pH-Responsive Cellulose/Silk/Fe3O4 Hydrogel Microbeads Designed for Biomedical Applications
Abstract
:1. Introduction
2. Results and Discussion
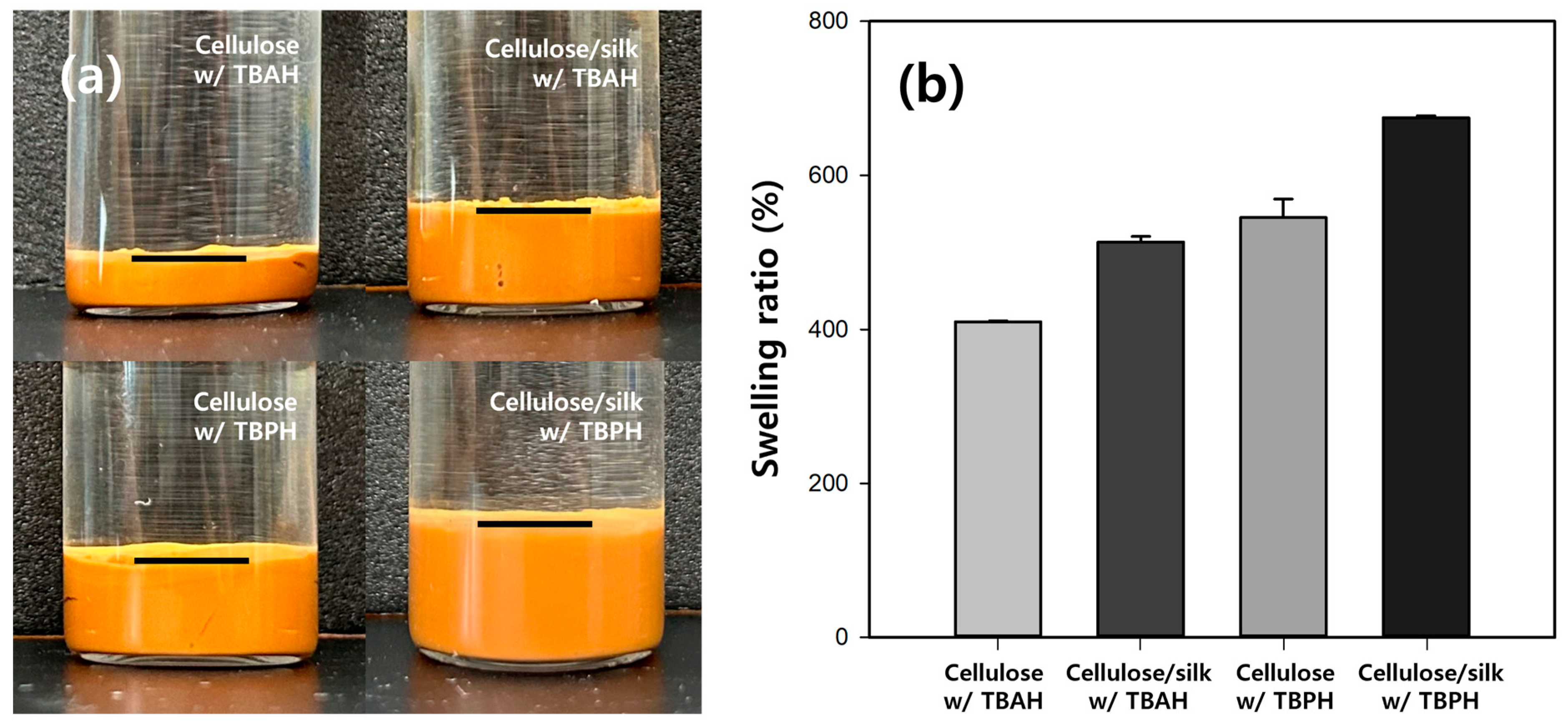
