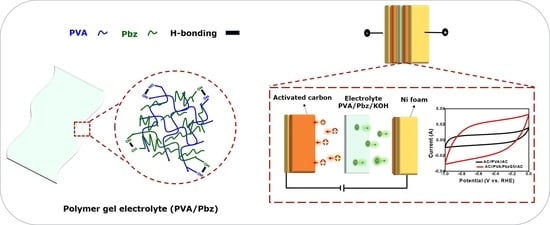
Flexible Composite Hydrogels Based on Polybenzoxazine for Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Electrochemical Measurments
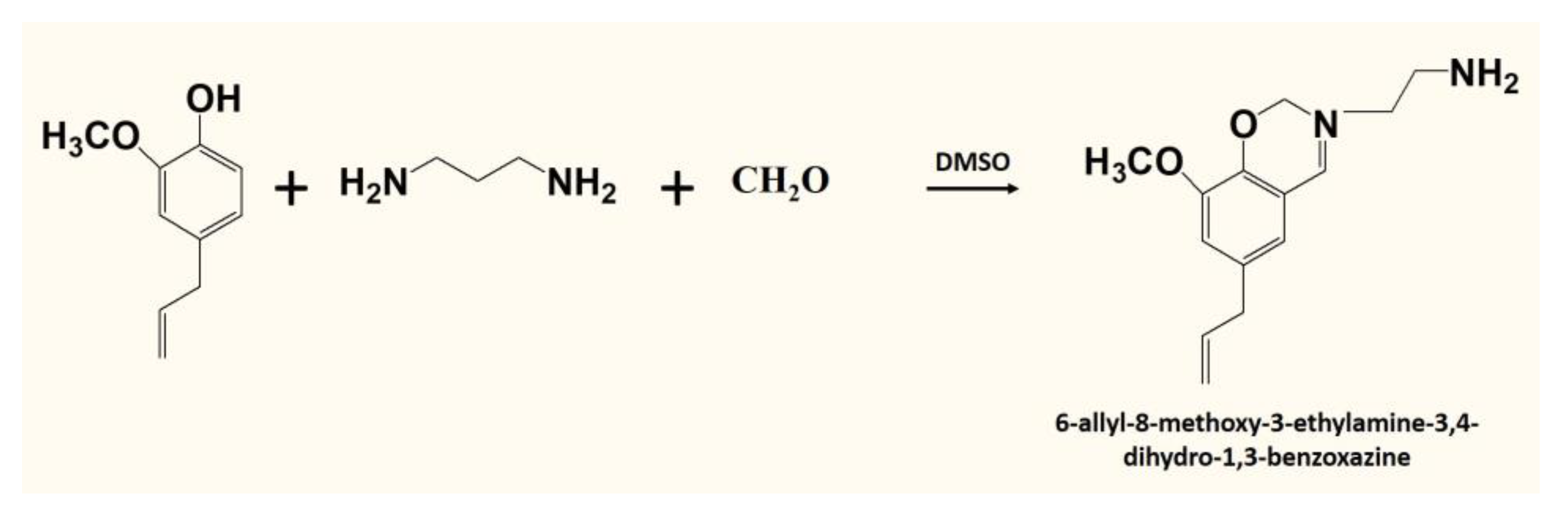
2.4. Synthesis of Benzoxazine Monomer (6-Allyl-8-methoxy-3-ethylamine-3,4-dihydro-1,3-benzoxazine) (Eu-Ed-Bzo)
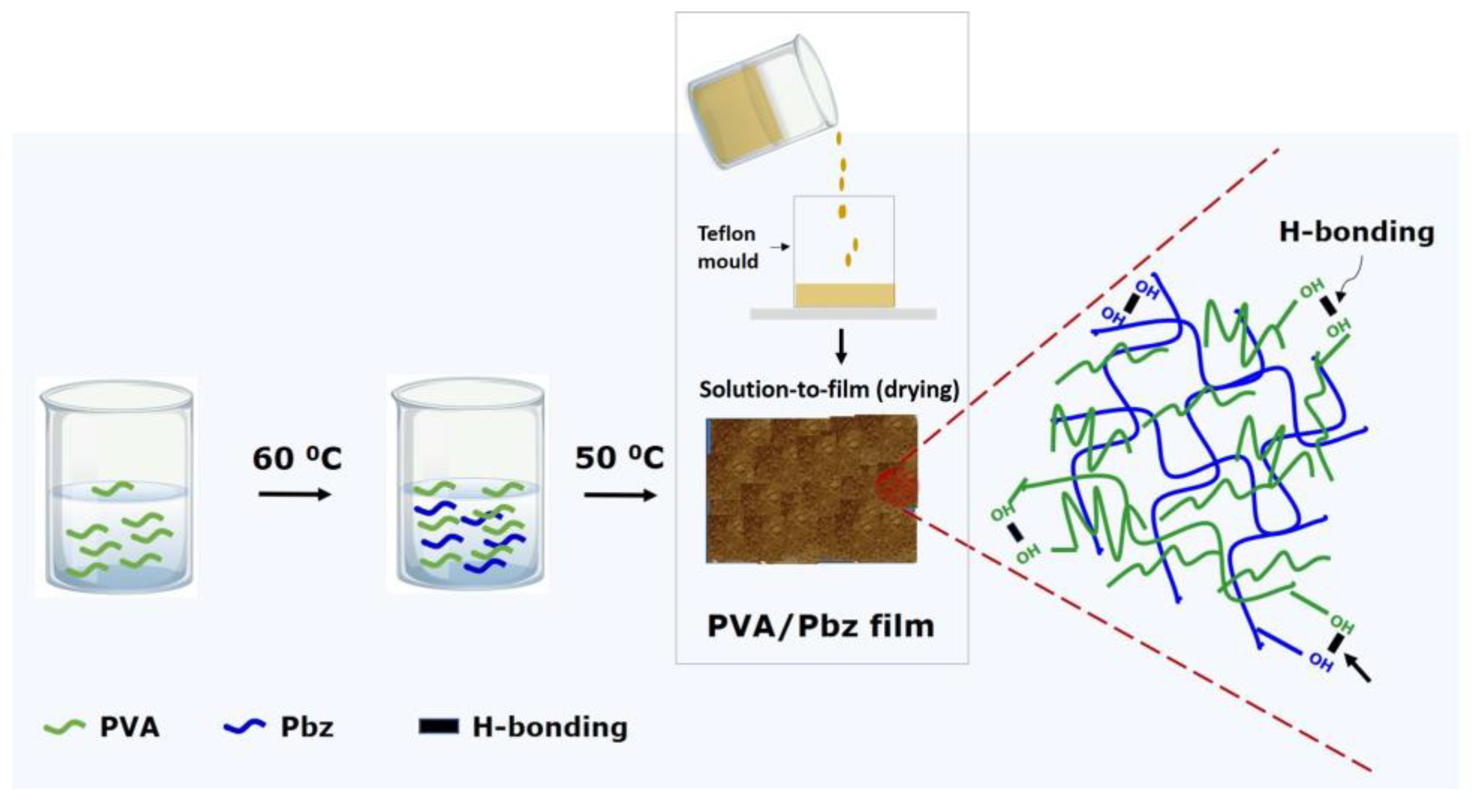
2.5. Preparation of PVA/Pbz Composite Films
3. Results and Discussion
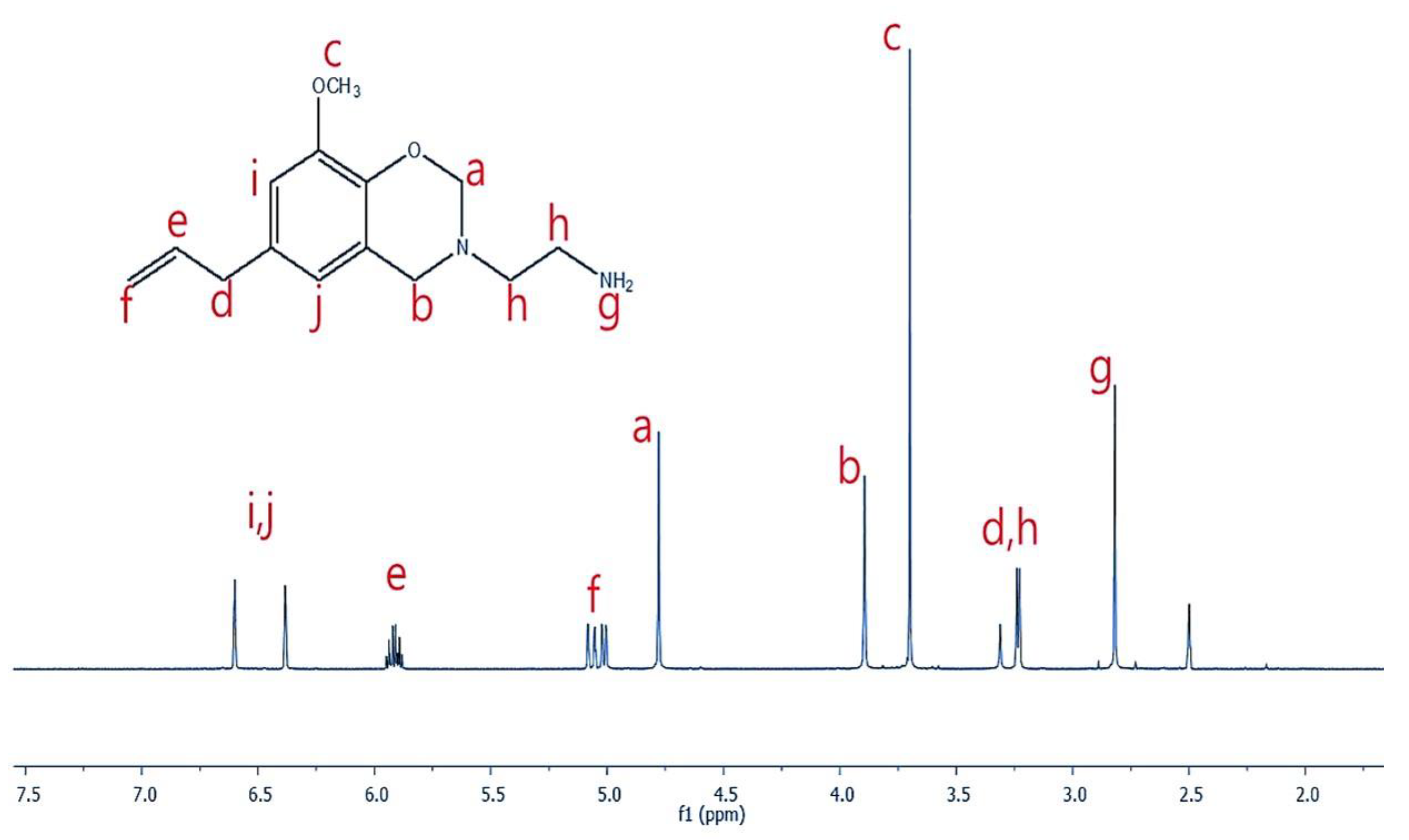
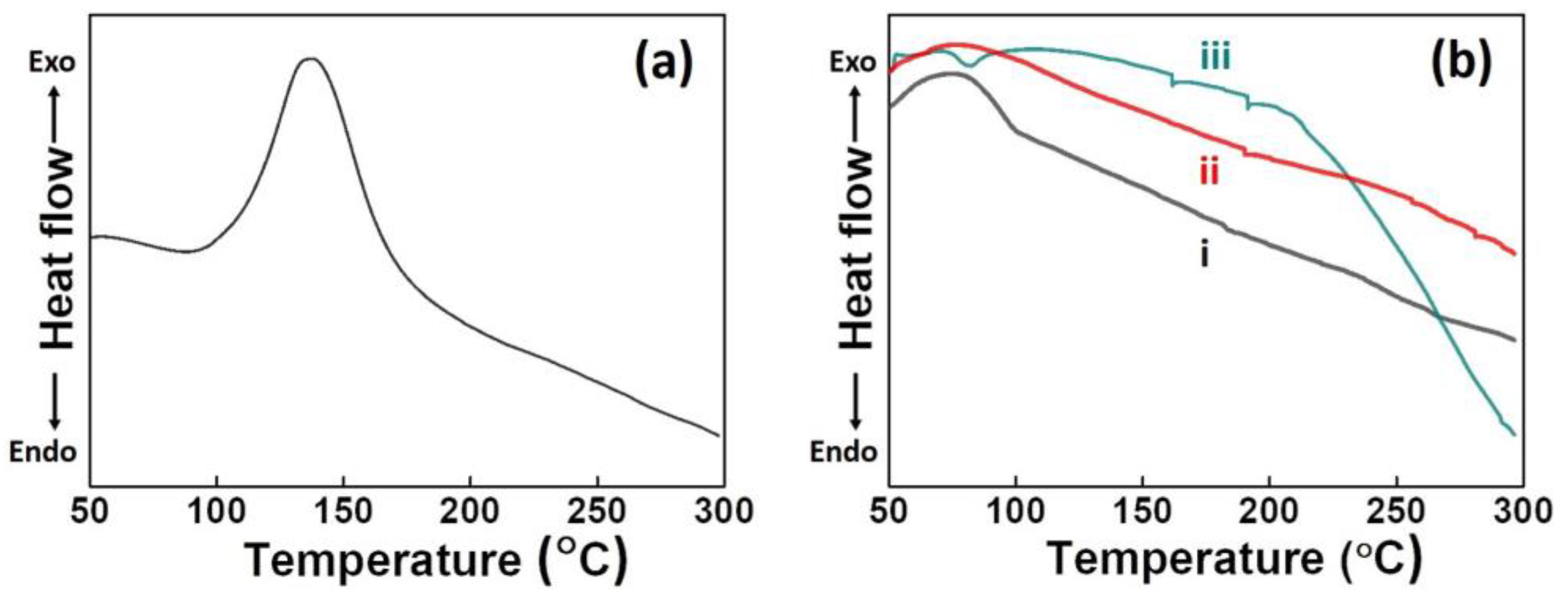
3.1. Structure Analysis of Eu-Ed-Bzo
3.2. DSC Analysis
3.3. Water Contact Angle Analysis
3.4. SEM Analysis
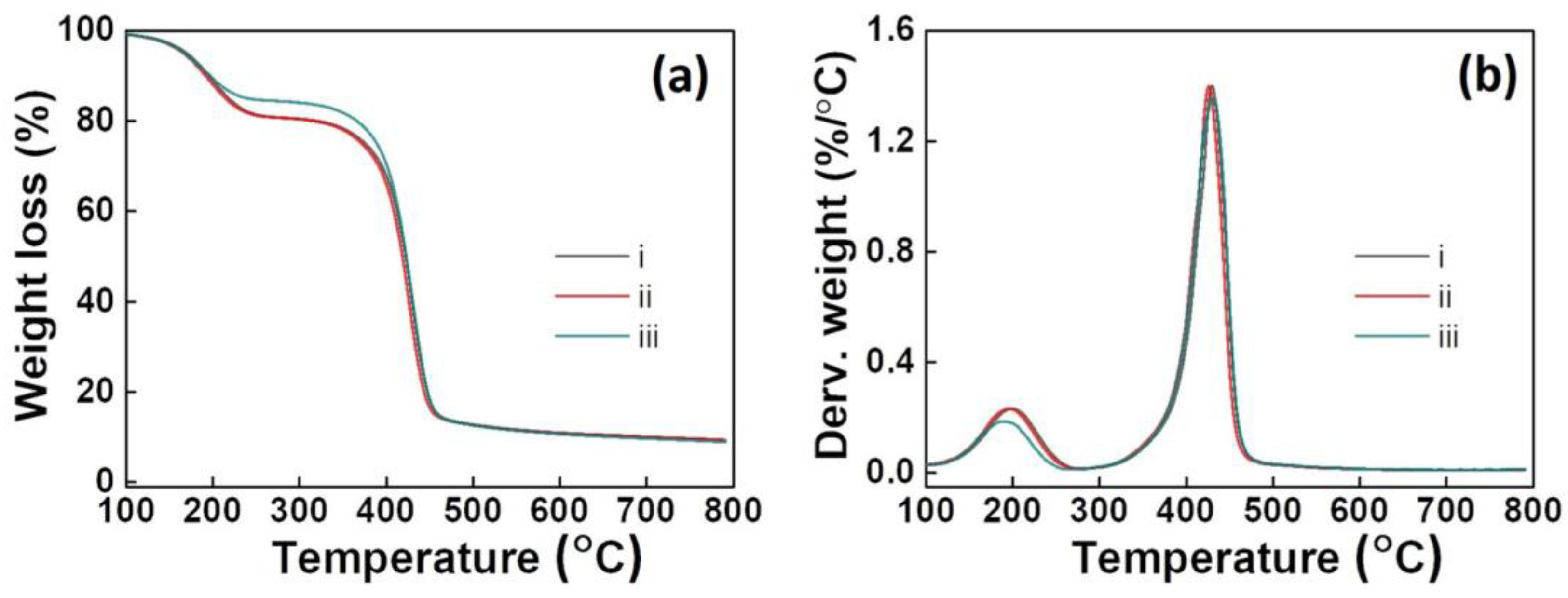
3.5. TGA Analysis
3.6. Swelling Behavior of the Composite Film
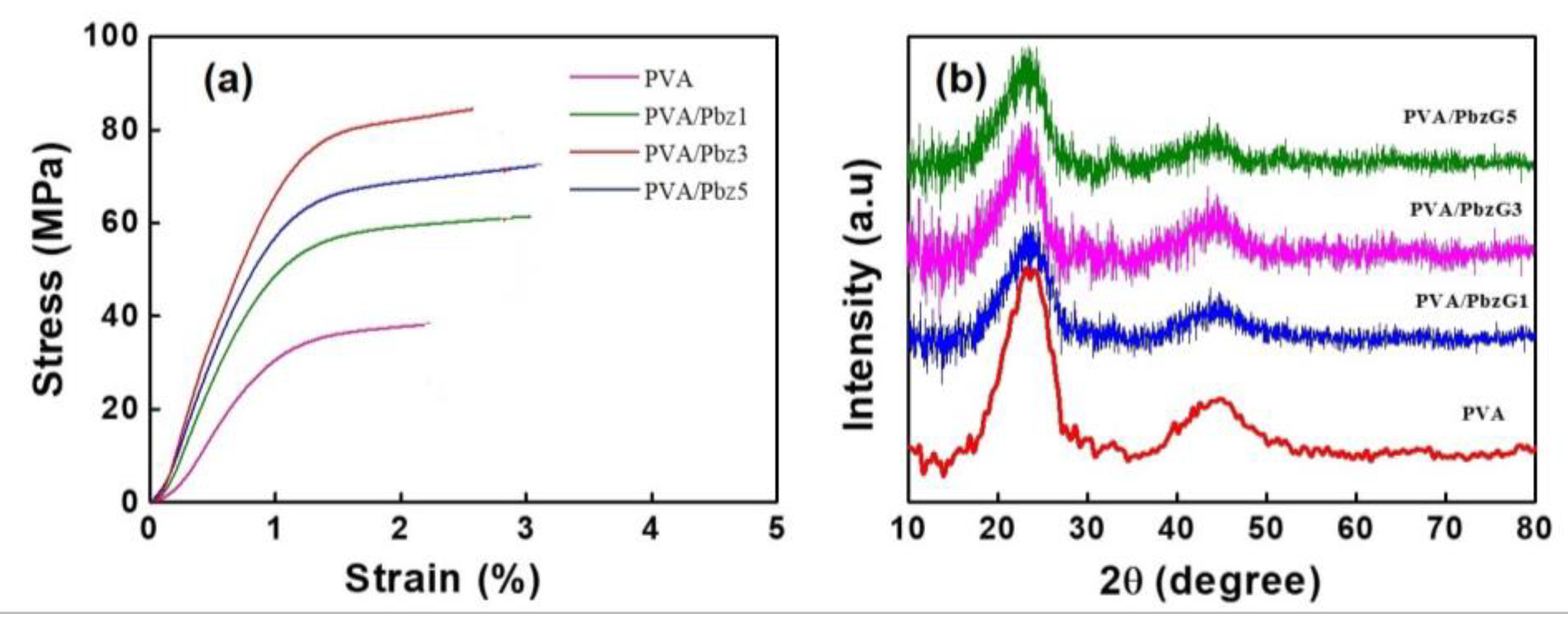
3.7. Tensile Property of the Composite Film
3.8. XRD Analysis
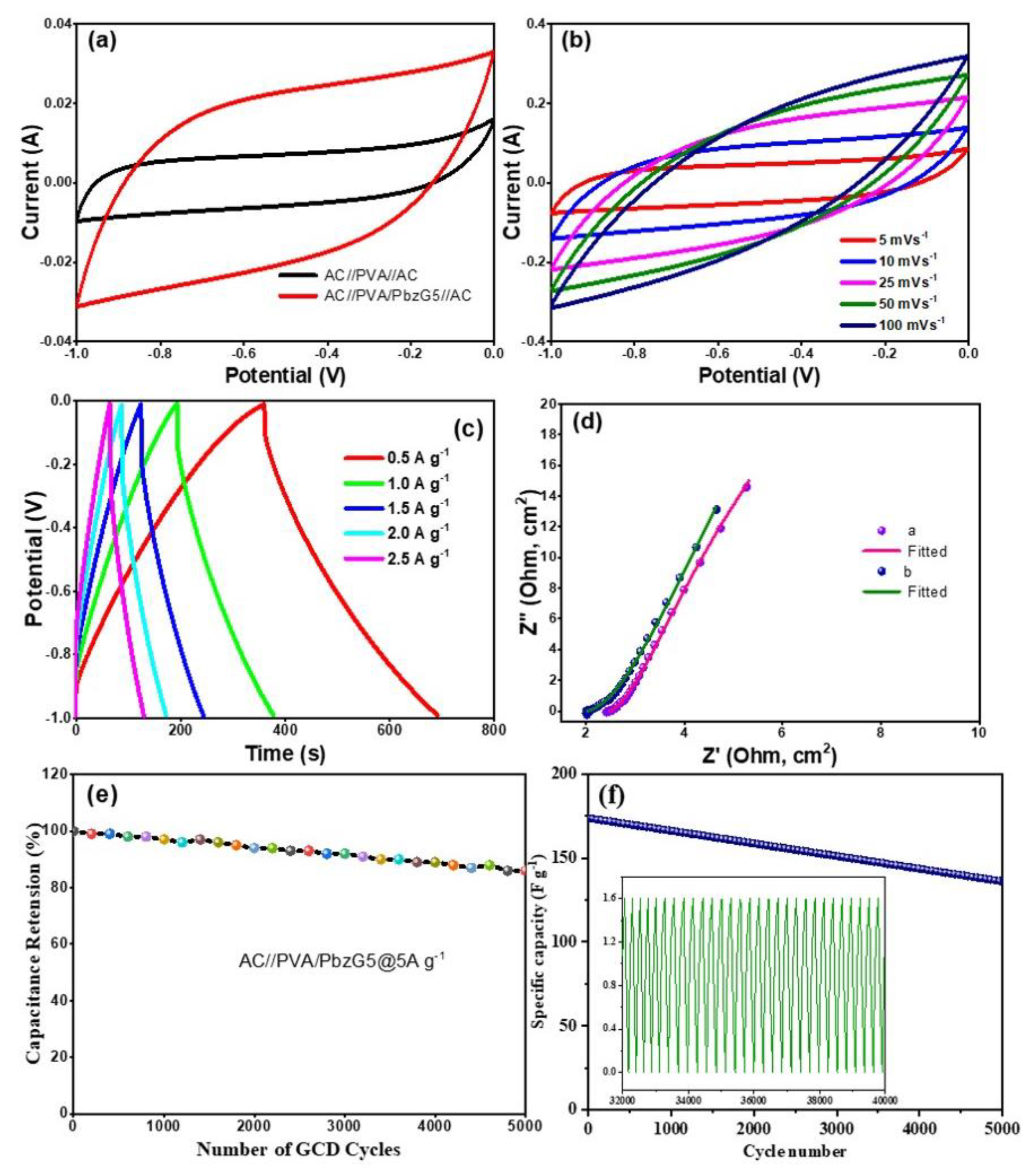
3.9. Performance of the SC Device
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishida, H.; Yee, H. A Study on the Volumetric Expansion of Benzoxazine-Based Phenolic Resin. Macromolecules 1997, 30, 1099–1106. [Google Scholar] [CrossRef]
- Rimdusit, S.; Ishida, H. Development of a New Class of Electronic Packaging Materials Based on Ternary Systems of Benzoxazine, Epoxy, and Phenolic Resins. Polymer 2000, 41, 7941–7949. [Google Scholar] [CrossRef]
