Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends
Abstract
:1. Introduction
2. Microbial Polysaccharides: A Brief Overview
3. Gellan Gum: An Overview of the Trends
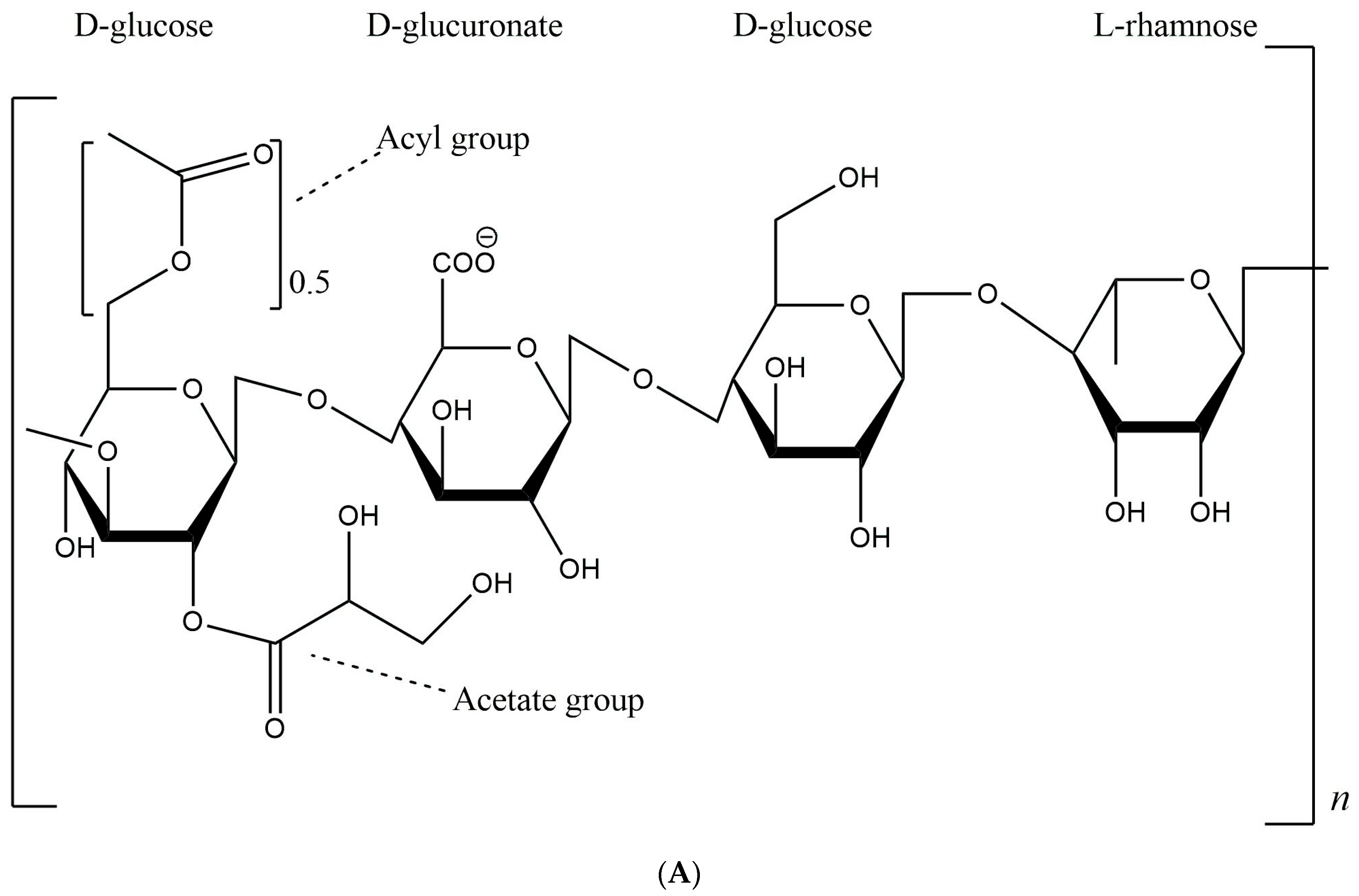
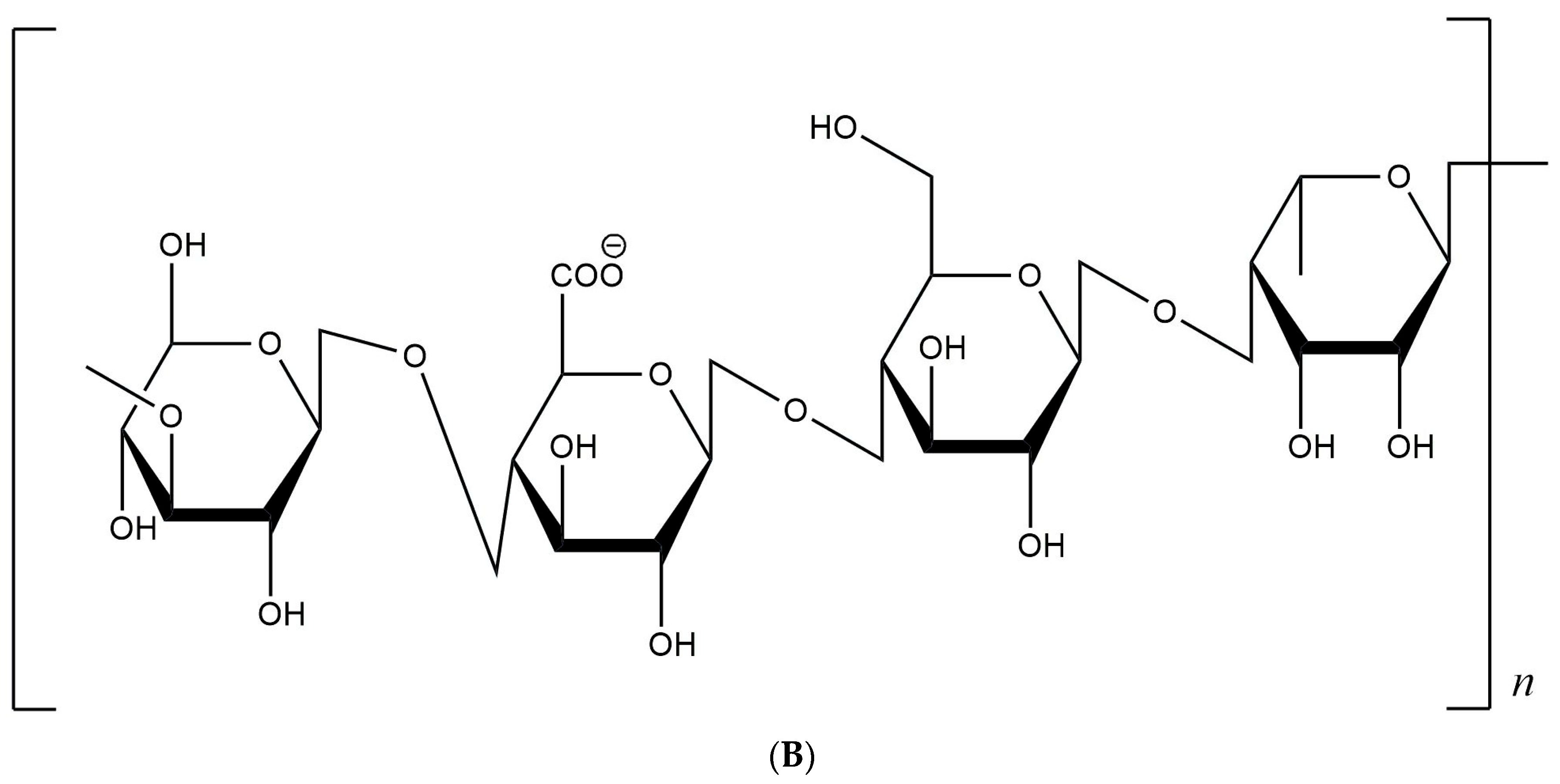
3.1. Chemical Composition of Gellan Gum
3.2. Physical and Chemical Properties of Gellan Gum
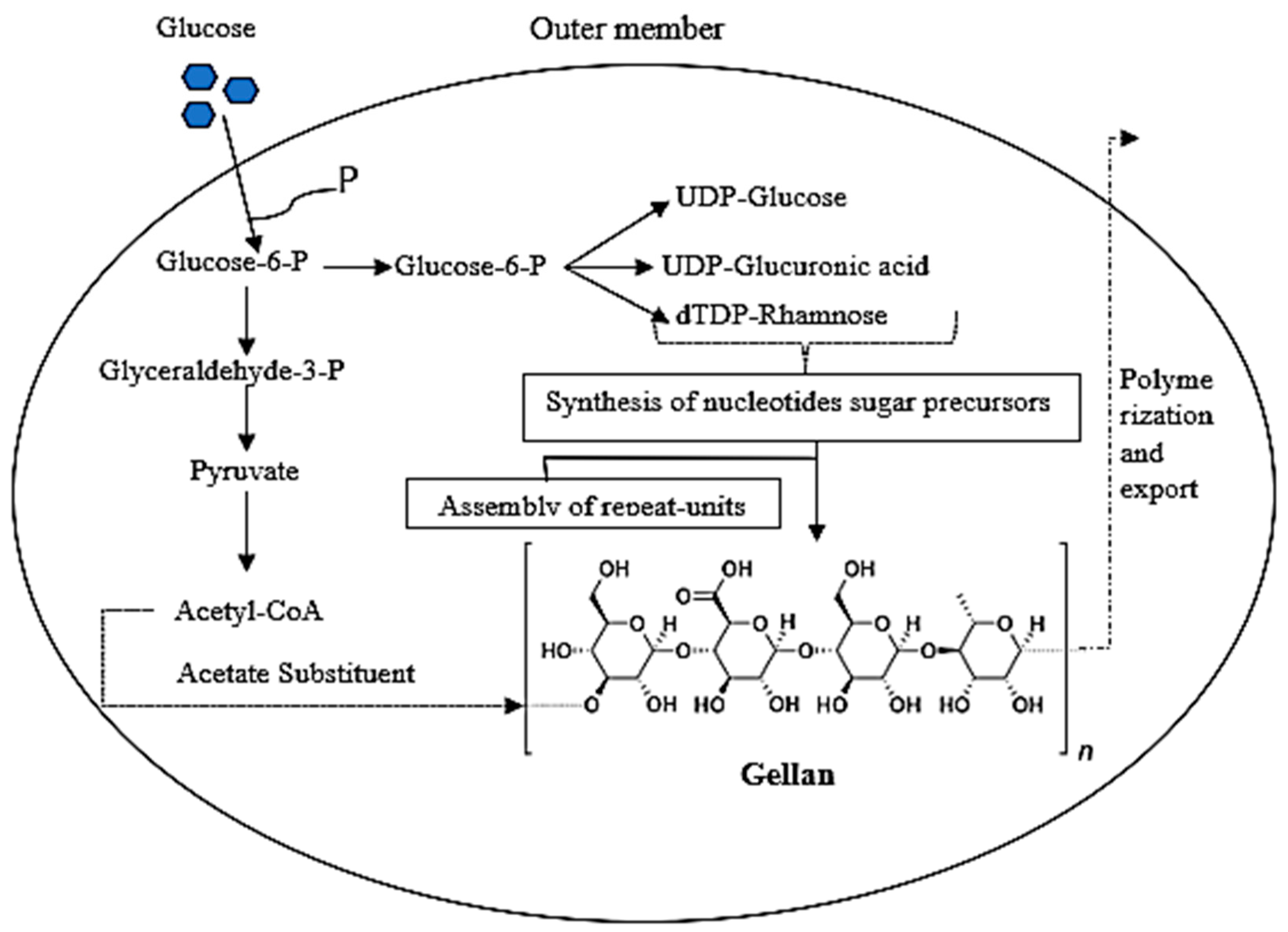
3.3. Biosynthesis of Gellan Gum
3.4. Extraction and Purification of Gellan Gum
4. Advantages and Disadvantages of Gellan Gum Compared to Other Polysaccharides
5. Applications of Gellan Gum
5.1. Food Applications
Production and Utilization of Edible Films
5.2. Medical and Pharmaceutical Applications
Responsive Systems for Biomedical Applications
5.3. Applications in the Cosmetics Industry
5.4. Biological Applications
6. Potential Future and Research Possibilities
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F. Theoretical study of molecular association and thermoreversible gelation in polymers. Polym. J. 2002, 34, 479–509. [Google Scholar] [CrossRef]
- Yang, L.; Han, Z.; Chen, C.; Li, Z.; Yu, S.; Qu, Y.; Zeng, R. Novel probiotic-bound oxidized Bletilla striata polysaccharide-chitosan composite hydrogel. Mater. Sci. Eng. C 2020, 117, 111265. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gong, T.; Lu, Y.H.; Li, A.; Sun, L.; Guo, Y. Compatibility of sodium alginate and konjac glucomannan and their applications in fabricating low-fat mayonnaise-like emulsion gels. Carbohydr. Polym. 2020, 229, 115468. [Google Scholar] [CrossRef] [PubMed]
- Shahbazizadeh, S.; Naji-Tabasi, S.; Shahidi-Noghabi, M.; Pourfarzad, A. Development of cress seed gum hydrogel and investigation of its potential application in the delivery of curcumin. J. Sci. Food Agric. 2021, 101, 6505–6513. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Goff, H.D.; Cui, S.W. Comparison of synergistic interactions of yellow mustard gum with locust bean gum or κ-carrageenan. Food Hydrocoll. 2022, 132, 107804. [Google Scholar] [CrossRef]
- Cairns, P.; Miles, M.J.; Morris, V.J.; Brownsey, G.J. X-ray fibre-diffraction studies of synergistic, binary polysaccharide gels. Carbohydr. Res. 1987, 160, 411–423. [Google Scholar] [CrossRef]
- Xu, X.; Li, B.; Kennedy, J.F.; Xie, B.J.; Huang, M. Characterization of konjac glucomannan–gellan gum blend films and their suitability for release of nisin incorporated therein. Carbohydr. Polym. 2007, 70, 192–197. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- de Souza, C.F.; Riegel-Vidotti, I.C.; Cardoso, M.B.; Ono, L.; Lucyszyn, N.; Lubambo, A.F.; Sens, C.V.; Grein-Iankovski, A.; Sierakowski, M.R. Nanometric organisation in blends of gellan/xyloglucan hydrogels. Carbohydr. Polym. 2014, 114, 48–56. [Google Scholar] [CrossRef]
- Tao, H.; Wang, B.; Wen, H.; Cui, B.; Zhang, Z.; Kong, X.; Wang, Y. Improvement of the textural characteristics of curdlan gel by the formation of hydrogen bonds with erythritol. Food Hydrocoll. 2021, 117, 106648. [Google Scholar] [CrossRef]
- Dev, M.J.; Warke, R.G.; Warke, G.M.; Mahajan, G.B.; Patil, T.A.; Singhal, R.S. Advances in fermentative production, purification, characterization and applications of gellan gum. Bioresour. Technol. 2022, 359, 127498. [Google Scholar] [CrossRef] [PubMed]
- Rühmann, B.; Schmid, J.; Sieber, V. Methods to identify the unexplored diversity of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 565. [Google Scholar]
- Fialho, A.M.; Moreira, L.M.; Granja, A.T.; Popescu, A.O.; Hoffmann, K.; Sá-Correia, I. Occurrence, production, and applications of gellan: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008, 79, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, K.; Kumar, A.; Kumar, S.; Permaul, K.; Singh, S. Production of gellan gum, an exopolysaccharide, from biodiesel-derived waste glycerol by Sphingomonas spp. 3 Biotech 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, S.; Li, C.; Zhang, C.; Ji, Y. Cost-effective optimization of gellan gum production by Sphingomonas paucimobilis using corn steep liquor. Prep. Biochem. Biotechnol. 2020, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kim, H.; Lee, S.; Kim, D.H.; Joe, M.H. Improved gellan gum production by a newly-isolated Sphingomonas azotifigens GL-1 in a cheese whey and molasses based medium. Process Biochem. 2020, 95, 269–278. [Google Scholar] [CrossRef]
- Bagheri, L.; Mousavi, M.E.; Madadlou, A. Stability and rheological properties of suspended pulp particles containing orange juice stabilized by gellan gum. J. Dispers. Sci. Technol. 2014, 35, 1222–1229. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Characteristics of gellan gum-based food gels. J. Texture Stud. 2010, 41, 459–471. [Google Scholar] [CrossRef]
- Sworn, G.; Stouby, L. Gellan gum. In Handbook of Hydrocolloids; Woodhead Publishing: Sawston, UK, 2021; pp. 855–885. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipic, M.; Frutos, M.J.; Frutos, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of gellan gum (E 418) as food additive. EFSA J. 2018, 16, e05296. [Google Scholar]
- Ahmad, N.H.; Mustafa, S.; Che Man, Y.B. Microbial polysaccharides and their modification approaches: A review. Int. J. Food Prop. 2015, 18, 332–347. [Google Scholar] [CrossRef]
