RNAPII Degradation Factor Def1 Is Required for Development, Stress Response, and Full Virulence of Magnaporthe oryzae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Growth Conditions
2.2. Gene Deletion and Complementation
2.3. CFW Staining Assay
2.4. Virulence Test and Infection Process Observation
2.5. Glycogen and Lipid Utilization Observation
2.6. ROS Accumulation Test
2.7. Stress Tolerance Assay
2.8. Western Blot
3. Results
3.1. Identification of Def1 in M. oryzae
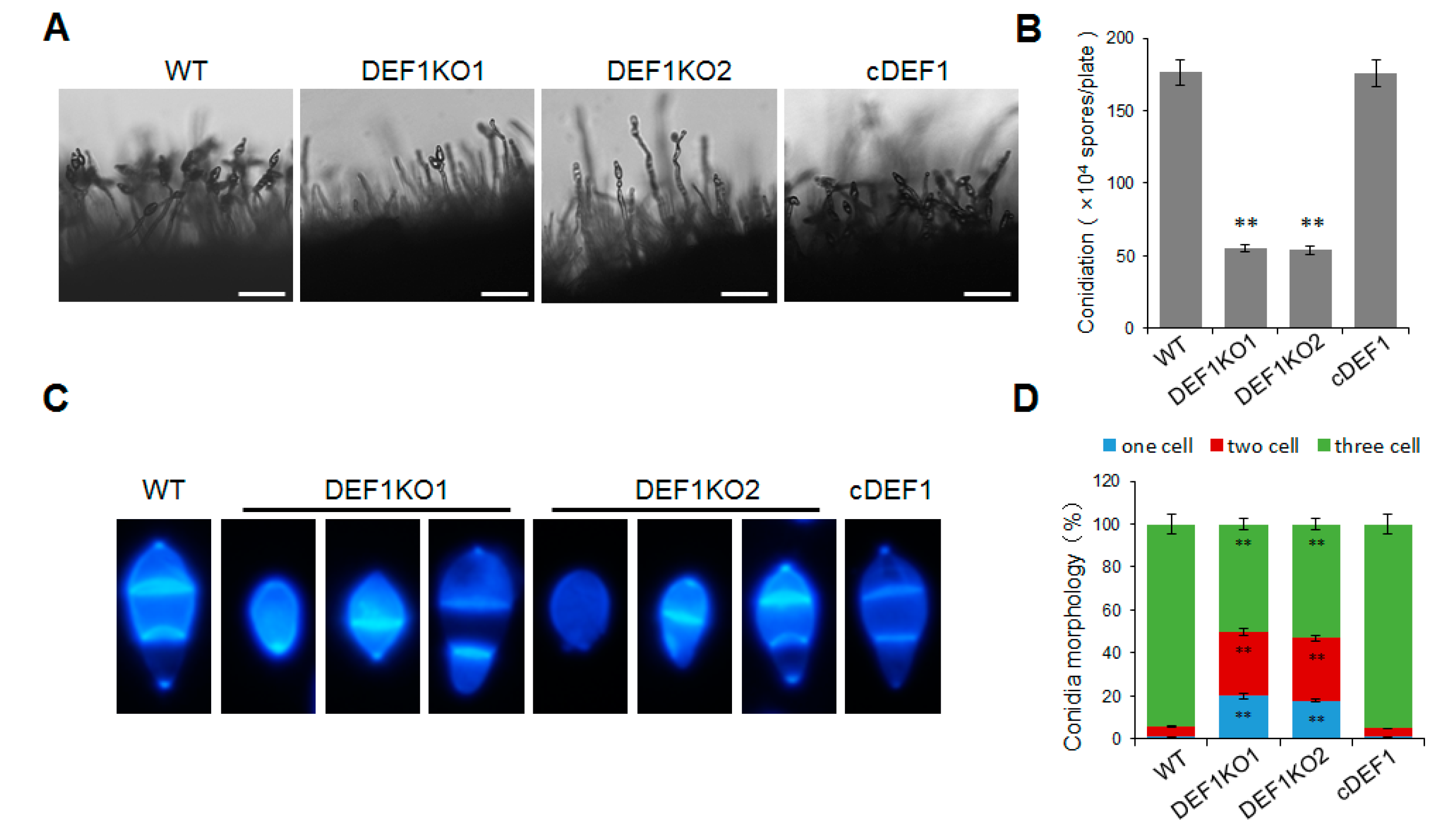
3.2. Def1 Contributes to M. oryzae Mycelial Growth and Conidia Formation
3.3. Def1 Is Required for Full Virulence of M. oryzae
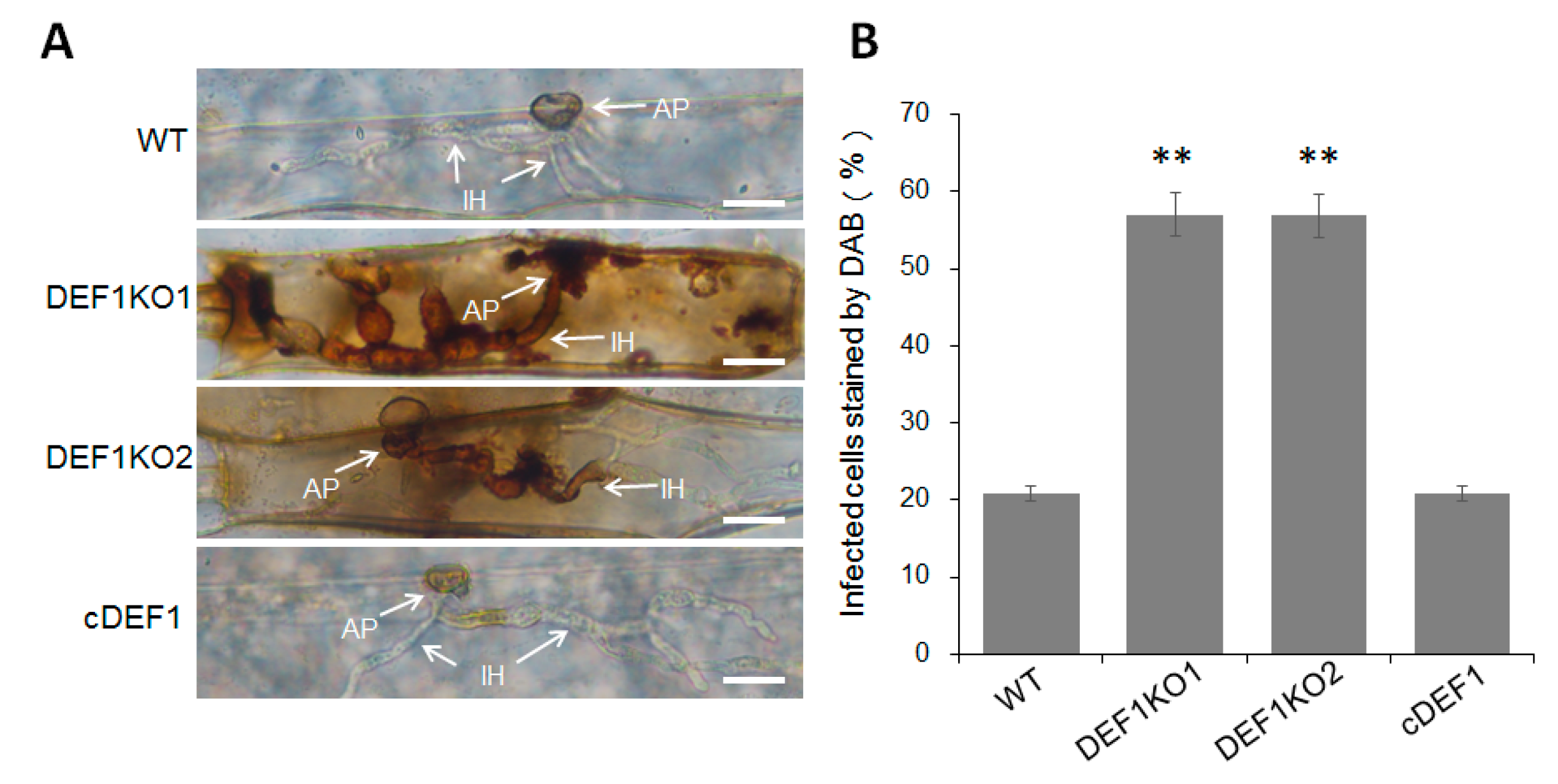
3.4. Def1 Is Important for Appressorial Penetration and Invasive Growth
3.5. Def1 Affects Utilization of Glycogen and Lipid during Appressorium Development
3.6. Deletion of Def1 Results in Accumulation of Host Reactive Oxygen Species (ROS)
3.7. Def1 Is Involved in Stress Response
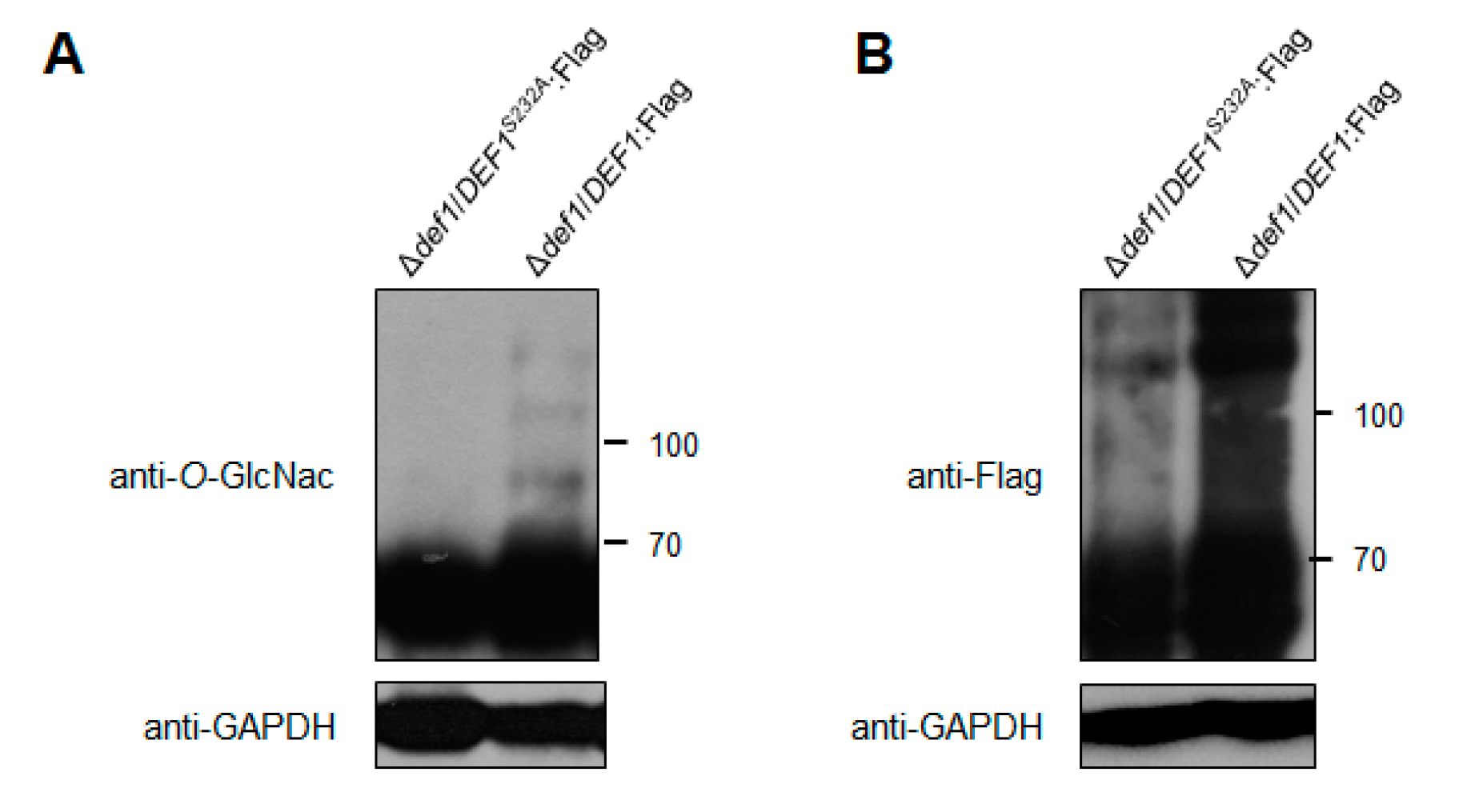
3.8. O-GlcNAc Modification Affects the Def1 Stability and Is Required for the Def1 Functions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ljungman, M.; Lane, D.P. Transcription—Guarding the genome by sensing DNA damage. Nat. Rev. Cancer 2004, 4, 727–737. [Google Scholar] [CrossRef]
- Wilson, M.D.; Harreman, M.; Taschner, M.; Reid, J.; Walker, J.; Erdjument-Bromage, H.; Tempst, P.; Svejstrup, J.Q. Proteasome-mediated processing of Def1, a critical step in the cellular response to transcription stress. Cell 2013, 154, 983–995. [Google Scholar] [CrossRef] [Green Version]
- Pani, B.; Nudler, E. Mechanistic insights into transcription coupled DNA repair. DNA Repair 2017, 56, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Lans, H.; Hoeijmakers, J.H.J.; Vermeulen, W.; Marteijn, J.A. The DNA damage response to transcription stress. Nat. Rev. Mol. Cell Biol. 2019, 20, 766–784. [Google Scholar] [CrossRef] [PubMed]
- Bohr, V.A.; Smith, C.A.; Okumoto, D.S.; Hanawalt, P.C. DNA repair in an active gene: Removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell 1985, 40, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Mellon, I.; Spivak, G.; Hanawalt, P.C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell 1987, 51, 241–249. [Google Scholar] [CrossRef]
- Mellon, I.; Hanawalt, P.C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature 1989, 342, 95–98. [Google Scholar] [CrossRef]