- Takeichi, T.; Agag, T.; Zeidam, R. Preparation and Properties of Polybenzoxazine/Poly(imidesiloxane) Alloys: In Situ Ring-Opening Polymerization of Benzoxazine in the Presence of Soluble Poly(imide-siloxane)s. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2633–2641. [Google Scholar] [CrossRef]
- Ishida, H.; Lee, Y.-H. Study of Hydrogen Bonding and Thermal Properties of Polybenzoxazine and Poly(caprolactone) Blends. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 736–749. [Google Scholar] [CrossRef]
- Zheng, S.; Lu, H.; Guo, Q. Thermosetting Blends of Polybenzoxazine and Poly(e-caprolactone): Phase Behavior and Intermolecular Specific Interactions. Macromol. Chem. Phys. 2004, 205, 1547–1558. [Google Scholar] [CrossRef]
- Jin, J.; Mengyao, W.; Wenli, Z.; Huihui, L.; Yan, W.; Ping, S. Hierarchical porous carbon materials derived from N, O, S-Containing Bio-Based polybenzoxazine for Supercapacitors. Eur. Polym. J. 2023, 191, 112054. [Google Scholar]
- Jingmiao, C.; Zhi-Xia, Z.; Hongying, Q.; Yi, H.; Shoujun, W.; Dezhi, C. Effect of various ammonium salts as activating additive on the capacitance performance of hierarchical porous carbon derived from camellia husk. J. Energy Storage 2022, 51, 104347. [Google Scholar]
