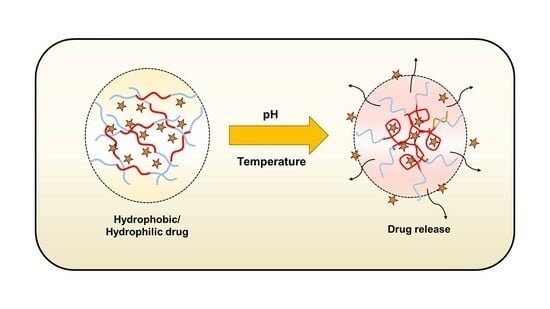
pNIPAm-Based pH and Thermoresponsive Copolymer Hydrogel for Hydrophobic and Hydrophilic Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
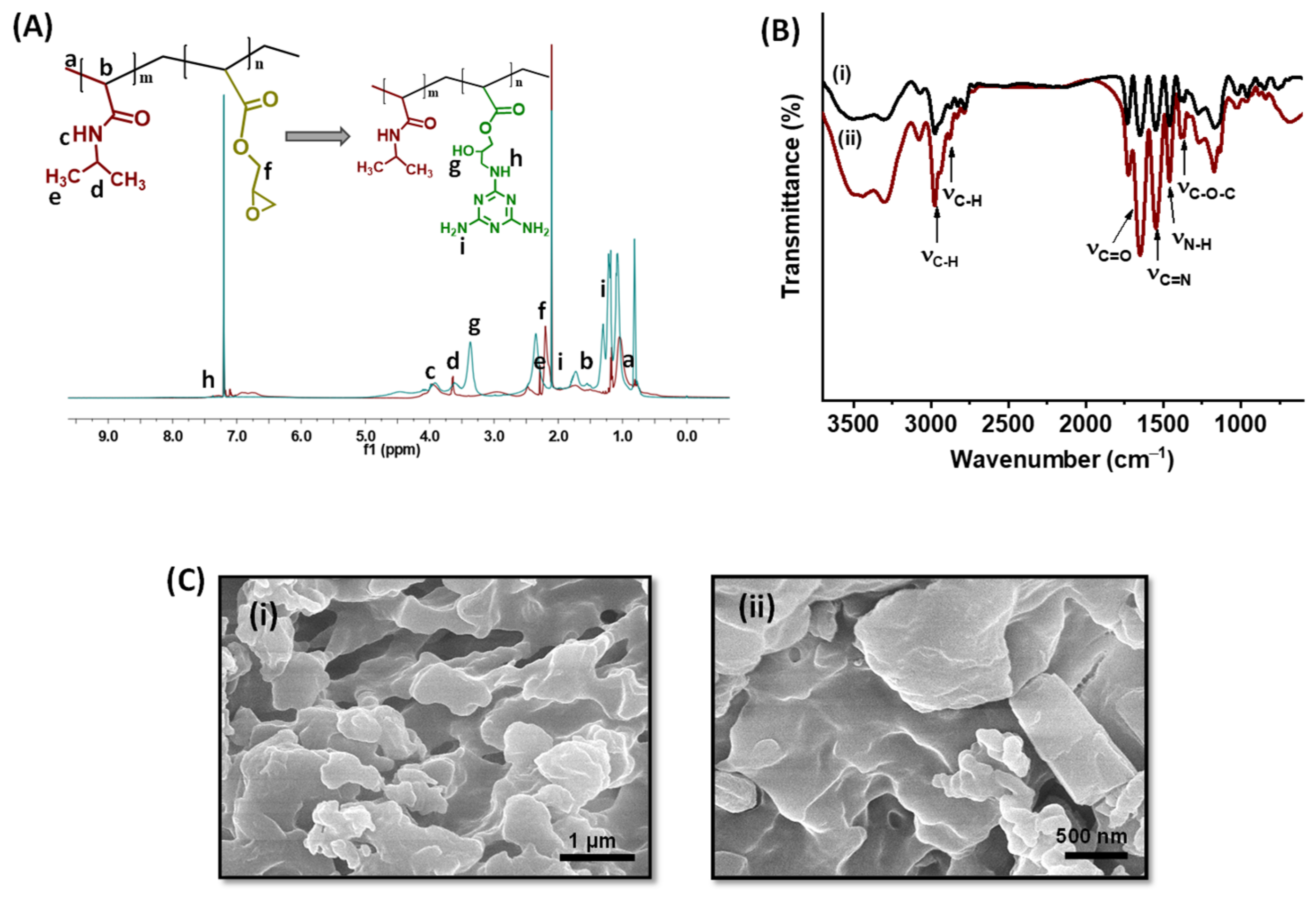
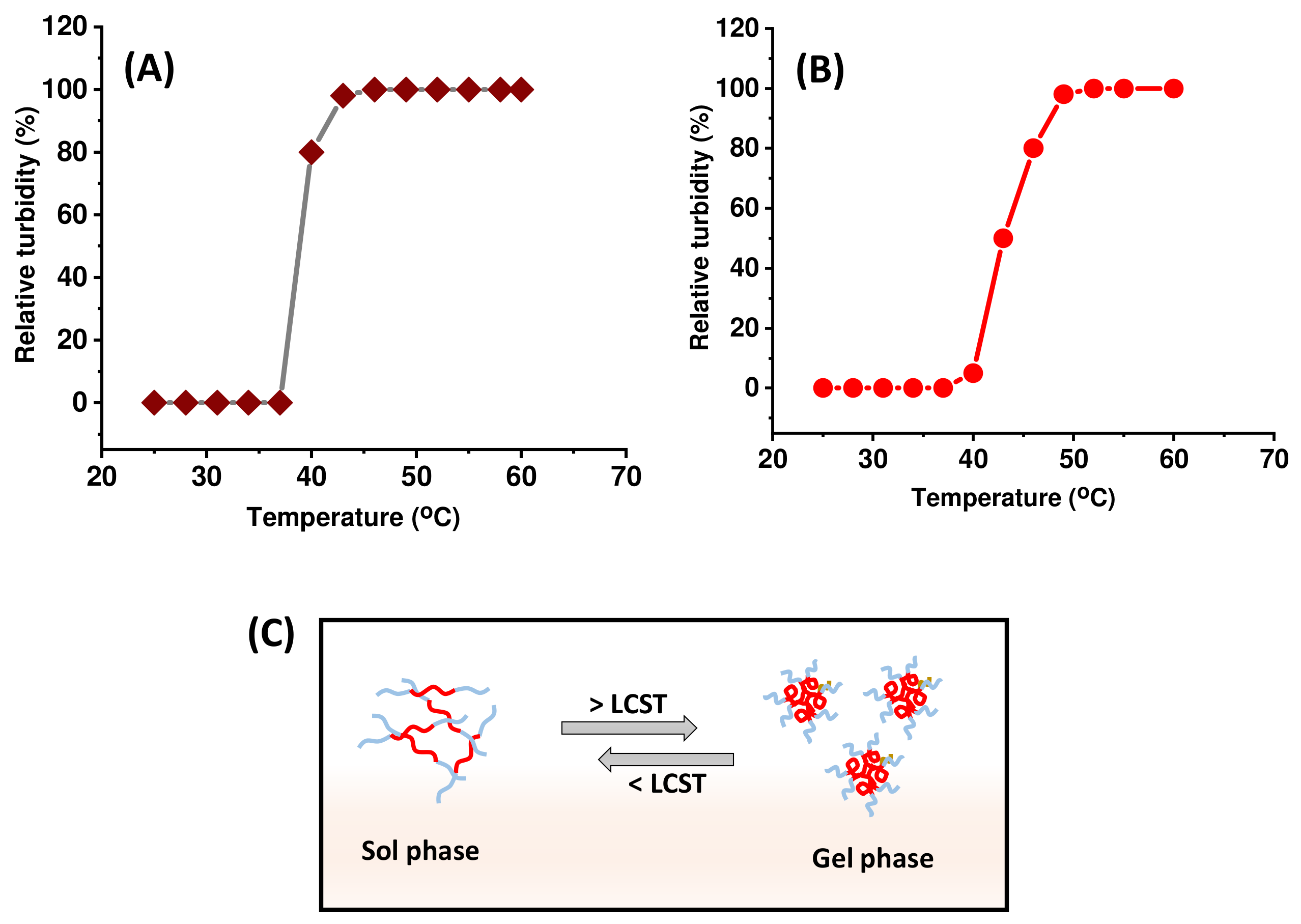
2.1. pNIPAm-co-pGMA and pNIPAm-co-pGMA-Mela Hydrogel Characterization
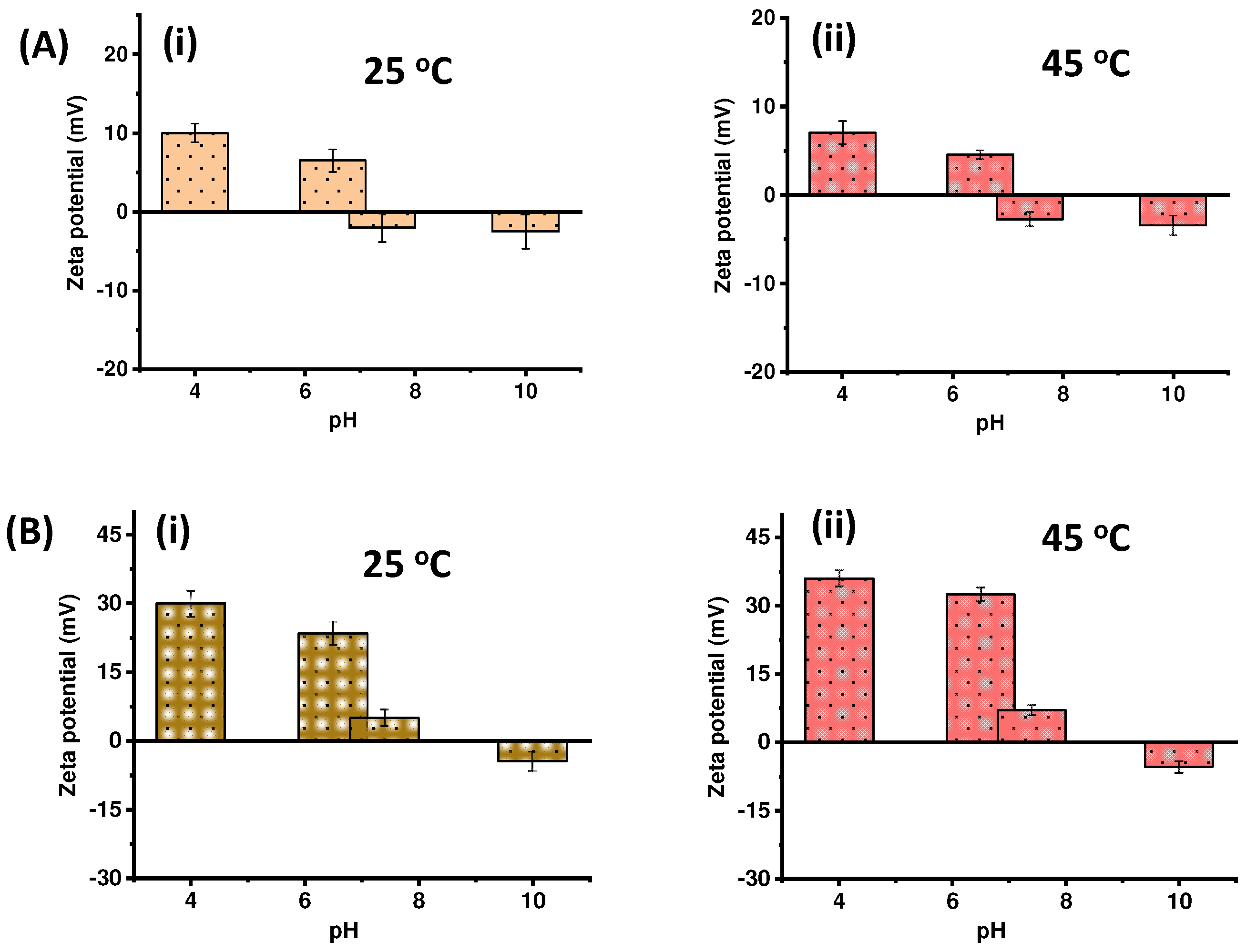
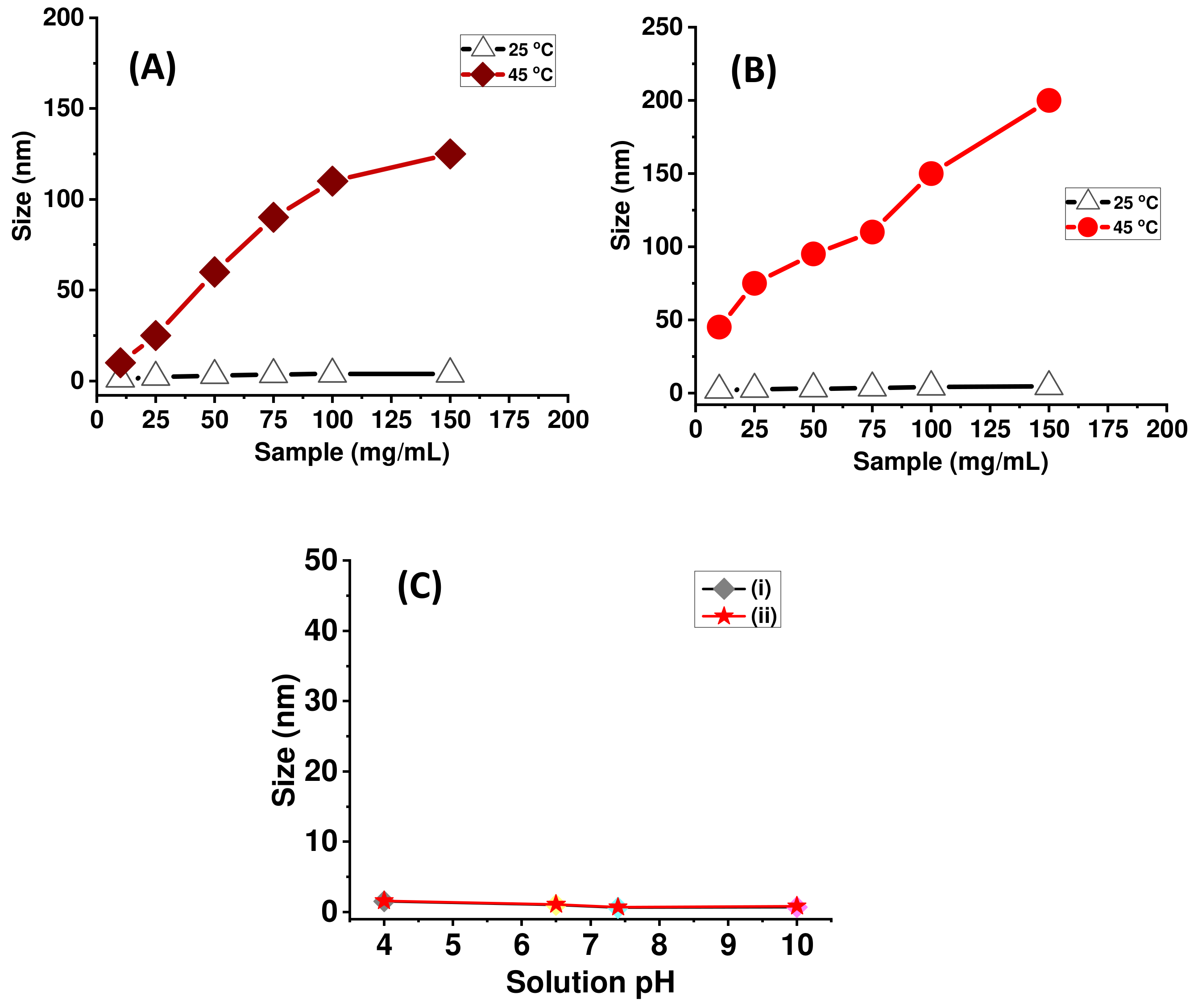
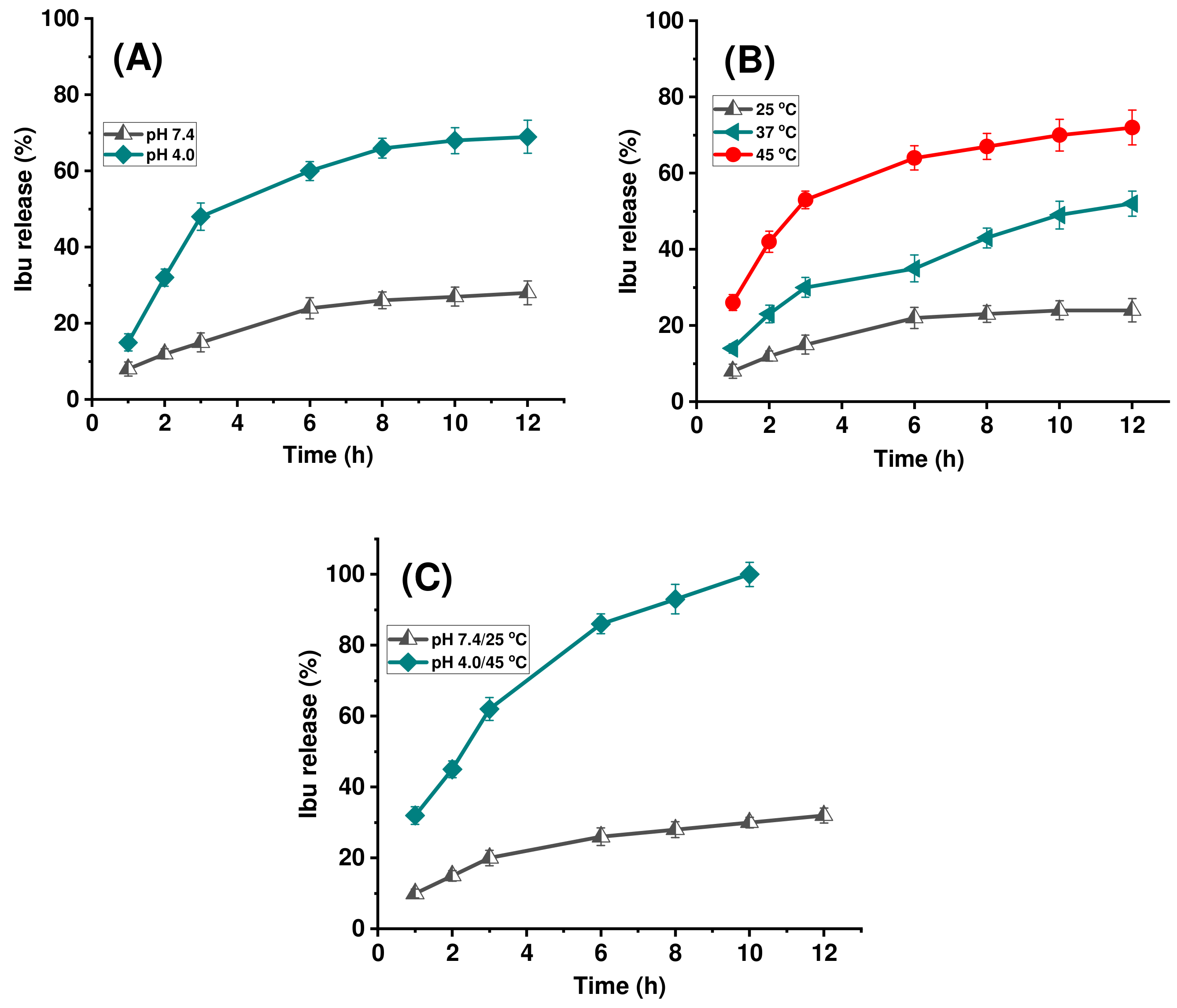
2.2. Stimuli-Responsive Drug Delivery Performance of pNIPAm-co-pGMA-Mela Hydrogel System
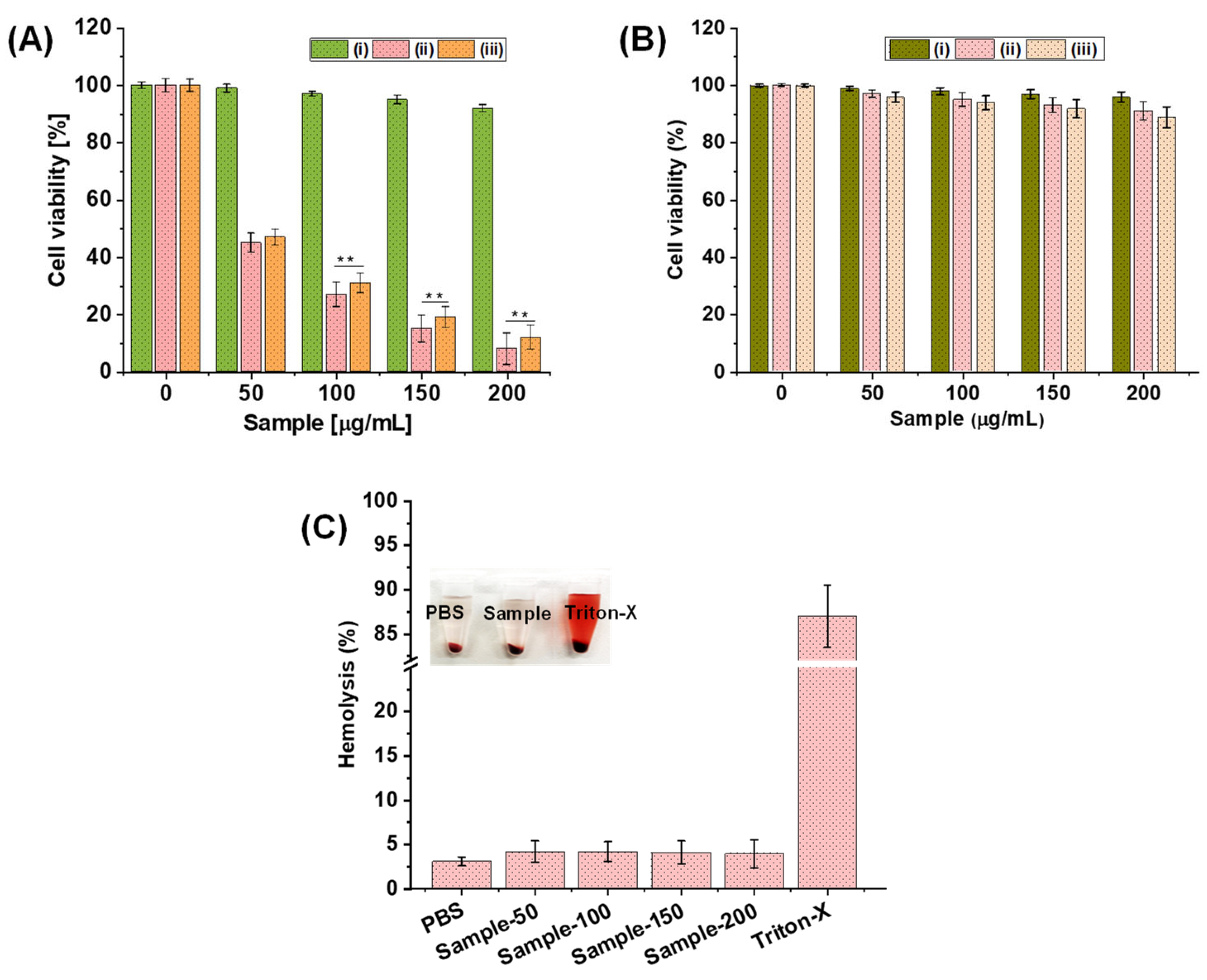
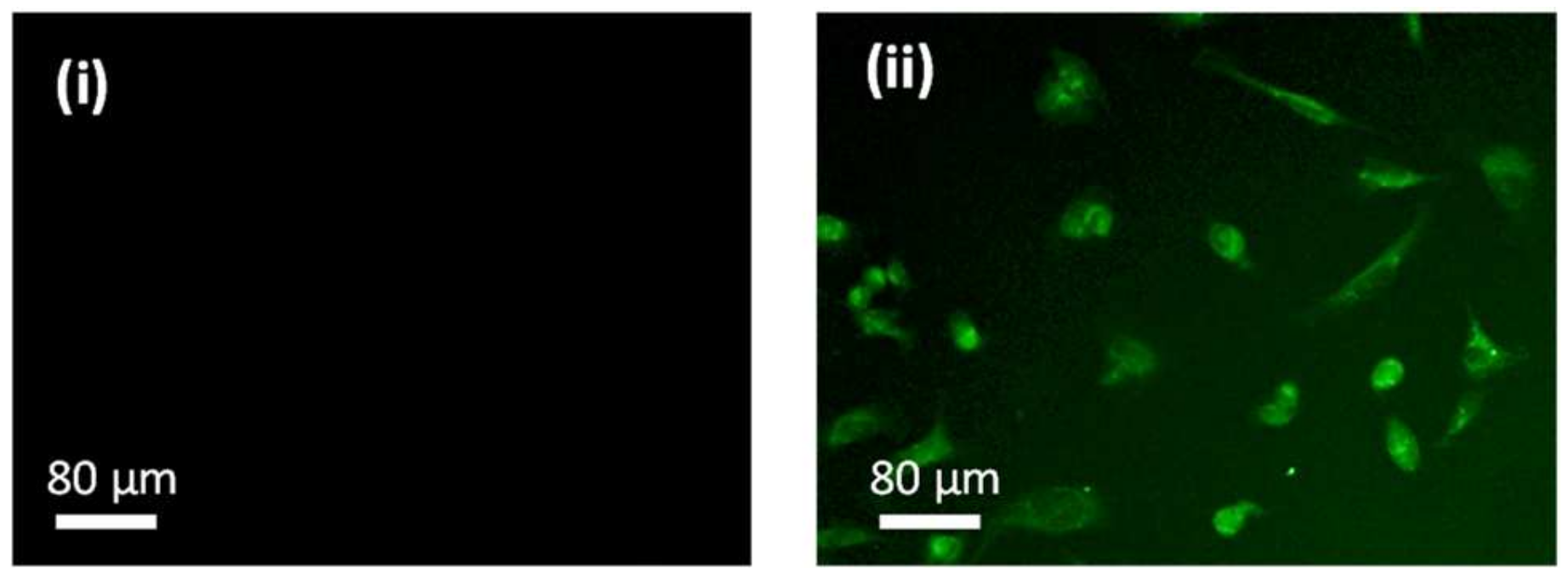
2.3. In Vitro Biocompatibility and Hemocompatibility Study
3. Conclusions
4. Experimental Section
4.1. Reagents
4.2. Synthesis of pNIAPAm-co-pGMA-Mela Copolymer Hydrogel
4.3. Characterizations
4.4. Turbidity Study
4.5. Drug Loading and pH and Temperature-Stimuli-Responsive Release Study
4.6. MTT Assay Study
4.7. Hemocompatibility Study
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, S.; Liu, L.; Wu, X.; Dai, H. Magnetically targeted co-delivery of hydrophilic and hydrophobic drugs with hollow mesoporous ferrite nanoparticles. RSC Adv. 2018, 8, 15326–15335. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nature Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Machtakova, M.; Therien-Aubin, H.; Landfester, K. Polymer nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chem. Soc. Rev. 2022, 51, 128–152. [Google Scholar] [CrossRef]
- Zhu, M.; Whittaker, A.K.; Han, F.Y.; Smith, M.T. Journey to the Market: The Evolution of Biodegradable Drug Delivery Systems. Appl. Sci. 2022, 12, 935. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahhverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Sheikhi, M.; Rafiemanzelat, F.; Moroni, L.; Setayeshmehr, M. Ultrahigh-water-content biocompatible gelatin-based hydrogels: Toughened through micro-sized dissipative morphology as an effective strategy. Mater. Sci. Eng. C 2021, 120, 111750. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer, S.; Schewaller, D.S.; Mesini, P.J. Beyond Sol-Gel: Molecular Gels with Different Transitions. Gels 2023, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Vermonden, T.; Hennik, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Larraneta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Matet. 2018, 91, 13. [Google Scholar] [CrossRef]
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of Nanozyme-Hydrogel for Enhanced Capture and Elimination of Bacteria. Adv. Funct. Mater. 2019, 29, 1900518. [Google Scholar] [CrossRef]
- Ayar, Z.; Shafieian, M.; Mahmoodi, N.; Sabzevari, O.; Hassannejad, Z. A rechargeable drug delivery system based on pNIPAM hydrogel for the local release of curcumin. J. Appl. Polym. Sci. 2021, 138, 51167. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, W.; Wang, R.; Yang, X.; Li, J.; Qui, T.; Xiao, X. The Preparation of Green Fluorescence-Emissioned Carbon Dots/Poly(N-Isopropylacrylamide) Temperature-Sensitive Hydrogels and Research on Their Properties. Polymers 2019, 11, 1171. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Tzoumani, I.; Beobide, A.S.; Iatridi, Z.; Voyiatzis, G.A.; Bokias, G.; Kallitsis, J.K. Glycidyl Methacrylate-Based Copolymers as Healing Agents of Waterborne Polyurethanes. Int. J. Mol. Sci. 2022, 23, 8118. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Mohan, A.; Mani, K.S.; Devendhiran, T.; Periyasami, G.; Kim, S.C.; Lin, M.C.; Kumarasamy, K.; Huang, P.J.; Ali, A. Synthesis of functionalized mesoporous silica nanoparticles for colorimetric and fluorescence sensing of selective metal (Fe3+) ions in aqueous solution. Methods 2024, 223, 26–34. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.A.; Mommer, S.; Parkins, C.C.; Scherman, A.O. Design Principles for Aqueous Interactive Materials: Lessons from Small Molecules and Stimuli-Responsive Systems. Adv. Mater. 2020, 32, 1906890. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, K.; Phan, T.T.; Santhamoorthy, M.; Ramkumar, V.; Kim, S.C. pH and Thermoresponsive PNIPAm-co-Polyacrylamide Hydrogel for Dual Stimuli-Responsive Controlled Drug Delivery. Polymers 2023, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Santhamoorthy, M.; Thirupathi, K.; Kumar, S.S.D.; Pandiaraj, S.; Rahaman, M.; Phan, T.T.V.; Kim, S.C. k-Carrageenan based magnetic@polyelectrolyte complex composite hydrogel for pH and temperature-responsive curcumin delivery. Int. J. Biomacromol. 2023, 224, 125467. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Selahi, Z.; Akrami, M.; Hosseinpour, M.; Jockenhövel, S.; Ghazanfari, S. 3D printed pH-responsive tablets containing N-acetylglucosamine-loaded methylcellulose hydrogel for colon drug delivery applications. Int. J. Pharm. 2023, 645, 123366. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Santhamoorthy, M.; Lee, Y.C. Recent advances in the pH-responsive organic–inorganic mesoporous hybrid silica for targeted drug delivery. Eur. Polym. J. 2024, 206, 112783. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Chiang, W.-Y.; Way, T.-F.; Tuan, H.N.A.; Chang, Y.-C. Study of the Thermo-/pH-Sensitivity of Stereo-Controlled Poly(N-isopropylacrylamide-co-IAM) Copolymers via RAFT Polymerization. Polymers 2018, 10, 512. [Google Scholar] [CrossRef]
- Mohan, A.; Suresh, R.; Ashwini, M.; Periyasami, G.; Guganathan, L.; Lin, M.C.; Kumarasamy, K.; Kim, S.C.; Santhamoorthy, M. Alginate functionalized magnetic-silica composites for pH-responsive drug delivery and magnetic hyperthermia applications. Mater. Lett. 2024, 361, 136088. [Google Scholar] [CrossRef]
- Piechocki, K.; Kozanecki, M.; Saramak, J. Water structure and hydration of polymer network in PMEO2MA hydrogels. Polymer 2020, 210, 122974. [Google Scholar] [CrossRef]
- Kim, S.; Lee, N.-K.; Chae, M.-K.; Johner, A.; Park, J.-M. Translocation of Hydrophobic Polyelectrolytes under Electrical Field: Molecular Dynamics Study. Polymers 2023, 15, 2550. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2023, 9, 35. [Google Scholar] [CrossRef]
