Hafner, R.; Kurzon, A.H.; Limjoco, M.T.; Spencer, P.; Larsen, R.A.; Hecht, F.M.; Podwerly, W. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trial Group. Am. J. Med. 1995, 98, 336–342. [Google Scholar] [CrossRef]
- Wheat, J.; Hafner, R.; Wulfsohn, M.; Spencer, P.; Squires, K.; Podwerly, W.; Wong, B.; Rinaldi, M.; Saag, M.; Hamill, R.; et al. National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Ann. Intern. Med. 1993, 118, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Wheat, J.; Saros, G.; McKinsey, D.; Hamill, R.; Dradsher, R.; Johnson, P.; Loyd, J.; Kauffman, C. Practice guidelines for the management of patients with histoplasmosis. Infectious Diseases Society of America. Clin. Infect. Dis. 2000, 30, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Pegu, R.; Borah, R.; Pratihar, S. Synthetic compounds for antifungal chemotherapy. In Recent Trends in Antifungal Agents and Antifungal Therapy; Basak, A., Chakraborty, R., Mandal, S.M., Eds.; Springer: New Delhi, India, 2016; pp. 191–216. ISBN 978-81-322-2782-3. [Google Scholar]
- Andriole, V.T. Current and future antifungal therapy: New targets for antifungal therapy. Int. J. Antimicrob. Agents 2000, 16, 317–321. [Google Scholar] [CrossRef]
- Li, R.K.; Ciblak, M.A.; Nordoff, N.; Pasarell, L.; Warnock, D.W.; McGinnis, M.R. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 2000, 44, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Connolly, P.; Wheat, J.; Schnizlein-Bick, C.; Durkin, M.; Kohler, S.; Smedema, M.; Goldberg, J.; Brizendine, E.; Loebenberg, D. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob. Agents Chemother. 1999, 43, 322–328. [Google Scholar]
- McKinsey, D.S.; Kauffman, C.A.; Pappas, P.G.; Cloud, G.A.; Girard, W.M.; Sharkey, P.K.; Hamill, R.J.; Thomas, C.J.; Dismukes, W.E. Fluconazole therapy for histoplasmosis. The National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin. Infect. Dis. 1996, 23, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Wheat, J.; Marichal, P.; Vanden Bossche, H.; Le Monte, A.; Connolly, P. Hypothesis on the mechanism of resistance to fluconazole in Histoplasma capsulatum. Antimicrob. Agents Chemother. 1997, 41, 410–414. [Google Scholar] [PubMed]
- Joffrion, T.M.; Cushion, M.T. Sterol biosynthesis and sterol uptake in the fungal pathogen Pneumocystis carinii. FEMS Microbiol. Lett. 2010, 311, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Raad, I.; Petrikkos, G.; Boogaerts, M.; Selleslag, D.; Petersen, F.B.; Sable, C.A.; Kartsonis, N.A.; Ngai, A.; Taylor, A.; et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 2004, 39, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Marco, F.; Pfaller, M.A.; Messer, S.A.; Jones, R.N. Activity of MK-0991 (L-743,872), a new echinocandin, compared with those of LY303366 and four other antifungal agents tested against blood stream isolates of Candida spp. Diagn. Microbiol. Infect. Dis. 1998, 32, 33–37. [Google Scholar] [CrossRef]
- Beltz, K.; Kramm, C.M.; Laws, H.J.; Schroten, H.; Wessalowski, R.; Göbel, U. Combined trimethoprim and caspofungin treatment for severe Pneumocystis jiroveci pneumonia in a five year old boy with acute lymphoblastic leukemia. Klin. Pediatr. 2006, 218, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Hof, H.; Schnülle, P. Pneumocystis jiroveci pneumonia in a patient with Wegener’s granulomatosis treated efficiently with caspofungin. Mycoses 2008, 51, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Utili, R.; Durante-Mangoni, E.; Basilico, C.; Mattei, A.; Ragone, E.; Grossi, P. Efficacy of caspofungin addition to trimethoprim-sulfamethoxazole treatment for severe pneumocystis pneumonia in solid organ transplant recipients. Transplantation 2007, 84, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.; Nelson, M. The use of caspofungin in HIV-infected individuals. Expert Opin. Investig. Drugs 2007, 16, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Cushion, M.T.; Collins, M.S. Susceptibility of Pneumocystis to echinocandins in suspension and biofilm cultures. Antimicrob. Agents Chemother. 2011, 55, 4513–4518. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Najvar, L.K.; Bocanergra, R.; Molina, D.; Olivo, M.; Graybill, J.R. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 2007, 51, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Aoki, W.; Ueda, M. Characterization of antimicrobial peptides toward the development of novel antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Ciociola, T.; Giovati, L.; Conti, S.; Magliani, W.; Santinoli, C.; Polonelli, L. Natural and synthetic peptides with antifungal activity. Future Med. Chem. 2016, 8, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, P.; Lourenço, A.; Pereira, M.O. New trends in peptide-based anti-biofilm strategies: A review of recent achievements and bioinformatic approaches. Biofouling 2012, 28, 1033–1061. [Google Scholar] [CrossRef] [PubMed]
- Landon, C.; Meudal, H.; Boulanger, N.; Bulet, P.; Vovelle, F. Solution structures of stomoxyn and spinigerin, two insect antimicrobial peptides with an alpha-helical conformation. Biopolymers 2006, 81, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of action of the antimicrobial peptide buforin II: Buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef]
- Singh, N.K.; Rani, J. Sequential and structural aspects of antifungal peptides from animals, bacteria and fungi based on bioinformatics tools. Probiotics Antimicrob. Proteins 2016, 8, 85–101. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Essig, A.; Hofmann, D.; Münch, D.; Gayathri, S.; Künzler, M.; Kallio, P.T.; Sahl, H.G.; Wider, G.; Schneider, T.; Aebi, M. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J. Biol. Chem. 2014, 289, 34953–34964. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Harries, E.; Carmona, L.; Campos-Soriano, L.; López, J.J.; Manzanares, P.; Gandía, M.; Coca, M.; Marcos, J.F. Concatemerization increases the inhibitory activity of short, cell-penetrating, cationic and tryptophan-rich antifungal peptides. Appl. Microbiol. Biotechnol. 2015, 99, 8011–8021. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.X.; Campagna, A.N.; Bobek, L.A. Factors affecting antimicrobial activity of MUC7 12-mer, a human salivary mucin-derived peptide. Ann. Clin. Microbiol. Antimicrob. 2007, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Guilhelmelli, F.; Vilena, N.; Albuquerque, P.; Derengowski, L.S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef] [PubMed]