- Yang, W.; Wang, P.; Tu, Z.; Hou, L.; Yan, L.; Jiang, B.; Zhang, C.; Huang, G.; Yang, F.; Li, Y. Heteroatoms-doped hierarchical porous carbon with multi-scale structure derived from petroleum asphalt for high-performance supercapacitors. Carbon 2022, 187, 338–348. [Google Scholar] [CrossRef]
- Quan, H.; Tao, W.; Wang, Y.; Chen, D. Enhanced supercapacitor performance of Camellia oleifera shell derived hierarchical porous carbon by carbon quantum dots. J. Energy Storage 2022, 55, 105573. [Google Scholar] [CrossRef]
- Takeichia, T.; Guoa, Y.; Rimdusit, S. Performance Improvement of Polybenzoxazine by Alloying with Polyimide: Effect of Preparation Method on the Properties. Polymer 2005, 46, 4909–4916. [Google Scholar] [CrossRef]
- Huang, J.-M.; Yang, S.-J. Studying the Miscibility and Thermal Behavior of Polybenzoxazine/Poly(3-caprolactone) Blends Using DSC, DMA, and Solid State 13C NMR Spectroscopy. Polymer 2005, 46, 8068–8078. [Google Scholar] [CrossRef]
- Rimdusit, S.; Pirstpindvong, S.; Tanthapanichakoon, W.; Damrongsakkul, S. Toughening of Polybenzoxazine by Alloying with Urethane Prepolymer and Flexible Epoxy: A Comparative Study. Polym. Eng. Sci. 2005, 45, 288–296. [Google Scholar] [CrossRef]