- Ravella, S.R.; Quiñones, T.S.; Retter, A.; Heiermann, M.; Amon, T.; Hobbs, P.J. Extracellular polysaccharide (EPS) production by a novel strain of yeast-like fungus Aureobasidium pullulans. Carbohydr. Polym. 2010, 82, 728–732. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.; Araújo, D.; Farinha, I.; Pereira, J.R.; Concórdio-Reis, P.; Reis, M.A. Advanced microbial polysaccharides. Biopolym. Biomed. Biotechnol. Appl. 2021, 2, 19–62. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.; Reis, M.A. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef]
- Kaneda, I.; Kobayashi, A.; Miyazawa, K.; Yanaki, T. Double helix of agrobacterium tumefaciens succinoglycan in dilute solution. Polymers 2002, 43, 1301–1305. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Vaishnavi, T.; Vidyasri, G.S.; Sathya, K.; Priyanka, P.; Venkatachalam, P.; Karuppiah, S. Production of bacterial cellulose using Gluconacetobacter kombuchae immobilized on Luffa aegyptiaca support. Sci. Rep. 2021, 11, 2912. [Google Scholar] [CrossRef] [PubMed]
- Al-Rumaidh, Y.S.; Al-Sahlany, S.T.; Ali, H.I. The Impact of Using Date Juice as a Carbon Source on Curdlan Produced by a Local Isolate of Agrobacterium leguminum. Basrah J. Agric. Sci. 2023, 36, 149–163. [Google Scholar] [CrossRef]
- Majumder, A.; Goyal, A. Rheological and gelling properties of a novel glucan from Leuconostoc dextranicum NRRL B-1146. Food Res. Int. 2009, 42, 525–528. [Google Scholar] [CrossRef]
- Besrour-Aouam, N.; Fhoula, I.; Hernández-Alcántara, A.M.; Mohedano, M.L.; Najjari, A.; Prieto, A.; Ruas-Madiedo, P.; López, P.; Ouzari, H.I. The role of dextran production in the metabolic context of Leuconostoc and Weissella Tunisian strains. Carbohydr. Polym. 2021, 253, 117254. [Google Scholar] [CrossRef]
- Schürks, N.; Wingender, J.; Flemming, H.; Mayer, C. Monomer composition and sequence of alginates from pseudomonas aeruginosa. Int. J. Biol. Macromol. 2002, 30, 105–111. [Google Scholar] [CrossRef]
- Mujumdar, S.; Joshi, P.; Karve, N. Production, characterization, and applications of bioemulsifiers (BE) and biosurfactants (BS) produced by Acinetobacter spp.: A review. J. Basic Microbiol. 2019, 59, 277–287. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Synthesis of the microbial polysaccharide gellan from dairy and plant-based processing coproducts. Polysaccharides 2021, 2, 234–244. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Niamah, A.K. Identification and antioxidant activity of hyaluronic acid extracted from local isolates of Streptococcus thermophilus. Mater. Today Proc. 2022, 60, 1523–1529. [Google Scholar] [CrossRef]
- Khassaf, W.H.; Niamah, A.K.; Al-Manhel, A.J. study of the Optimal Conditions of Levan Production from a Local Isolate of Bacillus subtilis subsp. subtilis w36. Basrah J. Agric. Sci. 2019, 32, 213–222. [Google Scholar] [CrossRef]
- Wani, S.M.; Mir, S.A.; Khanday, F.A.; Masoodi, F.A. Advances in pullulan production from agro-based wastes by Aureobasidium pullulans and its applications. Innov. Food Sci. Emerg. Technol. 2021, 74, 102846. [Google Scholar] [CrossRef]
- Boddapati, S.; Gummadi, S.N. A comprehensive review on mutan (a mixed linkage of α-1-3 and α-1-6 glucans) from bacterial sources. Biotechnol. Genet. Eng. Rev. 2021, 37, 208–237. [Google Scholar] [CrossRef]
- Al-Roomi, F.W.; Al-Sahlany, S.T. Identification and Characterization of Xanthan Gum Produced from Date Juice by a Local Isolate of Bacteria Xanthomonas campestris. Basrah J. Agric. Sci. 2022, 35, 35–49. [Google Scholar] [CrossRef]
- Liu, Z.H.; Niu, F.J.; Xie, Y.X.; Xie, S.M.; Liu, Y.N.; Yang, Y.Y.; Zhou, C.Z.; Wan, X.H. A review: Natural polysaccharides from medicinal plants and microorganisms and their anti-herpetic mechanism. Biomed. Pharmacother. 2020, 129, 110469. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Shu, Z.; Zheng, Y.; Hu, X.; Zhang, P.; Huang, H.; Sheng, L.; Zhang, P.; Wang, Q.; et al. Regulation strategy, bioactivity, and physical property of plant and microbial polysaccharides based on molecular weight. Int. J. Biol. Macromol. 2023, 244, 125360. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent advancements in microbial polysaccharides: Synthesis and applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (a review of current applications and upcoming potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Saudagar, P.S.; Singhal, R.S.; Pandey, A. Statistical approach to optimization of fermentative production of gellan gum from Sphingomonas paucimobilis ATCC 31461. J. Biosci. Bioeng. 2006, 102, 150–156. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. (Ed.) Gellans, curdlan, dextrans, levans, and pullulan. In Carbohydrate Chemistry for Food Scientists; Elsevier Inc: Amsterdam, The Netherlands, 2019; pp. 271–278. [Google Scholar]
- Ferris, C.J.; Gilmore, K.J.; Wallace, G.G. Modified gellan gum hydrogels for tissue engineering applications. Soft Matter 2013, 9, 3705–3711. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Millane, R.P.; Arnott, S.; Atkins, E.D. The crystal structure of gellan. Carbohydr. Res. 1988, 175, 1–15. [Google Scholar] [CrossRef]
- Miyoshi, E.; Nishinari, K. Rheological and thermal properties near the sol-gel transition of gellan gum aqueous solutions. In Physical Chemistry and Industrial Application of Gellan Gum; Nishinari, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 68–82. [Google Scholar] [CrossRef]
- Sworn, G.; Sanderson, G.R.; Gibson, W. Gellan gum fluid gels. Food Hydrocoll. 1995, 9, 265–271. [Google Scholar] [CrossRef]
- Farrés, I.F.; Norton, I.T. Formation kinetics and rheology of alginate fluid gels produced by in-situ calcium release. Food Hydrocoll. 2014, 40, 76–84. [Google Scholar] [CrossRef]