- Vriens, K.; Cammue, B.P.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Basak, A.; Chakraborty, R.; Mandal, S.M. Recent Trends in Antifungal Agents and Antifungal Therapy; Springer: New Delhi, India, 2016; pp. 1–250. ISBN 978-81-322-2782-3. [Google Scholar]
- Holfeld, L.; Herth, N.; Singer, D.; Hoffmann, R.; Knappe, D. Immunogenicity and pharmacokinetics of short, proline-rich antimicrobial peptides. Future Med. Chem. 2015, 7, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Risso, A.; Draidot, E.; Sordano, M.C.; Vianello, A.; Marci, F.; Skerlavaj, B.; Zanetti, M.; Renato, G.; Bernardi, P. BMAP-28, an Antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol. Cell Biol. 2002, 22, 1926–1935. [Google Scholar] [CrossRef]
- Skerlavaj, B.; Benincasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999, 463, 58–62. [Google Scholar] [CrossRef]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Oudhoff, M.J.; Blaauboer, M.E.; Nazmi, K.; Scheres, N.; Bolscher, J.G.; Veerman, E.C. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol. Chem. 2010, 391, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; Mignogna, G.; Canofeni, S.; Miele, R.; Mangoni, M.L.; Barra, D. Temporins, antimicrobial peptides from the European red frog Rana temporaria. Eur. J. Biochem. 1996, 242, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and mechanisms of action of naturally occuring antifungal peptides. Cell. Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Rinaldi, A.C.; Di Giulio, A.; Mignogna, G.; Bozzi, A.; Barra, D.; Simmaco, M. Structure-function relationships of temporins, small antimicrobial peptides from amphibian skin. Eur. J. Biochem. 2000, 267, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.; Silberring, J.; Soliymani, R.; Heikkinen, S.; Kilpelainen, I.; Lankinen, H.; Kilpelainen, I.; Lankinen, H.; Kuusela, P. Antibacterial activities of temporin A analogs. FEBS Lett. 2000, 479, 6–9. [Google Scholar] [CrossRef]
- Hujakka, H.; Ratilainen, J.; Korjamo, T.; Lankinen, H.; Kuusela, P.; Santa, H.; Laatikainen, R.; Narvanen, A. Synthesis and antimicrobial activity of the symmetric dimeric form of Temporin A based on 3-N,N-di(3-aminopropyl)amino propanoic acid as the branching unit. Bioorg. Med. Chem. 2001, 9, 1601–1607. [Google Scholar] [CrossRef]
- Carotenuto, A.; Malfi, S.; Saviello, M.R.; Campiglia, P.; Gomez-Monterrey, I.; Mangoni, M.L.; Gaddi, L.M.; Novellino, E.; Grieco, P. A different molecular mechanism underlying antimicrobial and hemolytic actions of temporins A and L. J. Med. Chem. 2008, 51, 2354–2362. [Google Scholar] [CrossRef]
- Warrington, R.; Silviu-Dan, F. Drug allergy. Allergy Asthma Clin. Immunol. 2011, 7, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Breff, M.H.; Hawkings, M.A.; Si Nardo, A.; Lopez-Garcia, B.; Howell, M.D.; Wong, C.; Lin, K.; Streib, J.E.; Dorschner, R.; Leung, D.Y.; et al. Structure-function relationships among human cathelicidin peptides: Dissociation of antimicrobial properties from host immunostimulatory activities. J. Immunol. 2005, 174, 4271–4278. [Google Scholar] [CrossRef]
- Sorensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Van Wetering, S.; Mannesse-Lazeroms, S.P.; Van Sterkenburg, M.A.; Daha, M.R.; Dijkman, J.H.; Hiemstra, P.S. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am. J. Physiol. 1997, 272, L888–L896. [Google Scholar] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, P.; Yoo, Y.J.; Yoon, Y.J.; Demain, A.L. Immunosuppressants: Remarkable Microbial Products. In Bioactive Natural Products: Chemistry and Biology; Wiley-VCH Verlag CmbH & Co.: Weinheim, Germany, 2015; pp. 43–82. ISBN 978-3-527-33794-1. [Google Scholar]
- Zasloff, M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J. Am. Soc. Nephrol. 2007, 18, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Bensaci, M.F.; Gurnev, P.A.; Bezrukov, S.M.; Takemoto, J.Y. Fungicidal activities and mechanisms of action of Pseudomonas syringae pv. syringae lipodepsipeptide Syringopeptins 22A and 25A. Front. Microbiol. 2011, 2, 216. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Jacks, T.J.; Takemoto, J.; Vinyard, B.; Peter, J.; Navarro, E.; Walsh, T.J. Fungal lethality, binding, and cytotoxicity of syringomycin-E. Antimicrob. Agents Chemother. 1999, 43, 371–373. [Google Scholar] [PubMed]
- De Lucca, A.J.; Walsh, T.J. Antifungal peptides: Novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 1999, 43, 1–11. [Google Scholar] [PubMed]
- Scholz-Schroeder, B.K.; Hutchison, M.L.; Grgurina, I.; Gross, D.C. The contribution of syringopeptin and syrigomycin to virulence of Pseudomonas syringae pv. syringage strain B301D on the basis of sypA and syrB1 biosynthesis mutant analysis. Mol. Plant-Microbe Interact. 2001, 14, 336–348. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: www.uniprot.org (accessed on 19 July 2017).
- Aranda, F.J.; Teruel, J.A.; Ortiz, A. Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim. Biophys. Acta 2005, 1713, 51–56. [Google Scholar] [CrossRef]
- Li, R.K.; Rinaldi, M.G. In vitro antifungal activity of nikkomycin Z in combination with fluconazole or itraconazole. Antimicrob. Agents Chemother. 1999, 43, 1401–1405. [Google Scholar]
- Nix, D.E.; Swezey, R.R.; Hector, R.; Galgiani, J.N. Pharmacokinetics of nikkomycin Z after single rising oral doses. Antimicrob. Agents Chemother. 2009, 53, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich. Available online: www.sigmaaldrich.com (accessed on 19 July 2017).