2.1. Preparation of Cellulose/Fe3O4 Hydrogel Microbeads with Various Cellulose Solvents
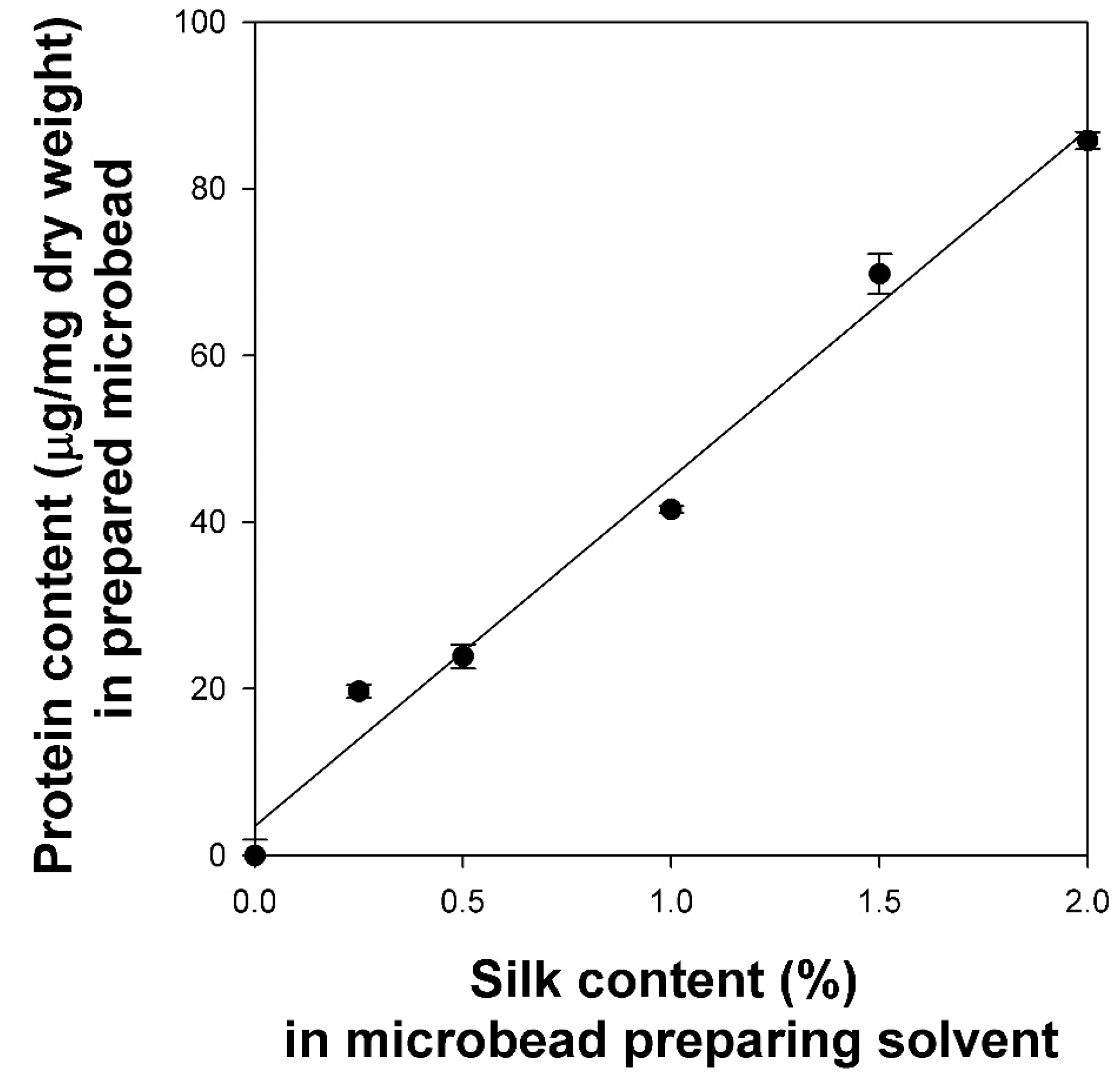
2.2. Preparation of Cellulose/Silk/Fe3O4 Hydrogel Microbeads
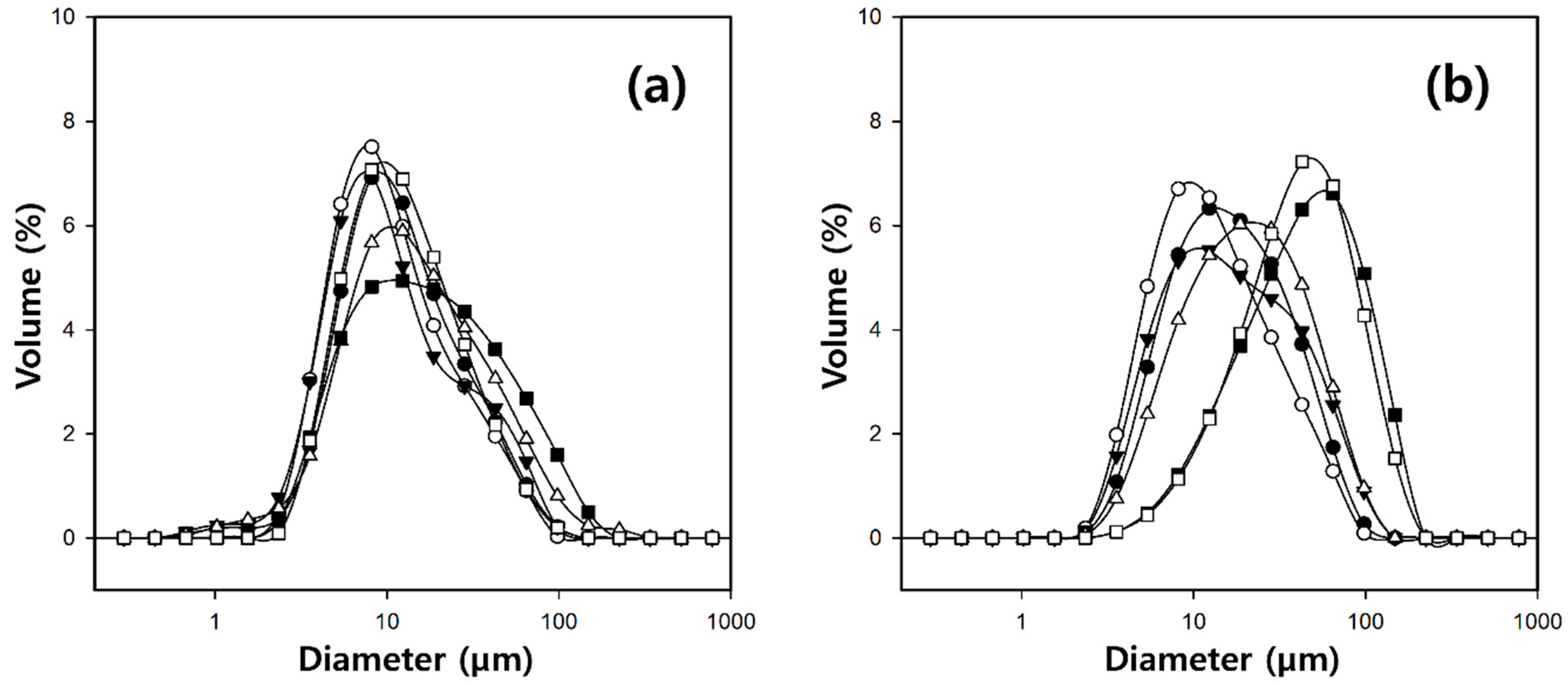