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T.A. Physics of agarose fluid gels: Rheological properties and microstructure. Curr. Res. Food Sci. 2021, 4, 436–448. [Google Scholar] [CrossRef]
- García, M.C.; Alfaro, M.C.; Muñoz, J. Yield stress and onset of nonlinear time-dependent rheological behaviour of gellan fluid gels. J. Food Eng. 2015, 159, 42–47. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Flahive III, J.J.; Foufopoulos, A.; Etzel, M.R. Alcohol precipitation of xanthan gum from pure solutions and fermentation broths. Sep. Sci. Technol. 1994, 29, 1673–1687. [Google Scholar] [CrossRef]
- Wu, X.; Wu, R.; Li, O.; Zhu, L.; Chen, Y.; Qian, C.; Chen, M. Yellow Pigments Generation Deficient Sphingomonas Strain and Application Thereof in Gellan Gum Production. U.S. Patent 8,685,698, 1 April 2014. [Google Scholar]
- Garcıa-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Zala, B.S.; Khutliwala, T.A. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr. Polym. 2013, 93, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, K.M.; Singhania, R.R.; Sabarinath, C.; Pandey, A. Fermentative production of gellan using Sphingomonas paucimobilis. Process Biochem. 2003, 38, 1513–1519. [Google Scholar] [CrossRef]
- Banik, R.M.; Santhiagu, A.; Upadhyay, S.N. Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour. Technol. 2007, 98, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Fialho, A.M.; Martins, L.O.; Donval, M.L.; Leitão, J.H.; Ridout, M.J.; Jay, A.J.; Morris, V.J.; Sá-Correia, I. Structures and properties of gellan polymers produced by Sphingomonas paucimobilis ATCC 31461 from lactose compared with those produced from glucose and from cheese whey. Appl. Environ. Microbiol. 1999, 65, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, N.K.; Shin, M.K.; Kim, S.K.; Kaplan, D.L.; Lee, J.W. Production of gellan gum by Sphingomonas paucimobilis NK2000 with soybean pomace. Biochem. Eng. J. 2003, 16, 357–360. [Google Scholar] [CrossRef]
- Martins, L.O.; Sá-Correia, I. Temperature profiles of gellan gum synthesis and activities of biosynthetic enzymes. Biotechnol. Appl. Biochem. 1994, 20, 385–395. [Google Scholar] [CrossRef]
- Kang, K.S.; Veeder, G.T.; Mirrasoul, P.J.; Kaneko, T.; Cottrell, I.W. Agar-like polysaccharide produced by a Pseudomonas species: Production and basic properties. Appl. Environ. Microbiol. 1982, 43, 1086–1091. [Google Scholar] [CrossRef]
- Manna, B.; Gambhir, A.; Ghosh, P. Production and rheological characteristics of the microbial polysaccharide gellan. Lett. Appl. Microbiol. 1996, 23, 141–145. [Google Scholar] [CrossRef]
- Coelho, J.; Eusébio, D.; Gomes, D.; Frias, F.; Passarinha, L.A.; Sousa, Â. Biosynthesis and isolation of gellan polysaccharide to formulate microspheres for protein capture. Carbohydr. Polym. 2019, 220, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.S.; Federico, S.; Pitarresi, G.; Fiorica, C.; Giammona, G. Gellan gum-based delivery systems of therapeutic agents and cells. Carbohydr. Polym. 2020, 229, 115430. [Google Scholar] [CrossRef]
- Fiorica, C.; Biscari, G.; Palumbo, F.S.; Pitarresi, G.; Martorana, A.; Giammona, G. Physicochemical and rheological characterization of different low molecular weight gellan gum products and derived ionotropic crosslinked hydrogels. Gels 2021, 7, 62. [Google Scholar] [CrossRef]
- Vilela, J.A.P.; Bonsanto, F.P.; Cunha, R.L. Mechanical properties of gellan gum beads prepared with potassium or calcium ions. J. Texture Stud. 2022, 53, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Guo, Y.; Ma, A.; Zhang, H. NMR analysis of the side-group substituents in welan gum in comparison to gellan gum. Int. J. Biol. Macromol. 2024, 254, 127847. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; McClements, D.J. Impact of heat-set and cold-set gelling polysaccharides on potato protein gelation: Gellan gum, agar, and methylcellulose. Food Hydrocoll. 2024, 149, 109535. [Google Scholar] [CrossRef]
- Sharma, S.; Bhende, M.; Goel, A. A review: Polysaccharide-based hydrogels and their biomedical applications. Polym. Bull. 2024, 1–22. [Google Scholar] [CrossRef]
- Zhan, L.; Lan, G.; Wang, Y.; Xie, S.; Cai, S.; Liu, Q.; Chen, P.; Xie, F. Mastering textural control in multi-polysaccharide gels: Effect of κ-carrageenan, konjac glucomannan, locust bean gum, low-acyl gellan gum, and sodium alginate. Int. J. Biol. Macromol. 2024, 254, 127885. [Google Scholar] [CrossRef]
- Chen, B.; Cai, Y.; Liu, T.; Huang, L.; Zhao, X.; Zhao, M.; Deng, X.; Zhao, Q. Formation and performance of high acyl gellan hydrogel affected by the addition of physical-chemical treated insoluble soybean fiber. Food Hydrocoll. 2020, 101, 105526. [Google Scholar] [CrossRef]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar] [CrossRef]
- Choudhury, A.R. Synthesis and rheological characterization of a novel thermostable quick setting composite hydrogel of gellan and pullulan. Int. J. Biol. Macromol. 2019, 125, 979–988. [Google Scholar] [CrossRef]
- Kanyuck, K.M.; Norton-Welch, A.B.; Mills, T.B.; Norton, I.T. Structural characterization of interpenetrating network formation of high acyl gellan and maltodextrin gels. Food Hydrocoll. 2021, 112, 106295. [Google Scholar] [CrossRef]
- Sapper, M.; Bonet, M.; Chiralt, A. Wettability of starch-gellan coatings on fruits, as affected by the incorporation of essential oil and/or surfactants. LWT 2019, 116, 108574. [Google Scholar] [CrossRef]