- Hawser, S.; Islam, K. Comparisons of the effects of fungicidal and fungistatic antifungal agents on the morphogenetic transformation of Candida albicans. J. Antimicrob. Chemother. 1999, 43, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Vriens, K.; Cools, T.L.; Harvey, P.J.; Craik, D.J.; Braem, A.; Vleugels, J.; De Coninck, B.; Cammue, B.P.; Thevissen, K. The radish defensins RsAFP1 and RsAFP2 act synergistically with caspofungin against Candida albicans biofilms. Peptides 2016, 75, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Terras, F.R.; Eggermont, K.; Kovaleva, V.; Raikhel, N.V.; Osborn, R.W.; Kester, A.; Rees, S.B.; Torrekens, S.; Van Leuven, F.; Vanderleyden, J.; et al. Small cysteine-rich antifungal proteins from Raddish: Their role in host defense. Plant Cell 1995, 7, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, A.F.; Vasconcelos, E.A.R.; Pelegrini, P.B.; Grossi de Sa, M.F. Antifungal defensins and their role in plant defense. Front. Microbiol. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Miwa, A.; Maeda, K.; Kimura, M.; Nishiuchi, T. The secreted antifungal protein thionin 2.4. in Arabidopsis thaliana suppresses the toxicity of a fungal fruit body lectin from Fusarium graminearum. PLoS Pathog. 2013, 9, e1003581. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Carvalho, A.O.; Rodrigues, R.; Trindade, F.G.; Da Cunha, M.; Gomes, V.M. Thionin-like peptide from Capsicum annuum fruits: Mechanism of action and synergism with fluconazole against Candida species. BMC Microbiol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Abad, R.L.; D’Urzo, M.P.; Liu, D.; Narasimhan, M.L.; Reuveni, M.; Zhu, J.K.; Niu, X.; Singh, N.K.; Hasegawa, P.M.; Bressan, R.A. Antifungal activity of tabacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 1996, 118, 11–23. [Google Scholar] [CrossRef]
- Stevens, D.A.; Calderon, L.; Martinez, M.; Clemons, K.V.; Wilson, S.J.; Selitrennikoff, C.P. Zeamatin, clotrimazole and nikkomycin Z in therapy of a Candida vaginitis model. J. Antimicrob. Chemother. 2002, 50, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Munks, R.J.; Hamilton, J.V.; Volvelle, F.; Brun, R.; Lehane, M.J.; Bulet, P. Epithelial innate immunity. A novel antimicrobial peptide with antiparasiting activity in the blood-sucking insect Stomoxys calcitrans. J. Biol. Chem. 2002, 277, 49921–49926. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, D.G. Melittin triggers apoptosis in Candida albicans through the reactive oxygen species-mediated mitochondria/caspase-dependent pathway. FEMS Microbiol. Lett. 2014, 355, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, D.G. Melittin induces apoptotic features in Candida albicans. Biochem. Biophys. Res. Commun. 2010, 394, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.T.; Zhu, S.Y. Drosomycin, an essential component of antifungal defence in Drosophila. Insect Mol. Biol. 2009, 18, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, D-H.; Lee, D.G.; Kim, K.L.; Lee, Y. Internalization of tenecin 3 by a fungal cellular process is essential for its fungicidal effect on Candida albicans. Eur. J. Biochem. 2001, 268, 4449–4458. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, M.S.; Kim, S.C. A novel antimicrobial peptide from Bufo bufo gargarizans. Biochem. Biophys. Res. Commun. 1996, 218, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Leng, Q.; Begum, M.D.; Woodle, M.C.; Scaria, P.; Chou, S.T.; Mixson, A.J. Peptide-based antifungal therapies against emerging infections. Drugs Future 2010, 35, 197. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Choda, N.; Matsuzaki, K. Magainin 2 in action: Distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophys. J. 2008, 95, 5757–5765. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.C.; Mangoni, M.L.; Rufo, A.; Luzi, C.; Barra, D.; Zhao, H.; Kinnunen, P.K.; Bozzi, A.; Di Giulio, A.; Simmaco, M. Temporin L: Antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 2002, 368, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.R.; Brand, G.D.; Silva, L.P.; Kückelhaus, S.A.; Bento, W.R.; Araújo, A.L.; Martins, G.R.; Lazzari, A.M.; Bloch, C., Jr. Dermatoseptins from Phyllomedusa oreades and Phyllomedusa distincta: Secondary structure, antimicrobial activity, and mammalian cell toxicity. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 336–343. [Google Scholar] [CrossRef]
- Mor, A.; Nicolas, P. Isolation and structure of novel defensive peptides from frog skin. Eur. J. Biochem. 1994, 219, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, A.; Veldhuizen, E.J.; Kalkhove, S.I.; Tjeerdsma-van Bokhoven, J.L.; Romijn, R.A.; Haagsman, H.P. The beta-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob. Agents Chemother. 2007, 51, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, H.; Yu, P.L. Avian antimicrobial peptides: The defense role of beta-defensins. Biochem. Biophys. Res. Commun. 2004, 323, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Thouzeau, C.; Le Maho, Y.; Froget, G.; Sabatier, L.; Le Bohec, C.; Hoffmann, J.A.; Bulet, P. Spheniscins, avian beta-defensins in preseved stomach contents of the king penguin, Aptenodytes patagonicus. J. Biol. Chem. 2003, 278, 51053–51058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Nguyen, T.; Liu, L.; Sacco, R.E.; Brogden, K.A.; Lehrer, R.I. Gallinacin-3, an inducible epithelial beta-defensin in the chicken. Infect. Immun. 2001, 69, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, S.R.; Amarullah, I.H.; Wubbolts, R.W.; Veldhuizen, E.J.; Haagsman, H.P. Fungicidal mechanisms of cathelicidins LL-37 and CATH-2 revealed by live-cell imaging. Antimicrob. Agents Chemother. 2014, 58, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.-L.; Yin, W.-B.; Chen, Y.-H.; Niu, L.-L.; Sun, Y.-R.; Zhao, S.-M.; Yang, F.-Q.; Wang, R.C.; Wu, Q.; Zhang, X-Q.; et al. A new strategy to produce a fefensin: Stable production of mutated NP-1 in nitrate reductase-deficient Chlorella ellipsoidea. PLoS ONE 2013, 8, e54966. [Google Scholar] [CrossRef]
- Schneider, J.J.; Unholzer, A.; Schaller, M.; Schäfer-Korting, M.; Korting, H.C. Human defensins. J. Mol. Med. 2005, 83, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Wyer, L.; Berghuis, L.; Bassel, L.L.; Clark, M.E.; Caswell, J.L. Regulation of tracheal antimicrobial peptide gene expression in airway epithelial cells of cattle. Vet. Res. 2016, 47, 44. [Google Scholar] [CrossRef]