- Hanawalt, P.C. Controlling the efficiency of excision repair. Mutat. Res. 2001, 485, 3–13. [Google Scholar] [CrossRef]
- Svejstrup, J.Q. Mechanisms of transcription-coupled DNA repair. Nat. Rev. Mol. Cell Biol. 2002, 3, 21–29. [Google Scholar] [CrossRef]
- Hanawalt, P.C.; Spivak, G. Transcription-coupled DNA repair: Two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008, 9, 958–970. [Google Scholar] [CrossRef]
- Donahue, B.A.; Yin, S.; Taylor, J.S.; Reines, D.; Hanawalt, P.C. Transcript cleavage by RNA polymerase II arrested by a cyclobutane pyrimidine dimer in the DNA template. Proc. Natl. Acad. Sci. USA 1994, 91, 8502–8506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.D.; Harreman, M.; Svejstrup, J.Q. Ubiquitylation and degradation of elongating RNA polymerase II: The last resort. Biochim. Biophys. Acta 2013, 1829, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ratner, J.N.; Balasubramanian, B.; Corden, J.; Warren, S.L.; Bregman, D.B. Ultraviolet radiation-induced ubiquitination and proteasomal degradation of the large subunit of RNA polymerase II. Implications for transcription-coupled DNA repair. J. Biol. Chem. 1998, 273, 5184–5189. [Google Scholar] [CrossRef] [Green Version]
- Woudstra, E.C.; Gilbert, C.; Fellows, J.; Jansen, L.; Brouwer, J.; Erdjument-Bromage, H.; Tempst, P.; Svejstrup, J.Q. A Rad26-Def1 complex coordinates repair and RNA pol II proteolysis in response to DNA damage. Nature 2002, 415, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Anindya, R.; Aygun, O.; Svejstrup, J.Q. Damage-induced ubiquitylation of human RNA polymerase II by the ubiquitin ligase Nedd4, but not Cockayne syndrome proteins or BRCA1. Mol. Cell. 2007, 28, 386–397. [Google Scholar] [CrossRef]
- Lommel, L.; Bucheli, M.E.; Sweder, K.S. Transcription-coupled repair in yeast is independent from ubiquitylation of RNA pol II: Implications for Cockayne’s syndrome. Proc. Natl. Acad. Sci. USA 2000, 97, 9088–9092. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ruggiero, C.; Li, S. Yeast Rpb9 plays an important role in ubiquitylation and degradation of Rpb1 in response to UV-induced DNA damage. Mol. Cell. Biol. 2007, 27, 4617–4625. [Google Scholar] [CrossRef] [Green Version]
- Ponting, C.P. Novel domains and orthologues of eukaryotic transcription elongation factors. Nucleic Acids Res. 2002, 30, 3643–3652. [Google Scholar] [CrossRef] [Green Version]