- Thirupathi, K.; Santhamoorthy, M.; Radhakrishnan, S.; Ulagesan, S.; Nam, T.J.; Phan, T.T.V.; Kim, S.C. Thermosensitive Polymer- Modified Mesoporous Silica for pH and Temperature-Responsive Drug Delivery. Pharmaceutics 2023, 15, 795. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Moorthy, M.S.; Manivasagan, P.; Bharathiraja, S.; Oh, J. Magnetic hyperthermia and pH-responsive effective drug delivery to the sub-cellular level of human breast cancer cells by modified CoFe2O4 nanoparticles. Biochimie 2017, 133, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Kim, H.-B.; Bae, J.-H.; Kim, S.-H.; Ha, C.-S. Design of core–shell magnetic mesoporous silica hybrids for pH and UV light stimuli-responsive cargo release. RSC Adv. 2016, 6, 29106–29115. [Google Scholar] [CrossRef]
- Balakrishnan, C.; Manonmani, M.; Santhamoorthy, M.; Rajasekar, M.; Vinitha, M.; Meenakshisundaram, S.P. Supramolecular structure of bis(1-methyl-1,3,5,7-tetraazatricyclo[3.3.1.13,7]decan-1-ium)2,5-dicarboxybenzene-1,4-dicarboxylate: Synthesis, spectral, structural and third-order nonlinear optical properties. J. Mol. Struct. 2024, 1296, 136822. [Google Scholar] [CrossRef]