- Vylkova, S.; Nayyar, N.; Li, W.; Edgerton, M. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disrubtion. Antimicrob. Agents Chemother. 2007, 51, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Tongaonkar, P.; Tran, P.; Roberts, K.; Schaal, J.; Osapay, G.; Tran, G.; Ouellette, A.J.; Selsted, M.E. Rhesus macaque θ-defensin isoforms: Expression, antimicrobial activities, and demonstration of a prominent role in neutrophil granule microbicidal activities. J. Leukoc. Biol. 2011, 89, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Tran, P.A.; Tang, Y.Q.; Yuan, J.; Cole, T.; Selsted, M.E. Homodimeric theta-defensins from rhesus macaque leukocytes: Isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 2002, 277, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Lee, P.H.A.; Gallo, R.L. Expression and potential function of cathelicidin antimicrobial peptides in dermatophytosis and tinea versicolor. J. Antimicrob. Chemother. 2006, 57, 877–882. [Google Scholar] [CrossRef]
- Benincasa, M.; Scocchi, M.; Pacor, S.; Tossi, A.; Nobili, D.; Basaglia, G.; Busetti, M.; Gennaro, R. Fungicidal activity of five cathelicidin peptides against clinical isolated yeasts. J. Antimicrob. Chemother. 2006, 58, 950–959. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczyk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Kim, H.K.; Kim, S.A.; Park, Y.; Park, S.C.; Jang, S.H.; Hahm, K.S. Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem. Biophys. Res. Commun. 2003, 305, 305–310. [Google Scholar] [CrossRef]
- Lawyer, C.; Pai, S.; Watabe, M.; Borgia, P.; Mashimo, T.; Eagleton, L.; Watabe, K. Antimicrobial activity of a 13 amino acid tryptophan-rich peptide derived from a putative porcine precursor protein of a novel family of antibacterial peptides. FEBS Lett. 1996, 390, 95–98. [Google Scholar] [CrossRef]
- Cho, Y.; Turner, J.S.; Dinh, N.N.; Lehrer, R.I. Activity of protegrins against yeast-phase Candida albicans. Infect. Immun. 1998, 66, 2486–2493. [Google Scholar] [PubMed]
- Han, J.; Jyoti, M.A.; Song, H.Y.; Jang, W.S. Antifungal activity and action mechanism of histidin 5-halocidin hybrid peptides against Candida spp. PLoS ONE 2016, 11, e0150196. [Google Scholar] [CrossRef]
- Puri, S.; Edgerton, M. How does it kill? Understanding the candidacidal mechanism of salivary histatin 5. Eukaryot. Cell 2014, 13, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luther, P.W.; Leng, Q.; Mixson, A.J. Synthetic histidine-rich peptides inhibit Candida species and other fungi in vitro: Role of endocytosis and treatment implications. Antimicrob. Agents Chemother. 2006, 50, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial activity of lactoferrin-related peptides and applications in human and veterinary medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, A.; Satoh, T.; Wakabayashi, H.; Ikeda, F. Effects of bovine lactorerrin to oral Candida albicans and Candida glabrata isolates recovered from saliva in elderly people. Odontology 2015, 103, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Takakura, N.; Wakabayashi, H.; Ishibashi, H.; Teraguchi, S.; Tamura, Y.; Yamaguchi, H.; Abe, S. Oral lactoferrin treatment of experimental oral candidiasis in mice. Antimicrob. Agents Chemother. 2003, 47, 2619–2623. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, S.; Hyun, B.; Suh, J.W.; Yon, C.; Kim, C.; Lim, Y.; Kim, C. Cepacidine A, a novel antifungal antibiotic produced by Pseudomonas cepacia. I. Taxonomy, production, isolation and biological activity. J. Antibiot. 1994, 47, 1402–1405. [Google Scholar] [CrossRef]
- Romero, D.; de Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.W.; Arrebola, E.; Cazorla, F.M.; Kuipers, O.P.; Paquot, M.; Pérez-García, A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant Microbe Interact. 2007, 20, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Sandovsky-Losica, H.; Shwartzman, R.; Lahat, Y.; Segal, E. Antifungal activity against Candida albicans of nikkomycin Z in combination with caspofungin, voriconazole or amphotericin B. J. Antimicrob. Chemother. 2008, 62, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Aoki, W.; Kitahara, N.; Miura, N.; Morisaka, H.; Yamamoto, Y.; Koruda, K.; Ueda, M. Candida albicans possesses Sap7 as a pepstatin A-insensitive secreted aspartic pro tease. PLoS ONE 2012, 7, e32513. [Google Scholar] [CrossRef] [PubMed]
- Cutfield, S.M.; Dodson, E.J.; Anderson, B.F.; Moody, P.C.; Marshall, C.J.; Sullivan, P.A.; Cutfield, J.F. The crystal structure of a major secreted aspartic proteinase from Candida albicans in complexes with two inhibitors. Structure 1995, 3, 1261–1271. [Google Scholar] [CrossRef]
- Marciniszyn, J., Jr.; Hartsuck, J.A.; Tang, J. Mode of inhibition of acid proteases by pepstatin. J. Biol. Chem. 1976, 251, 7088–7094. [Google Scholar] [PubMed]
- Marciniszyn, J., Jr.; Hartsuck, J.A.; Tang, J. Pepstatin inhibition mechanism. Adv. Exp. Med. Biol. 1977, 95, 199–210. [Google Scholar]
- Schild, L.; Heyken, A.; de Groot, P.W.; Hiller, E.; Mock, M.; de Koster, C.; Horn, U.; Rupp, S.; Hube, B. Proteolytic cleavage of covalently linked cell wall proteins by Candida albicans Sap9 and Sap10. Eukaryot. Cell 2011, 10, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Gauwerky, K.; Borelli, C.; Korting, H. Targeting virulence: A new paradigm for antifungals. Drug Discov. Today 2009, 14, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Rüchel, R.; Ritter, B.; Schaffrinski, M. Modulation of experimental systemic murine candidosis by intravenous pepstatin. Zentralbl. Bakterio. 1996, 273, 391–403. [Google Scholar] [CrossRef]
- Stotz, H.U.; Thomson, J.G.; Wang, Y. Plant defensins: Defense, development and application. Plant Signal. Behav. 2009, 4, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.M.; François, I.E.; Meert, E.M.; Li, Q.T.; Cammue, B.P.; Thevissen, K. The antifungal activity of RsAFP2, a plant defensin from raphanus sativus, involves the induction of reactive oxygen species in Candida albicans. J. Mol. Microbiol. Biotechnol. 2007, 13, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Selitrennikoff, C. Antifungal proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Faruck, M.O.; Yusof, F.; Chowdhury, S. An overview of antifungal peptides derived from insect. Peptides 2016, 80, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Andrä, J.; Berninghausen, O.; Leippe, M. Cecropins, antibacterial peptides from insects and mammals, are potently fungicidal against Candida albicans. Med. Microbiol. Immunol. 2001, 189, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhu, S. The drosomycin multigene family: Three-disulfide variants from Drosophila takahashii possess antibacterial activity. Sci. Rep. 2016, 6, 32175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Moon, H.J.; Kurata, S.; Natori, S.; Lee, B.L. Purification and cDNA cloning of an antifungal protein from the hemolymph of Holotrichia diomphalia larvae. Biol. Pharm. Bull. 1995, 18, 1049–1052. [Google Scholar] [CrossRef]