- Ponting, C.P. Proteins of the endoplasmic-reticulum-associated degradation pathway: Domain detection and function prediction. Biochem. J. 2000, 351 Pt 2, 527–535. [Google Scholar] [CrossRef]
- Biederer, T.; Volkwein, C.; Sommer, T. Role of Cue1p in ubiquitination and degradation at the ER surface. Science 1997, 278, 1806–1809. [Google Scholar] [CrossRef] [Green Version]
- Kang, R.S.; Daniels, C.M.; Francis, S.A.; Shih, S.C.; Salerno, W.J.; Hicke, L.; Radhakrishnan, I. Solution structure of a CUE-ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell 2003, 113, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchberger, A. From UBA to UBX: New words in the ubiquitin vocabulary. Trends Cell Biol. 2002, 12, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Prag, G.; Misra, S.; Jones, E.A.; Ghirlando, R.; Davies, B.A.; Horazdovsky, B.F.; Hurley, J.H. Mechanism of ubiquitin recognition by the CUE domain of Vps9p. Cell 2003, 113, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Harreman, M.; Taschner, M.; Sigurdsson, S.; Anindya, R.; Reid, J.; Somesh, B.; Kong, S.E.; Banks, C.A.; Conaway, R.C.; Conaway, J.W.; et al. Distinct ubiquitin ligases act sequentially for RNA polymerase II polyubiquitylation. Proc. Natl. Acad. Sci. USA 2009, 106, 20705–20710. [Google Scholar] [CrossRef] [Green Version]
- Damodaren, N.; Van Eeuwen, T.; Zamel, J.; Lin-Shiao, E.; Kalisman, N.; Murakami, K. Def1 interacts with TFIIH and modulates RNA polymerase II transcription. Proc. Natl. Acad. Sci. USA 2017, 114, 13230–13235. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.W.; Klein, F.; Leach, D.R. Novel roles for selected genes in meiotic DNA processing. PLoS Genet. 2007, 3, e222. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Byrum, S.; Fowler, F.C.; Pa, S.; Tackett, A.J.; Tyler, J.K. Proteomic identification of histone post-translational modifications and proteins enriched at a DNA double-strand break. Nucleic Acids Res. 2017, 45, 10923–10940. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.B.; Yang, C.P.; Li, R.X.; Zeng, R.; Zhou, J.Q. Def1p is involved in telomere maintenance in budding yeast. J. Biol. Chem. 2005, 280, 24784–24791. [Google Scholar] [CrossRef] [Green Version]
- Makovets, S.; Herskowitz, I.; Blackburn, E.H. Anatomy and dynamics of DNA replication fork movement in yeast telomeric regions. Mol. Cell Biol. 2004, 24, 4019–4031. [Google Scholar] [CrossRef] [Green Version]