- Rimdusit, S.; Mongkhonsi, T.; Kamonchaivanich, P.; Sujirote, K.; Thiptipakorn, S. Effects of Polyol Molecular Weight on Properties of Benzoxazine–Urethane Polymer Alloys. Polym. Eng. Sci. 2008, 48, 2238–2246. [Google Scholar] [CrossRef]
- Ardhyananta, H.; Wahid, M.H.; Sasaki, M.; Agag, T.; Kawauchi, T.; Ismail, H.; Takeichi, T. Performance Enhancement of Polybenzoxazine by Hybridization with Polysiloxane. Polymer 2008, 49, 4585–4591. [Google Scholar] [CrossRef]
- Kumar, K.S.S.; Nair, C.P.R.; Ninan, K.N. Investigations on the Cure Chemistry and Polymer Properties of Benzoxazine–Cyanate Ester Blends. Eur. Polym. J. 2009, 45, 494–502. [Google Scholar] [CrossRef]
- Tuzun, A.; Kiskan, B.; Alemdar, N.; Erciyes, A.T.; Yagci, Y. Benzoxazine Containing Polyester Thermosets with Improved Adhesion and Flexibility. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4279–4284. [Google Scholar] [CrossRef]
- Toncheva, N.; Jerome, R.; Mateva, R. Anionically Prepared Poly(e-caprolactam-co-e-caprolactone) and Poly(e-caprolactam-co-d-valerolactone) Copolymers: Thermal and Mechanical Properties. Eur. Polym. J. 2011, 47, 238–247. [Google Scholar] [CrossRef]