- Lamberty, M.; Zachary, D.; Lanot, R.; Bordereau, C.; Robert, A.; Hoffmann, J.A.; Bulet, P. Insect immunity. Constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. J. Biol. Chem. 2001, 276, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.; Jouvensal, L.; Lamberty, M.; Bulet, P.; Caille, A.; Vovelle, F. Solution structure of termicin, an antimicrobial peptide from the termite Pseudacanthotermes spiniger. Protein Sci. 2003, 12, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.; Sonnevend, A. Clinical applications of amphibian antimicrobial peptides. J. Med. Sci. 2011, 4, 62–72. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, Y.; Kim, P.I.; Jeong, H.G.; Woo, E.R.; Hahm, K.S. Influence on the plasma membrane of Candida albicans by HP (2-9)-magainin 2 (1-12) hybrid peptide. Biochem. Biophys. Res. Commun. 2002, 297, 885–889. [Google Scholar] [PubMed]
- Kobayashi, S.; Takeshima, K.; Park, C.B.; Kim, S.C.; Matsuzaki, K. Interactions of the novel antimicrobial peptide buforin 2 with lipid bilayers: Proline as a translocation promoting factor. Biochemistry 2000, 39, 8648–8654. [Google Scholar] [CrossRef]
- Amiche, M.; Galanth, C. Dermatoseptins as models for the elucidation of membrane-acting helical amphipathic antimicrobial peptides. Curr. Pharm. Biotechnol. 2011, 12, 1184–1193. [Google Scholar] [CrossRef]
- Mangoni, M.L. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. 2006, 63, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.L.; Choudhury, S.D.; Ahrens, K. Purification and characterization of the antimicrobial peptide, ostricacin. Biotechnol. Lett. 2001, 23, 207–210. [Google Scholar] [CrossRef]
- Silva, P.M.; Gonçalves, S.; Santos, N.C. Defensins: Antifungal lessons from eukaryotes. Front. Microbiol. 2014, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Szyk, A.; Wu, Z.; Tucker, K.; Yang, D.; Lu, W.; Lubkowski, J. Crystal structures of human alpha-defensins HNP4, HD5 and HD6. Protein Sci. 2006, 15, 2749–2760. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Li, X.S.; Berner, J.C.; Edgerton, M. Distinct antifungal mechanisms: Beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-fee cationic peptides. Antimicrob. Agents Chemother. 2005, 50, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Cuperus, T.; Coorens, M.; van Dijk, A.; Haagsman, H.P. Avian host defense peptides. Dev. Comp. Immunol. 2013, 41, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, H.A.; El-Hamidy, S.M.; Mahmoud, M.M.; Baeshen, M.N.; Almehdar, H.A.; Uversky, V.N.; Redwan, E.M.; Al-Maghrabi, O.A.; Elazzazy, A.M. Biocidal activity of chicken defensin-9 against microbial pathogens. Biochem. Cell. Biol. 2015, 94, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Landon, C.; Thouzeau, C.; Labbé, H.; Bulet, P.; Vovelle, F. Solution structure of spheniscin, a β-defensin from the penguin stomach. J. Biol. Chem. 2004, 279, 30433–30439. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.W.; Cheng, Y.L.; Hsieh, W.P.; Lan, C.Y. Responses of Candida albicans to the human antimicrobial peptide LL-37. J. Microbiol. 2014, 52, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Durnaś, B.; Wnorowska, U.; Pogoda, K.; Deptuła, P.; Wątek, M.; Piktel, E.; Głuszek, S.; Gu, X.; Savage, P.B.; Niemirowicz, K.; et al. Candidacidal activity of selected ceragenins and human cathelicidin LL-37 in experimental settings mimicking infection sites. PLoS ONE 2016, 11, e0157242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Prickett, M.D.; Gutowska, W.; Kuo, R.; Belov, K.; Burt, D.W. Evolution of the avian β-defensin and cathelicidin genes. BMC Evol. Biol. 2015, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, H.; Fitzgerald, D.H.; Sein, T.; Walsh, T.J.; O’Connel, B.C. Saliva affects the antifungal activity of exogenously added histatin 3 towards Candida albicans. FEMS Microbiol. Lett. 2005, 244, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Mehra, T.; Köberle, M.; Braunsdorf, C.; Mailänder-Sanchez, D.; Borelli, C.; Schaller, M. Alternative approaches to antifungal therapies. Exp. Dermatol. 2012, 21, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kakeya, H.; Miyazaki, T.; Izumikawa, K.; Yanagihara, K.; Ohno, H.; Yamamoto, Y.; Tashiro, T.; Kohno, S. Synergic antifungal effect of lactoferrin with azole antifungals against Candida albicans and a proposal for new treatment method for invasive candidiasis. Jpn. J. Infect. Dis. 2011, 64, 292–296. [Google Scholar] [PubMed]
- Jaskiewicz, M.; Orlowska, M.; Olizarowicz, G.; Migon, D.; Grzywacz, D.; Kamysz, W. Rapid screening of antimicrobial synthetic peptides. Int. J. Pept. Res. Ther. 2016, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Abe, S.; Okutomi, T.; Tansho, S.; Kawase, K.; Yamaguchi, H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 1996, 40, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Abe, S.; Teraguchi, S.; Hayasawa, H.; Yamaguchi, H. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin-related compounds. Antimicrob. Agents Chemother. 1998, 42, 1587–1591. [Google Scholar] [PubMed]
- Kuipers, M.E.; de Vries, H.G.; Eikelboom, M.C.; Meijer, D.K.F.; Swart, P.J. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob. Agents Chemother. 1999, 43, 2635–2641. [Google Scholar] [PubMed]