- Hart, G.W.; Housley, M.P.; Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446, 1017–1022. [Google Scholar] [CrossRef]
- Roquemore, E.P.; Chevrier, M.R.; Cotter, R.J.; Hart, G.W. Dynamic O-GlcNAcylation of the small heat shock protein alpha B-crystallin. Biochemistry 1996, 35, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Vercoutter-Edouart, A.S.; El Yazidi-Belkoura, I.; Guinez, C.; Baldini, S.; Leturcq, M.; Mortuaire, M.; Mir, A.M.; Steenackers, A.; Dehennaut, V.; Pierce, A.; et al. Detection and identification of O-GlcNAcylated proteins by proteomic approaches. Proteomics 2015, 15, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef] [Green Version]
- Chatham, J.C.; Marchase, R.B. Protein O-GlcNAcylation: A critical regulator of the cellular response to stress. Curr. Signal Transduct. Ther. 2010, 5, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Gu, Y.; Shan, H.; Mi, W.; Sun, J.; Shi, M.; Zhang, X.; Lu, X.; Han, F.; Gong, Q.; et al. O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat. Commun. 2017, 8, 1491. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Liang, Q.; Li, L.; Hu, Z.; Wu, F.; Zhang, P.; Ma, Y.; Zhao, B.; Kovacs, A.L.; Zhang, Z.; et al. O-GlcNAc-modification of SNAP-29 regulates autophagosome maturation. Nat Cell Biol. 2014, 16, 1215–1226. [Google Scholar] [CrossRef]
- Ruba, A.; Yang, W. O-GlcNAcylation in the nuclear pore complex. Cell Mol. Bioeng. 2016, 9, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Yan, X.; Talbot, N.J. Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr. Opin. Microbiol. 2016, 34, 147–153. [Google Scholar] [CrossRef] [Green Version]
- deJong, J.C.; McCormack, B.J.; Smirnoff, N.; Talbot, N.J. Glycerol generates turgor in rice blast. Nature 1997, 389, 244–245. [Google Scholar] [CrossRef]
- Kankanala, P.; Czymmek, K.; Valent, B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell. 2007, 19, 706–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, K.P.; Xu, J.R.; Smirnoff, N.; Talbot, N.J. Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 1999, 11, 2045–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, B.; Kumar, A.; Mehta, S.; Sheoran, N.; Chinnusamy, V.; Prakash, G. Hybrid de novo genome-reassembly reveals new insights on pathways and pathogenicity determinants in rice blast pathogen Magnaporthe oryzae RMg_Dl. Sci. Rep. 2021, 11, 22922. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Mallikarjuna, M.G.; Reddy, B.; Balamurugan, A.; Achary, V.M.M.; Reddy, M.K.; Kumar, A.; Prakash, G. Characterization and validation of hypothetical virulence factors in recently sequenced genomes of Magnaporthe species. Physiol. Mol. Plant Pathol. 2023, 124, 101969. [Google Scholar] [CrossRef]