- Rimdusit, S.; Bangsen, W.; Kasemsiri, P. Chemorheology and Thermomechanical Characteristics of Benzoxazine-Urethane Copolymers. J. Appl. Polym. Sci. 2011, 121, 3669–3678. [Google Scholar] [CrossRef]
- Jubsilp, C.; Takeichi, T.; Rimdusit, S. Property Enhancement of Polybenzoxazine Modified with Dianhydride. Polym. Degrad. Stab. 2011, 96, 1047–1053. [Google Scholar] [CrossRef]
- Rimdusit, S.; Kunopast, P.; Dueramae, I. Thermomechanical Properties of Arylamine-Based Benzoxazine Resins Alloyed with Epoxy Resin. Polym. Eng. Sci. 2011, 51, 1797–1807. [Google Scholar] [CrossRef]
- Malinova, L.; Stolinova, M.; Lubasova, D.; Martinova, L.; Brožek, J. Electrospinning of Polyesteramides Based on e-Caprolactam and e-Caprolactone from Solution. Eur. Polym. J. 2013, 49, 3135–3143. [Google Scholar] [CrossRef]
- Uchida, S.; Kawauchi, T.; Furukawa, N.; Takeichi, T. Polymer Alloys of High-Molecular-Weight Benzoxazine and Epoxy Resin. High Perform. Polym. 2014, 26, 846–855. [Google Scholar] [CrossRef]
- Ambrozic, R.; Sbenik, U.; Krajnc, M. Epoxy Emulsions Stabilized with Reactive Bio-Benzoxazine Surfactant from Epoxidized Cardanol for Coatings. Eur. Polym. J. 2016, 81, 138–151. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Hu, Y.; Zhang, S.; Xu, Z.; Feng, T.; Zhou, H.; Wu, M. Nitrogen-doped carbon coated zinc as powder-based anode with PVA-gel electrolyte enhancing cycling performance for zinc-ion batteries. Chem. Eng. J. 2023, 472, 145136. [Google Scholar] [CrossRef]
- Deng, M.-J.; Wu, Y.-S. 2.2 V wearable asymmetric supercapacitors based on Co oxide//Mn oxide electrodes and a PVA-KOH-urea-LiClO4 alkaline gel electrolyte. J. Alloys Compd. 2023, 945, 169285. [Google Scholar] [CrossRef]
- Aziz, S.B.; Asnawi, A.S.F.M.; Abdulwahid, R.T.; Ghareeb, H.O.; Alshehri, S.M.; Ahamad, T.; Hadi, J.M.; Kadir, M.F.Z. Design of potassium ion conducting PVA based polymer electrolyte with improved ion transport properties for EDLC device application. J. Mater. Res. Technol. 2021, 13, 933–946. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Zheng, X.; Yi, Y.; Chen, X.; Li, Y.; Sun, D.; Zhang, L. Multifunctional load-bearing hybrid hydrogel with combined drug release and photothermal conversion functions. NPG Asia Mater. 2020, 12, 18. [Google Scholar] [CrossRef]
- Ji, N.; Luo, J.; Zhang, W.; Sun, J.; Wang, J.; Qin, C.; Zhou, Q.; Dai, L. A Novel Polyvinyl Alcohol-Based Hydrogel with Ultra-Fast Self-Healing Ability and Excellent Stretchability Based on Multi Dynamic Covalent Bond Cross-Linking. Macromol. Mater. Eng. 2023, 308, 2200525. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.; Yu, X.; Zhang, W. Recent advances of PVA-based hydrogels in cartilage repair application. J. Mater. Res. Technol. 2023, 24, 2279–2298. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Shui, T.; Pan, M.; Li, A.; Fan, H.; Wu, J.; Liu, Q.; Zeng, H. Poly(vinyl Alcohol) (PVA)-Based Hydrogel Scaffold with Isotropic Ultratoughness Enabled by Dynamic Amine−Catechol Interactions. Chem. Mater. 2022, 34, 8613–8628. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, C.; Zhang, Y.; Wang, B.; Xiong, Q.; Zhao, M.; Ni, Y. Multi-layer hierarchical cellulose nanofibers/carbon nanotubes/vinasse activated carbon composite materials for supercapacitors and electromagnetic interference shielding. Nano Res. 2024, 17, 904–912. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Shakila Parveen, A.; Lee, Y.R.; Kim, S.-C. The synthesis of mechanically stable polybenzoxazine-based porous carbon and its application as high-performance supercapacitor electrodes. New J. Chem. 2021, 45, 8738. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Kim, S.-C. Nitrogen-Rich Porous Carbon/NiMn Hybrids as Electrode Materials for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 15605–15614. [Google Scholar]
- Shakila Parveen, A.; Thirukumaran, P.; Kim, S.-C. Enhanced electrochemical performance of HC/NiCo@ 800 C//HC using redox-active electrolytes showing increased energy density. J. Alloys Compd. 2024, 972, 172753. [Google Scholar]



| Sample | Film Weight (g) | Gel Weight (g) | Swelling (%) |
|---|---|---|---|
| PVA/Pbz1 | 0.0890 | 0.1784 | 100.44 |
| PVA/Pbz3 | 0.0628 | 0.1468 | 133.72 |
| PVA/Pbz5 | 0.1021 | 0.2588 | 153.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asrafali, S.P.; Periyasamy, T.; Bari, G.A.K.M.R.; Kim, S.-C. Flexible Composite Hydrogels Based on Polybenzoxazine for Supercapacitor Applications. Gels 2024, 10, 197. https://doi.org/10.3390/gels10030197
Asrafali SP, Periyasamy T, Bari GAKMR, Kim S-C. Flexible Composite Hydrogels Based on Polybenzoxazine for Supercapacitor Applications. Gels. 2024; 10(3):197. https://doi.org/10.3390/gels10030197
Chicago/Turabian StyleAsrafali, Shakila Parveen, Thirukumaran Periyasamy, Gazi A. K. M. Rafiqul Bari, and Seong-Cheol Kim. 2024. "Flexible Composite Hydrogels Based on Polybenzoxazine for Supercapacitor Applications" Gels 10, no. 3: 197. https://doi.org/10.3390/gels10030197