- Madhappan, S.; Kim, S.H.; Huh, P.; Jung, Y.S.; Kim, S.C. Dramatic reduction of toxicity of Poly(hexamethylene guanidine) disinfectant by charge neutralization. Environ. Res. 2023, 231, 116172. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Oh, Y.; Bharathiraja, S.; Manivasagan, P.; Rajarathinam, T.; Jang, B.; Phan, T.T.V.; Jang, H.; Oh, J. Synthesis of amine-polyglycidol functionalised Fe3O4@SiO2 nanocomposites for magnetic hyperthermia, pH-responsive drug delivery, and bioimaging applications. RSC Adv. 2016, 6, 110444–110453. [Google Scholar] [CrossRef]
- Ramkumar, V.; Raorane, C.J.; Christy, H.J.; Anandhi, S.; Santhamoorthy, M.; Kamachiappan, P.; Ashokkumar, A.; Balamurugan, S.; Kim, S.C. Hydrogen-bonded keto-enol mechanized chalcone material for optical and antibiofilm applications. J. Mol. Struct. 2023, 1292, 136109. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Shamszadeh, S.; Akrami, M.; Asgary, S. Size-dependent bioactivity of electrosprayed core–shell chitosan-alginate particles for protein delivery. Sci. Rep. 2022, 12, 20097. [Google Scholar] [CrossRef]