- Goswami, R.S. Targeted gene replacement in fungi using a split-marker approach. Methods Mol. Biol. 2012, 835, 255–269. [Google Scholar] [PubMed]
- Ruan, H.B.; Han, X.; Li, M.D.; Singh, J.P.; Qian, K.; Azarhoush, S.; Zhao, L.; Bennett, A.M.; Samuel, V.T.; Wu, J.; et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability. Cell Metab. 2012, 16, 226–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.D.; Ruan, H.B.; Hughes, M.E.; Lee, J.S.; Singh, J.P.; Jones, S.P.; Nitabach, M.N.; Yang, X. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 2013, 17, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, W.; Xu, L.; Chen, J.Y.; Chong, J.; Oh, J.; Leschziner, A.E.; Fu, X.D.; Wang, D. Cockayne syndrome B protein acts as an ATP-dependent processivity factor that helps RNA polymerase II overcome nucleosome barriers. Proc. Natl. Acad. Sci. USA 2020, 117, 25486–25493. [Google Scholar] [CrossRef]
- Somesh, B.P.; Reid, J.; Liu, W.F.; Sogaard, T.M.; Erdjument-Bromage, H.; Tempst, P.; Svejstrup, J.Q. Multiple mechanisms confining RNA polymerase II ubiquitylation to polymerases undergoing transcriptional arrest. Cell 2005, 121, 913–923. [Google Scholar] [CrossRef] [Green Version]
- Sigurdsson, S.; Dirac-Svejstrup, A.B.; Svejstrup, J.Q. Evidence that transcript cleavage is essential for RNA polymerase II transcription and cell viability. Mol. Cell. 2010, 38, 202–210. [Google Scholar] [CrossRef]
- Hobson, D.J.; Wei, W.; Steinmetz, L.M.; Svejstrup, J.Q. RNA polymerase II collision interrupts convergent transcription. Mol. Cell. 2012, 48, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owiti, N.; Lopez, C.; Singh, S.; Stephenson, A.; Kim, N. Def1 and Dst1 play distinct roles in repair of AP lesions in highly transcribed genomic regions. DNA Repair 2017, 55, 31–39. [Google Scholar] [CrossRef]