- Lai, Y.W.; Campbell, L.T.; Wilkins, M.R.; Pang, C.N.I.; Chen, S.; Carter, D.A. Synergy and antagonism between iron chelators and antifungal drugs in Cryptococcus. Int. J. Antimicrob. Agents 2016, 48, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Tabbene, O.; Azaiez, S.; Di Grazia, A.; Karkouch, I.; Ben Slimene, I.; Elkahoui, S.; Alfeddy, M.N.; Casciaro, B.; Luca, V.; Limam, F.; et al. Bacillomycin D and its combination with amphotericin B: Promising antifungal compounds with powerful antibiofilm activity and wound-healing potency. J. Appl. Microbiol. 2016, 120, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Mora-Navarro, C.; Caraballo-León, J.; Torres-Lugo, M.; Ortiz-Bermúdez, P. Synthetic antimicrobial β-peptide in dual treatment with fluconazole or ketoconazole enhances the in vitro inhibition of planktonic and biofilm Candida albicans. J. Pept. Sci. 2015, 21, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ortega, J.R.; van’t Hof, W.; Veerman, E.C.I.; Saugar, J.M.; Rivas, L. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 2008, 22, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Kim, Y.M.; Lee, J.K.; Kim, N.H.; Kim, E.J.; Heo, H.; Lee, M.Y.; Lee, J.R.; Jang, M.K. Targeting and synergistic action of an antifungal peptide in an antibiotic drug-delivery system. J. Control. Release 2017, 256, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, R.; Malmsten, M. Delivery systems for antimicrobial peptides. Adv. Colloid Interface Sci. 2017, 242, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Antimicrobial and antiviral hydrogels. Soft Matter 2011, 7, 8725–8736. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Vallet-Regi, M.; Kupferschmidt, N.; Terasaki, O.; Schmidtchen, A.; Malmsten, M. Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials 2009, 30, 5729–5736. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and in vivo Evaluation of a novel Histatin-5 bioadhesive hydrogel formulation against oral candidiasis. Antimicrob. Agents Chemother. 2016, 60, 881–889. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Cadicamo, C.D.; Mortier, J.; Wolber, G.; Hell, M.; Heinrich, I.E.; Michel, D.; Semlin, L.; Berger, U.; Korting, H.C.; Höltje, H.D.; et al. Design, synthesis, inhibition studies, and molecular modeling of pepstatin analogues addressing different secreted aspartic proteinases of Candida albicans. Biochem. Pharmacol. 2013, 85, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Maurya, I.K.; Pathak, S.; Sharma, M.; Sanwal, H.; Chaudhary, P.; Tupe, S.; Deshpande, M.; Chauhan, V.S.; Prasad, R. Antifungal activity of novel synthetic peptides by accumulation of reactive oxygen species (ROS) and disruption of cell wall against Candida albicans. Peptides 2011, 32, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Kim, P.I.; Park, Y.; Woo, E.R.; Choi, J.S.; Choi, C.H.; Hahm, K.S. Design of novel peptide analogs with potent fungicidal activity, based on PMAP-23 antimicrobial peptide isolated from porcine myeloid. Biochem. Biophys. Res. Commun. 2002, 293, 231–238. [Google Scholar] [CrossRef]
- Yu, H.Y.; Tu, C.H.; Yip, B.S.; Chen, H.L.; Cheng, H.T.; Huang, K.C.; Lo, H.J.; Cheng, J.W. Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob. Agents Chemother. 2011, 55, 4918–4921. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Kim, H.K.; Lee, K.Y.; Kim, S.A.; Han, Y.S.; Lee, I.H. Antifungal activity of synthetic peptide derived from halocidin, antimicrobial peptide from the tunicate, Halocynthia aurantium. FEBS Lett. 2006, 580, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Lopez-Ribot, J.L. Candidiasis drug discovery and development: New approaches targeting virulence for discovering and identifying new drugs. Expert Opin. Drug Discov. 2013, 8, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Romo, J.A.; Pierce, C.G.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Targeting Candida albicans filamentation for antifungal drug development. Virulence 2016, 8, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Leung, K.P.; Curt, S.; Rouabhia, M. Antimicrobial decapeptide KSL-W attenuates Candida albicans virulence by modulating its effects on Toll-like receptor, human β-defensin, and cytokine expression by engineered human oral mucosa. Peptides 2011, 32, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Presterl, E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 2012, 55, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.L.; Stark, M.; Chan, C.; Glukhov, E.; Sinnadurai, S.; Deber, C.M. Activity of novel non-amphipathic cationic antimicrobial peptides against Candida species. J. Antimicrob. Chemother. 2006, 57, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ren, B.; Tong, Y.; Dai, H.; Zhang, L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 2015, 6, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Szunerits, S.; Barras, A.; Khanal, M.; Pagneux, Q.; Boukherroub, R. Nanostructures for the inhibition of viral infections. Molecules 2015, 20, 14051–14081. [Google Scholar] [CrossRef] [PubMed]
- Niederhafner, P.; Bednarova, L.; Budesinsky, M.; Safarik, M.; Ehala, S.; Jezek, J.; Borovickova, L.; Fucik, V.; Cerovsky, V.; Slaninova, J. Melectin MAPs: The influence of dendrimerization on antimicrobial and hemolytic activity. Amino Acids 2010, 39, 1553–1561. [Google Scholar] [CrossRef]
- Liu, S.P.; Zhou, L.; Lakshminarayanan, R.; Beuerman, R.W. Multivalent antimicrobial peptides as therapeutics: Design principles and structural diversities. Int. J. Pept. Res. Ther. 2010, 16, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tam, J.P.; Lu, Y.A.; Yang, J.L. Antimicrobial dendrimeric peptides. Eur. J. Biochem. 2002, 269, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Giuliani, A.; Falciani, C.; Runci, Y.; Ricci, C.; Lelli, B.; Malossi, M.; Neri, P.; Rossolini, G.M.; Bracci, L. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob. Agents Chemother. 2005, 49, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhou, C.; Liu, Z.; Young, A.W.; Shi, Z.; Ren, D.; Kallenbach, N.R. Antimicrobial dendrimer active against Escherichia coli biofilms. Bioorg. Med. Chem. Lett. 2009, 19, 5478–5481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Young, A.W.; Hu, P.; Rice, A.J.; Zhou, C.; Zhang, Y.; Kallenbach, N.R. Tuning the membrane selectivity of antimicrobial peptides by using multivalent design. ChemBioChem 2007, 8, 2063–2065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deshazer, H.; Rice, A.J.; Chen, K.; Zhou, C.; Kallenbach, N.R. Multivalent antimicrobial peptides from a reactive polymer scaffold. J. Med. Chem. 2006, 49, 3436–3439. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P. Synthetic peptide vaccine design: Synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl. Acad. Sci. USA 1988, 85, 5409–5413. [Google Scholar] [CrossRef] [PubMed]