- Leal, A.R.; Oliveira, L.D.S.; Costa, J.N.D.; Alves, C.A.N.; Mata, P.; Sousa, P.H.M.D. In vitro bioaccessibility of antioxidant compounds from structured fruits developed with gellan gum and agar. Rev. Cienc. Agron. 2022, 53, e20207744. [Google Scholar] [CrossRef]
- Iurciuc, C.; Savin, A.; Lungu, C.; Martin, P.; Popa, M. Gellan. food applications. Cellul. Chem. Technol. 2016, 13, 1–13. [Google Scholar]
- Kong, X.; Xiao, Z.; Du, M.; Wang, K.; Yu, W.; Chen, Y.; Liu, Z.; Cheng, Y.; Gan, J. Physicochemical, Textural, and Sensorial Properties of Soy Yogurt as Affected by Addition of Low Acyl Gellan Gum. Gels 2022, 8, 453. [Google Scholar] [CrossRef]
- Mongkontanawat, N.; Khunphutthiraphi, T. Improved sensory acceptance and cytotoxicity to breast cancer cell line of instant germinated black rice yogurt supplemented with gellan gum. Food Agric. Sci. Technol. 2022, 8, 15–29. [Google Scholar]
- Xiao, G.; Zhu, Y.; Wang, L.; You, Q.; Huo, P.; You, Y. Production and storage of edible film using gellan gum. Procedia Environ. Sci. 2011, 8, 756–763. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Ehsani, A.; Moghaddas Kia, E.; Khezerlou, A. Microbial gums: Introducing a novel functional component of edible coatings and packaging. Appl. Microbiol. Biotechnol. 2019, 103, 6853–6866. [Google Scholar] [CrossRef]
- Zikmanis, P.; Juhņeviča-Radenkova, K.; Radenkovs, V.; Segliņa, D.; Krasnova, I.; Kolesovs, S.; Orlovskis, Z.; Šilaks, A.; Semjonovs, P. Microbial polymers in edible films and coatings of garden berry and grape: Current and prospective use. Food Bioprocess Technol. 2021, 14, 1432–1445. [Google Scholar] [CrossRef]
- Moghadam, F.A.M.; Khoshkalampour, A.; Moghadam, F.A.M.; PourvatanDoust, S.; Naeijian, F.; Ghorbani, M. Preparation and physicochemical evaluation of casein/basil seed gum film integrated with guar gum/gelatin based nanogel containing lemon peel essential oil for active food packaging application. Int. J. Biol. Macromol. 2023, 224, 786–796. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef]
- Maurizzi, E.; Bigi, F.; Volpelli, L.A.; Pulvirenti, A. Improving the post-harvest quality of fruits during storage through edible packaging based on guar gum and hydroxypropyl methylcellulose. Food Packag. Shelf Life 2023, 40, 101178. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Wang, T.; Li, Z.; Zou, X.; Huang, X.; Zhai, X.; Shi, J.; Shen, T.; Gong, Y.; et al. Novel gellan gum-based probiotic film with enhanced biological activity and probiotic viability: Application for fresh-cut apples and potatoes. Int. J. Biol. Macromol. 2023, 239, 124128. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Jafari, S.M. Gum-based nanocarriers for the protection and delivery of food bioactive compounds. Adv. Colloid Interface Sci. 2019, 269, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Thakur, N.; Kajla, P.; Thakur, S.; Punia, S. Application of encapsulation technology in edible films: Carrier of bioactive compounds. Front. Sustain. Food Syst. 2021, 5, 734921. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A comprehensive review of biodegradable polymer-based films and coatings and their food packaging applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, W.; Chen, L.; Liu, J.; Cao, J.; Jiang, W. Recent advances in guar gum-based films or coatings: Diverse property enhancement strategies and applications in foods. Food Hydrocoll. 2023, 136, 108278. [Google Scholar] [CrossRef]
- Yang, L. Physicochemical Properties of Biodegradable/Edible Films Made with Gellan Gum; DalTech-Dalhousie University: Halifax, NS, Canada, 1999. [Google Scholar]
- Rafe, A. Improving Texture of Foods using Emerging Hydrocolloids. Emerg. Nat. Hydrocoll. Rheol. Funct. 2019, 20, 499–523. [Google Scholar]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef]
- Vilela, J.A.P.; de Assis Perrechil, F.; Picone, C.S.F.; Sato, A.C.K.; da Cunha, R.L. Preparation, characterization and in vitro digestibility of gellan and chitosan–gellan microgels. Carbohydr. Polym. 2015, 117, 54–62. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, G.; Navarro, G.; Di Meo, C.; Matricardi, P.; Torchilin, V. Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: A multi-drug delivery system for a combination therapy in cancer treatment. Eur. J. Pharm. Biopharm. 2014, 87, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Patil, P.P. In situ cross linked chitosan-gellan gum polyelectrolyte complex based nanogels containing curcumin for delivery to cancer cells. Indian J. Pharm. Educ. Res. 2017, 51, s40–s45. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Cencetti, C.; Franzé, S.; Zoratto, N.; Di Meo, C.; Procacci, P.; Matricardi, P.; Cilurzo, F. Gellan nanohydrogels: Novel nanodelivery systems for cutaneous administration of piroxicam. Mol. Pharm. 2018, 15, 1028–1036. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Cieplak, T.; Cahú, T.B.; Blennow, A.; Knøchel, S.; Nielsen, D.S. Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct. 2018, 9, 5868–5879. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Prus-Walendziak, W.; Stachowiak, N.; Bajek, A.; Kazmierski, L.; Tylkowski, B. Modification of collagen/gelatin/hydroxyethyl cellulose-based materials by addition of herbal extract-loaded microspheres made from gellan gum and xanthan gum. Materials 2020, 13, 3507. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.A. An improved injectable polysaccharide hydrogel: Modified gellan gum for long-term cartilage regeneration in vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Silva, N.A.; Cooke, M.J.; Tam, R.Y.; Sousa, N.; Salgado, A.J.; Reis, R.L.; Shoichet, M.S. The effects of peptide modified gellan gum and olfactory ensheathing glia cells on neural stem/progenitor cell fate. Biomaterials 2012, 33, 6345–6354. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Alharbi, H.Y.; Alnoman, R.B.; Aljohani, M.S.; Al-Anazia, M.; Monier, M. Synthesis and characterization of gellan gum-based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2024, 258, 128828. [Google Scholar] [CrossRef]