- Oh, M.; Yoon, Y.; Lee, T.S. Synthesis of poly(N-isopropylacrylamide) polymer crosslinked with an AIE-active azonaphthol for thermoreversible fluorescence. RSC Adv. 2020, 10, 39277–39283. [Google Scholar] [CrossRef]
- Ekbrant, B.E.; Skov, A.L.; Daugaard, A.E. Epoxy-Rich Systems with Preference for Etherification over Amine-Epoxy Reactions for Tertiary Amine Accelerators. Macromolecules 2021, 54, 4280–4287. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, A.; Santhamoorthy, M.; Phan, T.T.V.; Kim, S.-C. pNIPAm-Based pH and Thermoresponsive Copolymer Hydrogel for Hydrophobic and Hydrophilic Drug Delivery. Gels 2024, 10, 184. https://doi.org/10.3390/gels10030184
Mohan A, Santhamoorthy M, Phan TTV, Kim S-C. pNIPAm-Based pH and Thermoresponsive Copolymer Hydrogel for Hydrophobic and Hydrophilic Drug Delivery. Gels. 2024; 10(3):184. https://doi.org/10.3390/gels10030184
Chicago/Turabian StyleMohan, Anandhu, Madhappan Santhamoorthy, Thi Tuong Vy Phan, and Seong-Cheol Kim. 2024. "pNIPAm-Based pH and Thermoresponsive Copolymer Hydrogel for Hydrophobic and Hydrophilic Drug Delivery" Gels 10, no. 3: 184. https://doi.org/10.3390/gels10030184