- Defoort, J.P.; Nardelli, B.; Huang, W.; Tam, J.P. A rational design of synthetic peptide vaccine with a built-in adjuvant. A modular approach for unambiguity. Int. J. Pept. Protein Res. 1992, 40, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.; Christie, M.P.; Moyle, P.M.; Toth, I. Peptide based DNA nanocarriers incorporating a cell-penetrating peptide derived from neurturin protein and poly-L-lysine dendrons. Bioorg. Med. Chem. 2015, 23, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, J.; Sowinska, M.; Rajnisz, A.; Solecka, J.; Lacka, I.; Milewski, S.; Urbanczyk-Lipkowska, Z. Novel dendrimeric lipopeptides with antifungal activity. Bioorg. Med. Chem. Lett. 2012, 22, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, R.; Liu, S.; Li, J.; Nandhakumar, M.; Aung, T.T.; Goh, E.; Ting Chang, J.Y.; Saraswathi, P.; Tang, C.; Safie, S.R.B.; et al. Synthetic multivalent antifungal peptides effective against fungi. PLoS ONE 2014, 9, e87730. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Vasileva-Tonkova, E.; Makki, M.S.I.; Sobahi, T.R.; Abdfl-Rahman, R.M.; Boyaci, I.H.; Abdullah, M.; Asiri, A.M.; Grabchev, I. Synthesis and spectral characterization of a new PPA dendrimer modified with 4-bromo-1,8-naphthalimide and in vitro antimicrobial activity of its Cu(II) and Zn(II) metal complexes. Tetrahedron 2015, 71, 1080–1087. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Yordanova, S.; Cangiotti, M.; Vasileva-Tonkova, E.; Coppola, C.; Stoyanov, S.; Grabchev, I. Spectral characterization and in vitro microbiological activity of new bis-1,8-naphthalimides and their Cu(II) complexes. J. Mol. Struct. 2016, 1110, 72–82. [Google Scholar] [CrossRef]
- Carta, F.; Osman, S.M.; Vullo, D.; AlOthman, Z.; Del Prete, S.; Capasso, C.; Supuran, C.T. Poly(amidoamine) dendrimers show carbonic anhydrase inhibitory activity against alpha-, beta-, gamma- and eta-class enzymes. Bioorg. Med. Chem. 2015, 23, 6794–6798. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and PAMAM dendrimers: Formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, K.; Szekalska, M.; Winnicka, K. The effect of PAMAM dendrimers with amine or hydroxyl terminal groups on the bioadhesive properties of hydrogels with clotrimazole. Polimery 2016, 61, 322–326. [Google Scholar] [CrossRef]
- Felczak, A.; Wronska, N.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Appelhans, D.; Voit, B.; Rozalska, S.; Lisowska, K. Antimicrobial activity of poly(propylene imine) dendrimers. New J. Chem. 2012, 36, 2215–2222. [Google Scholar] [CrossRef]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, J.; Urbanczyk-Lipkowska, Z. Amphiphilic dendrimeric peptides as model non-sequential pharmacophores with antimicrobial properties. J. Mol. Biol. Microbiol. 2007, 13, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, J.; Swieton, J.; Lipkowski, A.W.; Urbanczyk-Lipkowska, Z. Low molecular mass peptide dendrimers that express antimicrobial activity. Bioorg. Med. Chem. Lett. 2003, 13, 3711–3713. [Google Scholar] [CrossRef] [PubMed]
- Polcyn, P.; Jurczak, M.; Rajnisz, A.; Solecka, J.; Urbanczyk-Lipkowska, Z. Design of antimicrobially active small amphiphilic peptide dendrimers. Molecules 2009, 14, 3881–3905. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, L.W.; da Silva, A.; Leite, A.; Valente, A.P.; Almeida, F.C.L. NMR structure of PW2 bound to SDS micelles—A tryptophan-rich anticoccidial peptide selected from phage display libraries. J. Biolog. Chem. 2002, 277, 36351–36356. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Bondaryk, M.; Zielińska, P.; Urbańczyk-Lipkowska, Z. The in vitro effects of new D186 dendrimer on virulence factors of Candida albicans. J. Antibiot. 2014, 67, 425–432. [Google Scholar] [CrossRef]
- Zielińska, P.; Staniszewska, M.; Bondaryk, M.; Koronkiewicz, M.; Urbańczyk-Lipkowska, Z. Design and studies of multiple mechanism of anti-Candida activity of a new potent Trp-rich peptide dendrimers. Eur. J. Med. Chem. 2015, 105, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffman, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
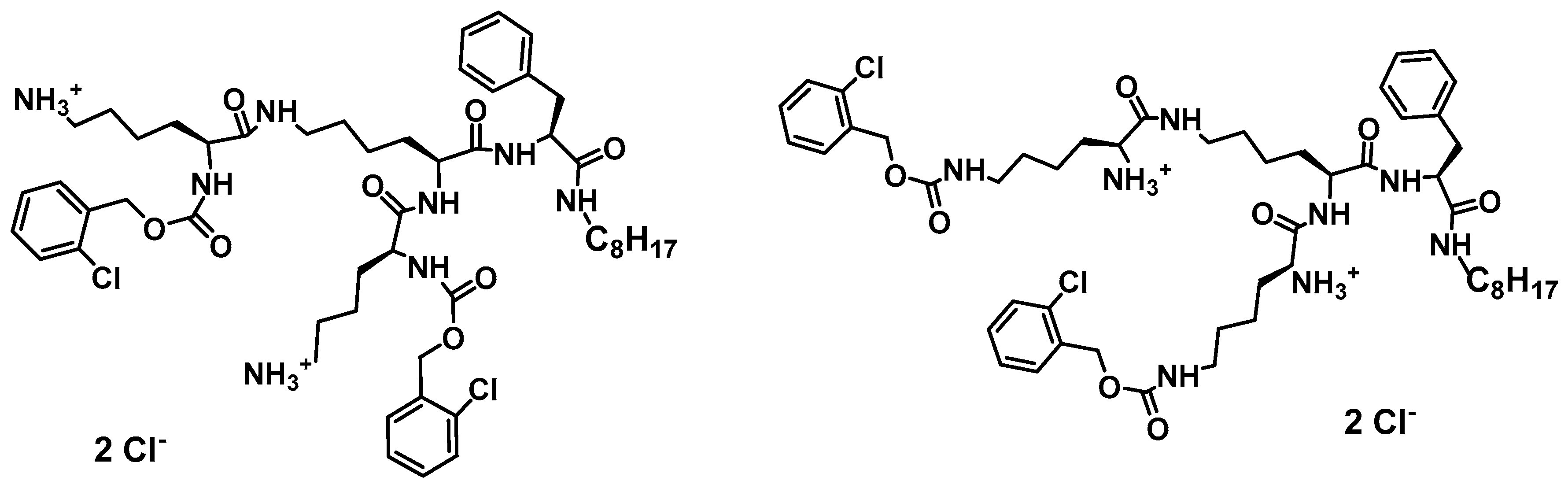
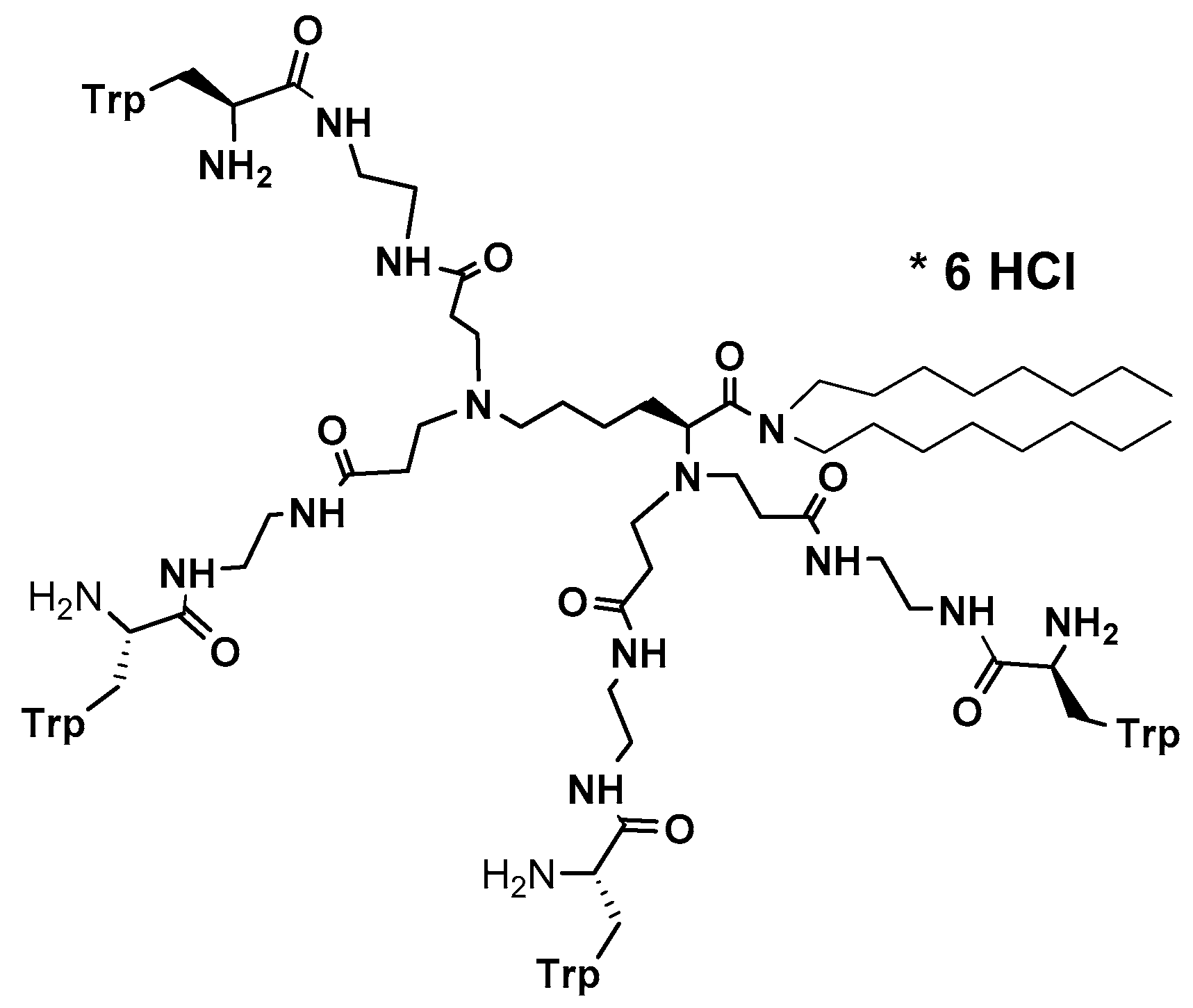
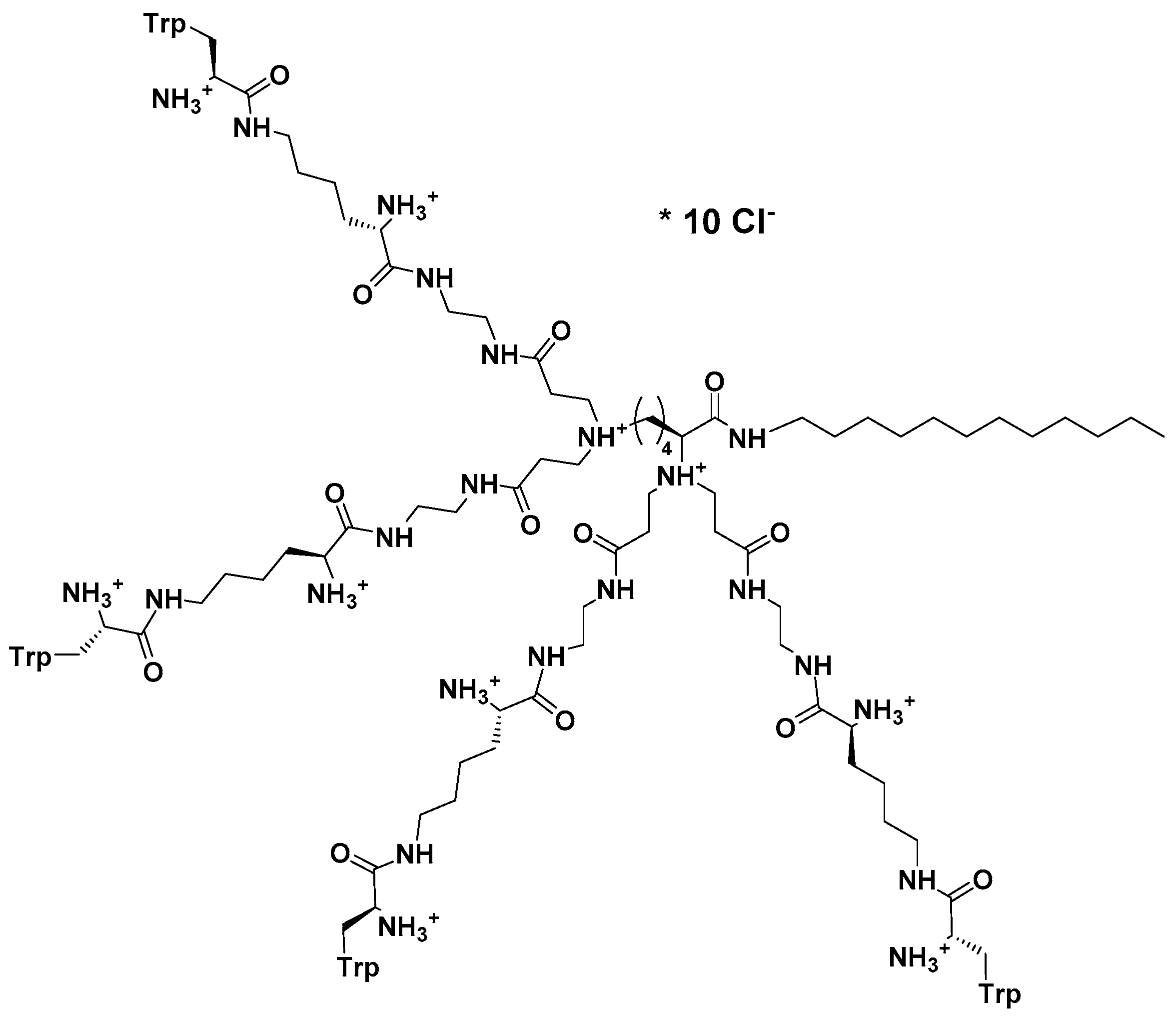
| Risk Factors | References |
|---|---|
| Abdominal surgery/recent major surgery | [3,5,9,10] |
| Deep burns | [11,12] |
| Diabetes mellitus | [9,10,12] |
| Dialysis | [12,13] |
| Disturbance of natural skin or mucosal barriers | [10,12] |
| Exposure to broad-spectrum antibiotics | [3,10,12] |
| Extremes of age | [5,10] |
| HIV/AIDS | [9,13] |
| Immune disorders | [5,11] |
| Local disorders of the gastrointestinal tract | [5,10,12] |
| Long-term catheterization | [3,5,13] |
| Malignancies | [3,5] |
| Mechanical ventilation | [10,13] |
| Parenteral nutrition | [5,10,13] |
| Premature very low birth weight infants | [11] |
| Prolonged hospitalization | [12] |
| Renal failure | [9,11,12] |
| Solid organ or bone marrow transplantation | [5] |
| Treatment with corticosteroids | [3,5] |
| Use of immunosuppressive drugs | [5,10,12] |
| Group Name | Group Member/s | Mode of Action | Resistance Mechanism | References |
|---|---|---|---|---|
| Fluorinated Pyrimidine Analogs | Flucytosine (5-FC) | Inhibition of RNA and/or DNA synthesis |
| [15,52] |
| Polyenes | Nystatin Natamycin Amphotericin B | Alteration of the membrane function by binding of ergosterol (depleting cells of ergosterol) |
| [18,52] |
| Echinocandins | Caspofungin Micafungin Anidulafungin | Alteration of cell wall biosynthesis by inhibition of β(1,3)-glucan synthase Fks1p or Fks2p |
| [16,19] |
| Allylamines | Terbinafine Naftifine | Inhibition of the ergosterol biosynthesis by inhibition of squalene epoxidase (Erg1) and/or accumulation of toxic sterol intermediates |
| [16,19] |
| Azoles | Fluconazole Posoconazole Voriconazole | Inhibition of cytochrome P450 14α-lanosterol demethylase (encoded by ERG11) in ergosterol biosynthesis pathway |
| [16,19,52] |
| Name | Origin | Sequence or Molecular Formula | Mode of Action | Reference |
|---|---|---|---|---|
| Bacterial Peptides | ||||