- Ghandforoushan, P.; Alehosseini, M.; Golafshan, N.; Castilho, M.; Dolatshahi-Pirooz, A.; Hanaee, J.; Davaran, S.; Orive, G. Injectable hydrogels for cartilage and bone tissue regeneration: A review. Int. J. Biol. Macromol. 2023, 246, 125674. [Google Scholar] [CrossRef] [PubMed]
- Carrêlo, H.; Cidade, M.T.; Borges, J.P.; Soares, P. Gellan gum/alginate microparticles as drug delivery vehicles: DOE production optimization and drug delivery. Pharmaceuticals 2023, 16, 1029. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S. (Eds.) Advanced Biopolymeric Systems for Drug Delivery; Springer: Cham, Switzerland, 2020; pp. 373–384. [Google Scholar]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2023, 24, e202300217. [Google Scholar] [CrossRef]
- Garcia, M.T.; Carmo, P.H.F.d.; Figueiredo-Godoi, L.M.A.; Gonçalves, N.I.; Lima, P.M.N.d.; Ramos, L.d.P.; Oliveira, L.D.d.; Borges, A.L.S.; Shukla, A.; Junqueira, J.C. Gellan-Based Hydrogel as a Drug Delivery System for Caffeic Acid Phenethyl Ester in the Treatment of Oral Candida albicans Infections. Pharmaceutics 2024, 16, 298. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Ahlfeld, T.; Funk, A.; Waske, A.; Lode, A.; Gelinsky, M. Highly concentrated alginate-gellan gum composites for 3D plotting of complex tissue engineering scaffolds. Polymers 2016, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xiao, R.; Wu, Y.; Xu, L. Advances in tissue engineering of gellan gum-based hydrogels. Carbohydr. Polym. 2023, 324, 121484. [Google Scholar] [CrossRef] [PubMed]
- Feketshane, Z.; Alven, S.; Aderibigbe, B.A. Gellan gum in wound dressing scaffolds. Polymers 2022, 14, 4098. [Google Scholar] [CrossRef] [PubMed]
- Bal-Öztürk, A.; Torkay, G.; İdil, N.; Özkahraman, B.; Özbaş, Z. Gellan gum/guar gum films incorporated with honey as potential wound dressings. Polym. Bull. 2024, 81, 1211–1228. [Google Scholar] [CrossRef]
- Ramaprabha, K.; Kumar, V.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Kamyab, H.; Vasseghian, Y. Exploring the diverse applications of Carbohydrate macromolecules in food, pharmaceutical, and environmental technologies. Environ. Res. 2024, 240, 117521. [Google Scholar]
- Mousavi, S.S.; Keshvari, H.; Daemi, H. Partial sulfation of gellan gum produces cytocompatible, body temperature-responsive hydrogels. Int. J. Biol. Macromol. 2023, 235, 123525. [Google Scholar] [CrossRef]
- Xu, S.Q.; Du, Y.N.; Zhang, Z.J.; Yan, J.N.; Sun, J.J.; Zhang, L.C.; Wang, C.; Lai, B.; Wu, H.T. Gel properties and interactions of hydrogels constructed with low acyl gellan gum and puerarin. Carbohydr. Polym. 2024, 326, 121594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zheng, X.; Yi, K.; Du, X.; Wang, C.; Cui, P.; Jiang, P.; Ni, X.; Qiu, L.; Wang, J. Temperature-Ion-pH Triple Responsive Gellan Gum as In Situ Hydrogel for Long-Acting Cancer Treatment. Gels 2022, 8, 508. [Google Scholar] [CrossRef]
- Razali, M.H.; Ismail, N.A.; Zulkafli, M.F.A.M.; Amin, K.A.M. 3D Nanostructured materials: TiO2 nanoparticles incorporated gellan gum scaffold for photocatalyst and biomedical Applications. Mater. Res. Express 2018, 5, 035039. [Google Scholar] [CrossRef]
- Lameirinhas, N.S.; Teixeira, M.C.; Carvalho, J.P.; Valente, B.F.; Pinto, R.J.; Oliveira, H.; Luís, J.L.; Pires, P.L.; Oliveira, J.M.; Vilela, C.; et al. Nanofibrillated cellulose/gellan gum hydrogel-based bioinks for 3D bioprinting of skin cells. Int. J. Biol. Macromol. 2023, 229, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Santos, T.C.; Martins, L.; Picciochi, R.; Marques, A.P.; Castro, A.G.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum injectable hydrogels for cartilage tissue engineering applications: In vitro studies and preliminary in vivo evaluation. Tissue Eng. Part A 2010, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Paolicelli, P.; Dreesen, I.; Kobayashi, S.; Vitalone, A.; Casadei, M.A. Injectable and photocross-linkable gels based on gellan gum methacrylate: A new tool for biomedical application. Int. J. Biol. Macromol. 2015, 72, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Posadowska, U.; Brzychczy-Włoch, M.; Drożdż, A.; Krok-Borkowicz, M.; Włodarczyk-Biegun, M.; Dobrzyński, P.; Chrzanowski, W.; Pamuła, E. Injectable hybrid delivery system composed of gellan gum, nanoparticles and gentamicin for the localized treatment of bone infections. Expert Opin. Drug Deliv. 2016, 13, 613–620. [Google Scholar] [CrossRef]
- Cho, H.H.; Choi, J.H.; Been, S.Y.; Kim, N.; Choi, J.M.; Kim, W.; Kim, D.; Jung, J.J.; Song, J.E.; Khang, G. Development of fluorescein isothiocyanate conjugated gellan gum for application of bioimaging for biomedical application. Int. J. Biol. Macromol. 2020, 164, 2804–2812. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Moretti, G.; Petralito, S.; Di Giacomo, S.; Vitalone, A.; Casadei, M.A. Gellan gum methacrylate and laponite as an innovative nanocomposite hydrogel for biomedical applications. Eur. Polym. J. 2016, 77, 114–123. [Google Scholar] [CrossRef]