- Stepchenkova, E.I.; Shiriaeva, A.A.; Pavlov, Y.I. Deletion of the DEF1 gene does not confer UV-immutability but frequently leads to self-diploidization in yeast Saccharomyces cerevisiae. DNA Repair 2018, 70, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, H.; Tous, C.; Botet, J.; Gonzalez-Aguilera, C.; Quintero, M.J.; Viladevall, L.; Garcia-Rubio, M.L.; Rodriguez-Gil, A.; Marin, A.; Arino, J.; et al. Genome-wide analysis of factors affecting transcription elongation and DNA repair: A new role for PAF and Ccr4-not in transcription-coupled repair. PLoS Genet. 2009, 5, e1000364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paeschke, K.; Bochman, M.L.; Garcia, P.D.; Cejka, P.; Friedman, K.L.; Kowalczykowski, S.C.; Zakian, V.A. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature 2013, 497, 458–462. [Google Scholar] [CrossRef] [Green Version]
- Morshed, S.; Mochida, T.; Shibata, R.; Ito, K.; Mostofa, M.G.; Rahman, M.A.; Ushimaru, T. Def1 mediates the degradation of excess nucleolar protein Nop1 in budding yeast. Biochem. Biophys. Res. Commun. 2019, 519, 302–308. [Google Scholar] [CrossRef]
- Akinniyi, O.T.; Reese, J.C. DEF1: Much more than an RNA polymerase degradation factor. DNA Repair 2021, 107, 103202. [Google Scholar] [CrossRef]
- Slawson, C.; Copeland, R.J.; Hart, G.W. O-GIcNAc signaling: A metabolic link between diabetes and cancer? Trends Biochem. Sci. 2010, 35, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Ruan, H.B.; Nie, Y.Z.; Yang, X.Y. Regulation of protein degradation by O-GlcNAcylation: Crosstalk with ubiquitination. Mol. Cell. Proteom. 2013, 12, 3489–3497. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liu, T.W.; Madden, Z.; Yuzwa, S.A.; Murray, K.; Cecioni, S.; Zachara, N.; Vocadlo, D.J. Post-translational O-GlcNAcylation is essential for nuclear pore integrity and maintenance of the pore selectivity filter. J. Mol. Cell. Biol. 2016, 8, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Oses-Ruiz, M.; Sakulkoo, W.; Littlejohn, G.R.; Martin-Urdiroz, M.; Talbot, N.J. Two independent S-phase checkpoints regulate appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Proc. Natl. Acad. Sci. USA 2017, 114, E237–E244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Yokoyama, A.; Tsuji, T.; Ikeshima, E.; Nakashima, K.; Ikushima, S.; Kobayashi, C.; Yoshida, S. Identification and characterization of genes involved in glutathione production in yeast. J. Biosci. Bioeng. 2011, 112, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Jelinsky, S.A.; Estep, P.; Church, G.M.; Samson, L.D. Regulatory networks revealed by transcriptional profiling of damaged Saccharomyces cerevisiae cells: Rpn4 links base excision repair with proteasomes. Mol. Cell. Biol. 2000, 20, 8157–8167. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, K.D.; Wang, T.; Gordon, D.B.; Gifford, D.K.; Stormo, G.D.; Fraenkel, E. An improved map of conserved regulatory sites for Saccharomyces cerevisiae. BMC Bioinform. 2006, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Venters, B.J.; Wachi, S.; Mavrich, T.N.; Andersen, B.E.; Jena, P.; Sinnamon, A.J.; Jain, P.; Rolleri, N.S.; Jiang, C.; Hemeryck-Walsh, C.; et al. A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol. Cell. 2011, 41, 480–492. [Google Scholar] [CrossRef] [Green Version]
- Narukawa-Nara, M.; Sasaki, K.; Ishii, A.; Baba, K.; Amano, K.; Kuroki, M.; Saitoh, K.-I.; Kamakura, T. Identification and characterization of a novel gene encoding the NBS1 protein in Pyricularia oryzae. Biosci. Biotechnol. Biochem. 2015, 79, 1183–1190. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, J.; Jeon, J.; Kim, S.; Park, S.-Y.; Jeon, J.; Lee, Y.-H. Role of the histone acetyltransferase Rtt109 in development and pathogenicity of the rice blast fungus. Mol. Plant Microbe Interact. 2018, 31, 1200–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Cao, H.; Shi, Y.; Huang, P.; Dong, B.; Liu, X.; Lin, F.; Lu, J. The regulatory factor X protein MoRfx1 is required for development and pathogenicity in the rice blast fungus Magnaporthe oryzae. Mol. Plant Pathol. 2017, 18, 1075–1088. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, D.; Zhu, J.; Zheng, J.; Li, H.; He, Q.; Peng, J.; Chen, S.; Chen, X.-L.; Wang, W. RNAPII Degradation Factor Def1 Is Required for Development, Stress Response, and Full Virulence of Magnaporthe oryzae. J. Fungi 2023, 9, 467. https://doi.org/10.3390/jof9040467
Zhang X, Li D, Zhu J, Zheng J, Li H, He Q, Peng J, Chen S, Chen X-L, Wang W. RNAPII Degradation Factor Def1 Is Required for Development, Stress Response, and Full Virulence of Magnaporthe oryzae. Journal of Fungi. 2023; 9(4):467. https://doi.org/10.3390/jof9040467
Chicago/Turabian StyleZhang, Xinrong, Dong Li, Jun Zhu, Jing Zheng, Hongye Li, Qixuan He, Jun Peng, Shen Chen, Xiao-Lin Chen, and Weixiang Wang. 2023. "RNAPII Degradation Factor Def1 Is Required for Development, Stress Response, and Full Virulence of Magnaporthe oryzae" Journal of Fungi 9, no. 4: 467. https://doi.org/10.3390/jof9040467