| Syringomycin | Pseudomonas syringae pv. syringae | MSLQANTAPVFADEQQTDAPTWPDRAADPSVRLSLLATGNSLPVVIEPTADGLDPVQWASARREAIETLLCRHGAVLFRGFDLPSVAAFEGFAEALSPGLHGTYGDLPKKEGGRNVYRSTPYPEREMILYHNESSHLESWPRKQWFFCEQPSRVGGATPLADIRQVLAYLPKEVVERFESKGLLYSRTFTAGVEPSWESFFGTSERSVIEQRCREQGTDFEWLDGDTLQLRTQCPAVITHPFTGERCFFNQVQLHHPYCMGEELREDLLDMFGPDRLPRLVSYGDGSAIEDPVMALIGEAYEACAVRFEWRKGDVVMLDNMLAAHARDPYEEPRLIVVAMGEMTARGDVWQPA | Cell lysis | [84,85,86,87,88] |
| SyrP protein | ||||
| Iturin A | Bacillus subtilis | KIYGVYMDRPLSAGEEVRMMAAVSAEKREKCRRFYHKEDAHRTLIGDMLIRTAAAKAYGLDPAGISFGVQEYGKPYIPALPDMHFNISHSGRWIVCAVDSKPIGIDIEKMKPGTIDIAKRFFSPTEYSDLQAKHPDQQTDYFYHLWSMKESFIKQAGKGLSLPLDSFSVRLKDDGHVSIEL | Cell lysis | [86,88,89] |
| Nikkomycin | Streptomyces spp. |  | Inhibition of chitin biosynthesis | [90,91,92] |
| Pepstatin A | Streptomyces spp. |  | Inhibition of aspartic proteases | [92] |
| Fungal Peptides | ||||
| Aculeacin A | Aspergillus aculeatus |  | Inhibition of 1,3-β-d-glucan synthase | [92,93] |
| Plant Peptides | ||||
| Defensins | ||||
| Rs-AFP1 | Raphanus sativus | QKLCERPSGTWSGVCGNNNACKNQCINLEKARHGSCNYVFPAHKCICYFPC | Membrane permeabilization | [62,88,94,95,96] |
| Rs-AFP2 | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKAWGSCNYVFPAHKCICYFPC | |||
| Thionins | ||||
| CaThi | Capsicum annuum | KEICCKVPTTPFLCTNDPQCKTLCSKVNYEDGHCFDILSKCVCMNRCVQDAKTLAAELIEEEFLKQ | Membrane permeabilization | [88,97,98] |
| Thaumatin-like (TL) proteins | ||||
| Osmotin | Nicotiana tabacum | ATIEVRNNCPYTVWAASTPIGGGRRLDRGQTWVINAPRGTKMARVWGRTNCNFNAAGRGTCQTGDCGGVLQCTGWGKPPNTLAEYALDQFSGLDFWDISLVDGFNIPMTFAPTNPSGGKCHAIHCTANINGECPRELRVPGGCNNPCTTFGGQQYCCTQGPCGPTFFSKFFKQRCPDAYSYPQDDPTSTFTCPGGSTNYRVIFCPNGQAHPNFPLEMPGSDEVAK | Cell wall perturbations; spore lysis | [86,88,99,100] |
| Zeamatin | Zea mays | AAVFTVVNQCPFTVWAASVPVGGGRQLNRGESWRITAPAGTTAARIWARTGCKFDASGRGSCRTGDCGGVLQCTGYGRAPNTLAEYALKQFNNLDFFDISLIDGFNVPMSFLPDGGSGCSRGPRCAVDVNARCPAELRQDGVCNNACPVFKKDEYCCVGSAANDCHPTNYSRYFKGQCPDAYSYPKDDATSTFTCPAGTNYKVVFCP | Cell lysis | |
| Insect Peptides | ||||
| Cecropins | ||||
| Stomoxyn | Stomoxys calcitrans | RGFRKHFNKLVKKVKHTISETAHVAKDTAVIAGSGAAVVAAT | Cell lysis | [54,88,101] |
| Melittin | Apis mellifera | GIGAVLKVLTTGLPALISWIKRKRQQ-CONH2 | Proapoptotic activity | [102,103] |
| Defensins | ||||
| Drosomycin | Drosophila melanogaster | DCLSGRYKGPCAVWDNETCRRVCKEEGRSSGHCSPSLKCWCEGC | Cell lysis | [86,88,104] |
| Tenecin 3 | Tenebrio molitor | DHHDGHLGGHQTGHQGGQQGGHLGGQQGGHLGGHQGGQPGGHLGGHQGGIGGTGGQQHGQHGPGTGAGHQGGYKTHGH | Unknown | [71,88,105] |
| Holotricin 3 | Holotrichia diomphalia | YGPGDGHGGGHGGGHGGGHGNGQGGGHGHGPGGGFGGGHGGGHGGGGRGGGGSGGGGSPGHGAGGGYPGGHGGGHHGGYQTHGY | Growth inhibition | |
| Termicin | Pseudacanthotermes spiniger | ACNFQSCWATCQAQHSIYFRRAFCDRSQCKCVFVRG | ||
| Amphibian Peptides | ||||
| Magainin 2 | Xenopus laevis | GIGKFLHSAKKFGKAFVGEIMNS | Disrubtion of plasma membrane | [106,107,108] |
| Buforin I | Bufo bufo garagriozans | AGRGKQGGKVRAKAKTRSSRAGLQFPVGRVHRLLRKGNY | Cell lysis | [55,106] |
| Buforin II | TRSSRAGLQFPVGRVHRLLRK | |||
| Temporin A | Rana temporaria | FLPLIGRVLSGIL | Cell lysis | [56,88,109] |
| Dermaseptin-1 | Phyllomedusa hypochondrialis | GLWSTIKNVGKEAAIAAGKAALGAL | Membrane permeabilization | [86,88,110,111] |
| Avian Peptides | ||||
| Avian β-defensins | ||||
| Gallinacins | Gallus gallus | GRKSDCFRKSGFCAFLKCPSLTLISGKCSRFYLCCKRIWG | Cell lysis | [86,88,112,113,114,115] |
| β-defensin-4 | IVLLFVAVHGAVGFSRSPRYHMQCGYRGNFCTPGKCPHGNAYPGLCRPKYSCCRW | |||
| THP-1 | Meleagris gallopavo | GKREKCLRRNGFCAFLKCPTLSVISGTCSRFQVCC | ||
| Spheniscin-1 | Aptenodytes patagonicus | SFGLCRLRRGFCAHGRCRFPSIPIGRCSRFVQCCRRVW | ||
| Cathelicidins | ||||
| Cathelicidin-2 | Gallus gallus | LVQRGRFGRFLRKIRRFRPKVTITIQGSARFG | Cell lysis | [88,116] |
| Mammalian Peptides | ||||
| α-defensins | ||||
| HNP-1 | Homo sapiens | ACYCRIPACIAGERRYGTCIYQGRLWAFCC | Cell lysis | [86,88,117,118] |
| HNP-2 | CYCRIPACIAGERRYGTCIYQGRLWAFCC | |||
| NP-1 | Rabbit bocaparvovirus | MSSRHSPYPRKTSGDTTGSKTSWASSGSRENKGNHKNPSFSTASRPFLTRQQKKEILKPRALRKDPPKVFCATHRADSPDAPAVCGFFWHSNRIAGKGTDWIFTRGKQLFQERAKNNVIDWDMARDLLFSFKRECDQWYRNMLYHFRLGEPCDKCNYWDGAYRKYCARVNADYEKEINATSASQELTDEEAAAALDAAMADASH | ||
| β-defensins | ||||
| HBD-1 | Homo sapiens | DHYNCVSSGGQCLYSACPIFTKIQGTCYRGKAKCCK | Cell lysis | [88,118,119,120] |
| HBD-2 | TCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | |||
| HBD-3 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | |||
| HBD-4 | ELDRICGYGTARCRKKCRSQEYRIGRCPNTYACCLRK | |||
| Tracheal antimicrobial peptide (TAP) | Bos taurus | MRLHHLLLALLFLVLSAWSGFTQGVGNPVSCVRNKGICVPIRCPGSMKQIGTCVGRAVKCCRKK | ||
| Lingual antimicrobial peptide (LAP) | VRNSQSCRRNKGICVPIRCPGSMRQIGTCLGAQVKCCRRK | |||
| θ-defensins | ||||
| RTD-1 | Macaca mulatta | GFCRCLCRRGVCRCICTR | Cell lysis | [88,121,122] |
| Cathelicidins | ||||
| LL-37 | Homo sapiens | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | Destabilization of plasma membrane | [88,123] |
| Indolicidin | Bos taurus | ILPWKWPWWPWRR | Destabilization of plasma membrane | [88,124,125,126] |
| Tritrpticin | Sus scrofa | VRRFPWWWPFLRR | Destabilization of plasma membrane | [88,127] |
| Protegrin-1 | RGGRLCYCRRRFCVCVGR | Destabilization of plasma membrane | [88,124,128] | |
| Histatins | ||||
| Histatin-5 | Homo sapiens | DSHAKRHHGYKRKFHEKHHSHRGY | Osmotic stress | [88,129,130,131] |
| Lactoferrin-derived peptides | ||||
| Lactoferricin H | Homo sapiens | GRRRSVQWCAVSQPEATKCFQWQRNMRKVRGPPVSCIKRDSPIQCIQA | Disruption of plasma membrane | [88,132,133,134,135] |
| Lactoferricin B | Bos taurus | FKCRRWQWRMKKLGAPSITCVRRAF | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondaryk, M.; Staniszewska, M.; Zielińska, P.; Urbańczyk-Lipkowska, Z. Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds. J. Fungi 2017, 3, 46. https://doi.org/10.3390/jof3030046
Bondaryk M, Staniszewska M, Zielińska P, Urbańczyk-Lipkowska Z. Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds. Journal of Fungi. 2017; 3(3):46. https://doi.org/10.3390/jof3030046
Chicago/Turabian StyleBondaryk, Małgorzata, Monika Staniszewska, Paulina Zielińska, and Zofia Urbańczyk-Lipkowska. 2017. "Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds" Journal of Fungi 3, no. 3: 46. https://doi.org/10.3390/jof3030046