- Nayak, A.K.; Bera, H.; Hasnain, M.S.; De, A.; Pal, D.; Samanta, A. Gellan gum-based nanomaterials in drug delivery applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 313–336. [Google Scholar]
- Freitas, F.; Alves, V.D.; Reis, M.A. Bacterial polysaccharides: Production and applications in cosmetic industry. In Polysaccharides; Springer: Cham, Switzerland, 2015; pp. 2017–2043. [Google Scholar]
- Iurciuc, C.E.; Lungu, C.; Martin, P.; Popa, M. Gellan. Pharmaceutical, medical and cosmetic applications. Cellul. Chem Technol. 2017, 51, 187–202. [Google Scholar]
- Lee, C.H.; Kim, H.T.; Yun, E.J.; Lee, A.R.; Kim, S.R.; Kim, J.H.; Choi, I.G.; Kim, K.H. A novel agarolytic β-galactosidase acts on agarooligosaccharides for complete hydrolysis of agarose into monomers. Appl. Environ. Microbiol. 2014, 80, 5965–5973. [Google Scholar] [CrossRef]
- Sanchez-Cardozo, J.; Quintanilla-Carvajal, M.X.; Ruiz-Pardo, R.; Acosta-González, A. Evaluating gelling-agent mixtures as potential substitutes for bacteriological agar: An approach by mixture design. Dyna 2019, 86, 171–176. [Google Scholar] [CrossRef]
- Descallar, F.B.A.; Matsukawa, S. Change of network structure in agarose gels by aging during storage studied by NMR and electrophoresis. Carbohydr. Polym. 2020, 245, 116497. [Google Scholar] [CrossRef]
| Producing Microorganisms | Type of Sugar Produced | Reference |
|---|---|---|
| Agrobacterium tumefaciens | Succinoglycan | [26] |
| Gluconacetobacter, Sarcina Agrobacterium, Rhizobium | Cellulose | [27] |
| Agrobacterium leguminum | Curdlan | [28] |
| Leuconostoc dextranicum | Glucan | [29] |
| Leuconostoc, Streptococcus, Weissella, Pediococcus Lactobacillus | Dextran | [30] |
| Pseudomonas aeruginosa | Algins | [31] |
| Acinetobacter sp. | Emulsan | [32] |
| Sphingomonas paucimobilis | Gellan | [33] |
| Streptococcus equisimilis, S. Pyogenes, S. thermophilus, S. equi | Hyaluronic acid | [34] |
| Acetobacter, Bacillus, Brenneria, Geobacillus, Halomonas, Lactobacillus, Zymomonas, Saccharomyces | Levan | [35] |
| Aureobasidium pullulans, Cytaria spp., Teloschistes flavicans, Rhodototula bacarum, Cryphonectria parasitica | Pullulan | [36] |
| Streptococcus mutans | Mutan | [37] |
| Xanthomonas spp. | Xanthan gum | [38] |
| Features of Polysaccharides | Microorganism Polysaccharides | Plant Polysaccharides |
|---|---|---|
| Source | Produced by microorganisms like bacteria, yeast, and fungi. Some examples are xanthan gum (bacterial), dextran (bacterial), and pullulan (fungal). | Derived from botanical sources. Some examples are cellulose, hemicellulose, pectin, and starch. |
| Structure | They frequently exhibit a more intricate and varied composition. Illustrations feature branched structures in xanthan gum and linear structures in dextran. | Usually exhibit well-defined and consistent structures. Illustrations consist of the straight arrangement of cellulose and the intricate arrangement of pectin. |
| Function | Frequently produced by microorganisms for functions like protection, attachment, or energy retention. Applications in industries involve serving as thickeners, stabilizers, and gelling agents in the food and pharmaceutical sectors. | Plants perform a range of functions, including providing structural support (cellulose), storing energy (starch), and maintaining cell wall integrity (hemicellulose and pectin). They are applied in a variety of industrial settings, such as food, pharmaceuticals, and paper production. |
| Production Method | Produced via fermentation techniques with microorganisms in controlled environments. | Derived from plant tissues using a combination of physical and chemical methods, which may include breaking down cell walls to release polysaccharides. |
| Solubility | Certain microbial polysaccharides, such as xanthan gum, exhibit water solubility and produce viscous solutions. | The solubility of plant polysaccharides differs from one another. As an illustration, starch does not dissolve in cold water, whereas pectin does. |
| Gelling Properties | Certain microbial polysaccharides like GG demonstrate gelling properties and can produce firm, flexible gels. | Agar and pectin, plant-derived polysaccharides, are recognized for their gel-forming properties and are widely utilized as gelling agents in the food and pharmaceutical industries. |
| Applications | Commonly utilized in the food, pharmaceutical, and cosmetic sectors for their thickening, stabilizing, and gelling properties. | Applied in a wide range of industries, such as food (for thickening and gelling), pharmaceuticals, textiles, and paper production. |
| Tests | Property | Value |
|---|---|---|
| Physicochemical | Molecular weight | 500 KDa |
| Appearance | Yellowish white powder | |
| Functional use | Thickener and stabilizer | |
| Solubility | Dissolved in water, forming a viscous solution, insoluble in ethanol and other organic solvents | |
| Weight loss | No more than 15% when using a temperature of 105 °C for 2.5 h | |
| Lead | No more than 2 mg/kg | |
| Nitrogen | No more than 3% | |
| Microbial | Total bacterial count | No more than 1 × 104 CFU/g |
| E. coli | There was no growth | |
| Salmonella | There was no growth | |
| Yeasts and molds | No more than 5 × 102 CFU/g |
| Features | Critical Remarks |
|---|---|
| Benefits | |
| Thermoreversibility | GG develops thermoreversible gels, which can solidify when cooled and return to a liquid form when heated. This feature is advantageous in scenarios where precise temperature management is crucial, enabling the creation of distinct textures in food and pharmaceuticals. |
| Low concentration requirement | GG can produce gels at lower concentrations in comparison to certain other polysaccharides, such as agar. This can offer benefits in terms of cost-effectiveness and sensory impact. |
| Resistance to syneresis | GG gels typically exhibit resistance to syneresis, the phenomenon where a liquid is released from a gel. This characteristic plays a role in maintaining the stability and visual appeal of products that contain GG as time passes. |
| Clarity | GG gels are known for their excellent clarity, rendering them ideal for uses in applications that require a transparent or semi-transparent look, like specific desserts or beverages. |
| Film-forming ability | GG possesses film-forming properties, which render it ideal for use in scenarios where thin, pliable films are needed, like in edible films for food encapsulation. |
| Drawbacks | |
| Texture sensitivity to ions | The texture of GG gels can be affected by the specific ions and their concentrations in the formulation. Its sensitivity could restrict its use in specific circumstances. |
| Limited familiarity | When compared to other polysaccharides, such as agar or xanthan gum, GG might not be as familiar or commonly utilized in specific industries or sectors, potentially affecting its adoption and accessibility. |
| Cost | GG might have a higher price compared to certain other hydrocolloids, which could be an important factor to keep in mind when cost is a crucial consideration in formulations. |
| Main Food Field | Representative Food Product | References |
|---|---|---|
| Beverages | Beverages with jelly and fruit | [74] |
| Sugar | Starch, jelly, stuffing, and candy floss | [61] |
| Jam | Low-heat jam, synthetic jam, and bread stuffing | [62] |
| Synthetic food | Synthetic fruits, synthetic vegetables, and synthetic meat | [18,19] |
| Water-based gel | Dessert gel and decorative jelly | [47] |
| Pie stuffing and pudding | Fast-food dessert, tinned pudding, pre-cooked pudding, and pie stuffing | [75] |
| Pet food | Tinned meat segment and gel pet food | [71] |
| Sugar coating and sugar frost | Sugar coatings for cakes and tinned sugar frost | [75] |
| Milk products | Ice cream, jelly milk, yogurt, and frozen milk | [60] |
| Aspects of Edible Film Production | Remarks |
|---|---|
| Ingredient selection | GG has been chosen as the primary hydrocolloid for film formation. Additional components can be incorporated to improve the characteristics of the film, including plasticizers like glycerol and sorbitol, antimicrobial agents, antioxidants, or flavorings. |
| Solution preparation | GG is commonly dispersed in water or a water-based solution. Next, the solution is heated to fully hydrate and dissolve the GG. |
| Film formation | Once the solution is ready, it is poured or cast into a mold or onto a flat surface to create a thin layer. The film-forming solution can be dried using techniques such as air drying, hot air drying, or freeze-drying based on the desired properties of the film. |
| Control of film properties | By adjusting the concentration of GG and other additives along with the drying conditions, it is possible to control the properties of the edible film, including the thickness, transparency, and mechanical strength. |
| Multiple Applications within the Food Sector | Remarks | References |
|---|---|---|
| Food packaging | Edible films composed of GG are used as packaging for a variety of food items. These films serve as protective shields that block out moisture, oxygen, and other environmental elements, ultimately prolonging the shelf life of perishable items. | [86] |
| Coatings for fresh produce | Edible films developed from GG are suitable for coating fresh fruits and vegetables. The films aid in decreasing water loss and preserving freshness and can also be used to transport additional nutrients or preservatives. | [87,88,89] |
| Encapsulation of bioactive compounds | GG films are ideal for encapsulating and protecting bioactive compounds like antioxidants, vitamins, or antimicrobial agents. This enables a regulated release of these substances within the food matrix. | [75,90,91] |
| Flavor films | GG-based films have the capability to incorporate flavors or aromas, offering a distinctive and personalized sensory encounter when utilized as coverings for candies, confections, or other flavored food items. | [92,93] |
| Edible strips and wrappers | GG films can be shaped into strips or wrappers that are convenient to use and eat. This is especially beneficial for products that require a thin, dissolvable layer, like single-serving condiment packets. | [94] |
| Improvement of texture | Edible films composed of GG have the potential to enhance the texture of specific food products, resulting in a more pleasing mouthfeel and improved crispiness. | [95,96] |
| Other innovative uses | Exploring GG-based edible films in different innovative applications such as edible food labels, decorations, and interactive food experiences. | [41] |
| Drug/Application Formulation | Fabrication Procedure | Most Important Type | Results | Reference |
|---|---|---|---|---|
| Model microgels | Ionotropic gelation with CaCl2 or KCl, coating with chitosan | Microgels | Good stability in aqueous media except for KCl-crosslinked microgels; the particles were stable in gastric conditions; chitosan-coated microgels were less susceptible to degradation in intestinal fluid | [98] |
| Prednisolone, paclitaxel/cancer | Self-assembly | Nanogels | Prednisolone acted as a hydrophobic moiety in nanogel self-assembly; increased cytotoxic efficacy towards different cancer cell lines | [99] |
| Curcumin/cancer | Polyelectrolyte complexation | Nanogels | Prolonged curcumin release; good hemocompatibility and non-toxicity | [100] |
| Piroxicam/non-melanoma skin cancers | Self-assembly | Nanogels | Nanogels enhanced drug retention in the epidermis; nanogels permeated across the stratum corneum and released the drug in the viable epidermis | [101] |
| Probiotic bacteria/gut microbiota dysbiosis | Ionic crosslinking with CaCl2, freeze-drying | Microcapsules | Improved survival rate during simulated gastrointestinal tract passage | [102] |
| Calendula officinalis extract/cosmetic applications | Ionotropic gelation with CaCl2 (extrusion or emulsion | Microspheres | The size and entrapment efficiency of microspheres depended on the fabrication method | [103] |
| Innovative Trend | Biomedical Application | References |
|---|---|---|
| Smart drug delivery systems | GG has been utilized in the development of advanced drug delivery systems that react to different stimuli, like temperature, pH, or specific ions. These systems can offer the controlled and targeted delivery of therapeutic substances, enhancing the effectiveness of drugs while reducing potential negative reactions | [107,110,111,112] |
| Temperature-responsive hydrogels | Hydrogels composed of GG have been formulated to demonstrate temperature-sensitive properties, enabling them to transition between sol and gel states based on temperature variations. This characteristic is especially valuable in scenarios where in situ gelation is required, like injectable hydrogels for minimally invasive drug delivery or tissue engineering. | [107,118,119] |
| Ion-responsive systems | GG can produce gels when exposed to certain ions, like calcium or potassium. This characteristic has been utilized in the creation of ion-responsive systems, which are designed to release drugs or bioactive agents in response to certain ions found in the body. | [72,120] |
| 3D bioprinting and tissue engineering | When combined with other biomaterials, GG has been studied for its potential in 3D bioprinting for tissue engineering. Due to GG’s capability of producing thermoreversible gels, it enables the development of intricate structures with improved mechanical characteristics, ideal for scaffold production. | [113,114,121,122] |
| Wound healing and dressings | Research has been conducted on hydrogels composed of GG for potential uses in promoting wound healing. These hydrogels offer a moist environment, strong adherence to the wound site, and the potential release of bioactive compounds to improve the healing process. Features can be integrated to cater to particular wound conditions. | [115,116] |
| Injectable systems for minimally invasive procedures | The thermoreversible gelation property of GG has been utilized to develop injectable systems for minimally invasive procedures. These systems have the capability to be administered in a liquid state and then undergo gelation in situ, which renders them ideal for various applications like tissue augmentation or local drug delivery. | [123,124,125] |
| Diagnostic applications | GG has been investigated for its use in producing diagnostic devices and biosensors. Utilizing the unique properties of GG, it is possible to develop sensing platforms capable of detecting particular biomolecules or variations in physiological conditions. | [111,112,114,117,126] |
| Combination with nanoparticles | GG can be combined with nanoparticles, like drug-loaded nanoparticles or imaging agents, to develop multifunctional responsive systems with improved therapeutic or diagnostic capabilities. | [121,127,128] |
| Industrial Field | Major Area | New Possibilities for Study and Investigation |
|---|---|---|
| Food industry | Functional foods |
|
| Clean-label solutions and applications |
| |
| Biopharmaceuticals | Biotherapeutics |
|
| Pharmaceutical excipients |
| |
| Tablet formulation |
| |
| Oral drug delivery: |
| |
| Biomedical applications | Biosensors |
|
| Personalized drug formulations |
| |
| Drug delivery systems |
| |
| Biocompatible scaffolds |
| |
| Wound healing |
| |
| Biotechnology | Microbial production optimization |
|
| Strain improvement |
| |
| Synthetic polysaccharides |
| |
| Genome editing |
| |
| Environmental applications | Wastewater treatment |
|
| Bioremediation |
| |
| Biodegradable polymers |
| |
| Materials science | Hydrogel applications |
|
| Biodegradable films |
| |
| Nanostructured materials |
| |
| Responsive materials |
| |
| Advanced analytical applications | Characterization techniques |
|
| Molecular studies |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdl Aali, R.A.K.; Al-Sahlany, S.T.G. Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends. Gels 2024, 10, 183. https://doi.org/10.3390/gels10030183
Abdl Aali RAK, Al-Sahlany STG. Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends. Gels. 2024; 10(3):183. https://doi.org/10.3390/gels10030183
Chicago/Turabian StyleAbdl Aali, Raghad Abdl Karim, and Shayma Thyab Gddoa Al-Sahlany. 2024. "Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends" Gels 10, no. 3: 183. https://doi.org/10.3390/gels10030183