2.3. Characteristics of Cellulose/Silk/Fe3O4 Hydrogel Microbeads with Various Silk Contents
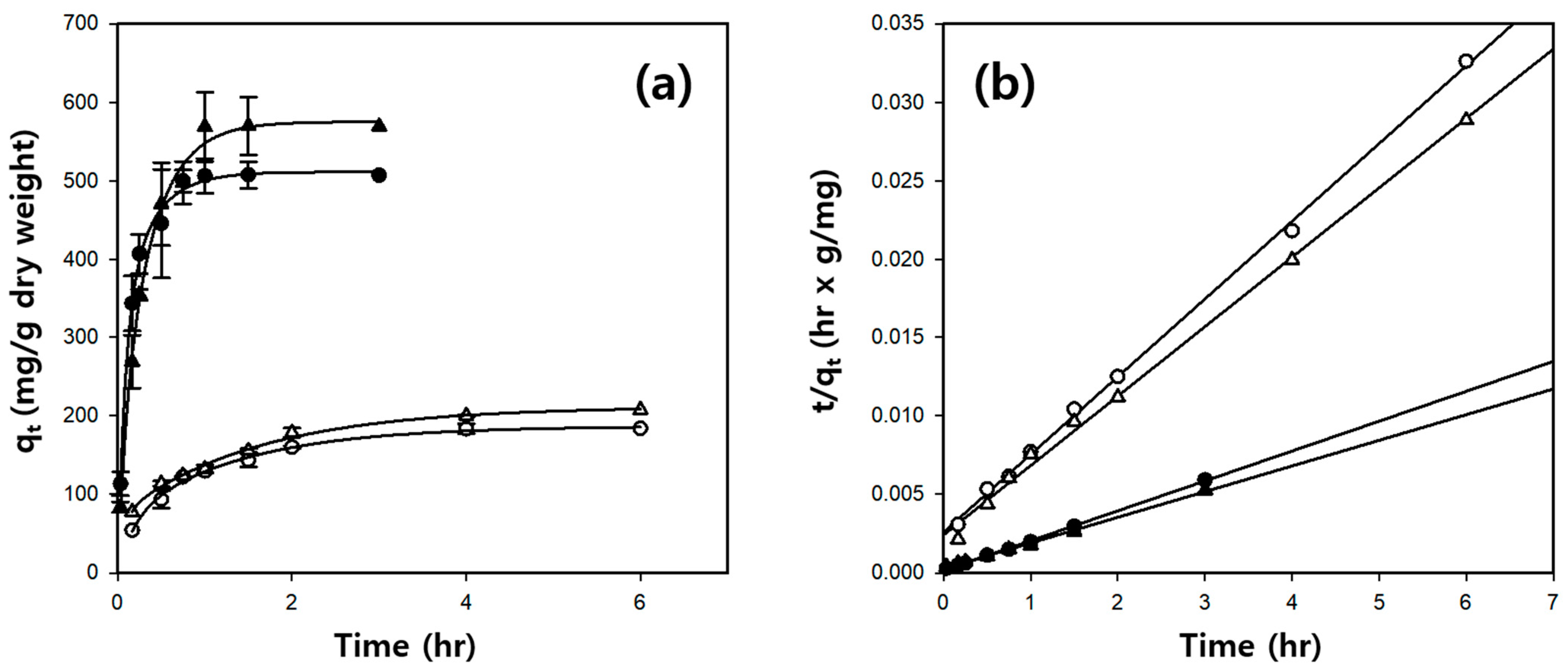
2.4. Kinetic Study of BSA Adsorption on Cellulose/Silk/Fe3O4 Hydrogel Microbeads
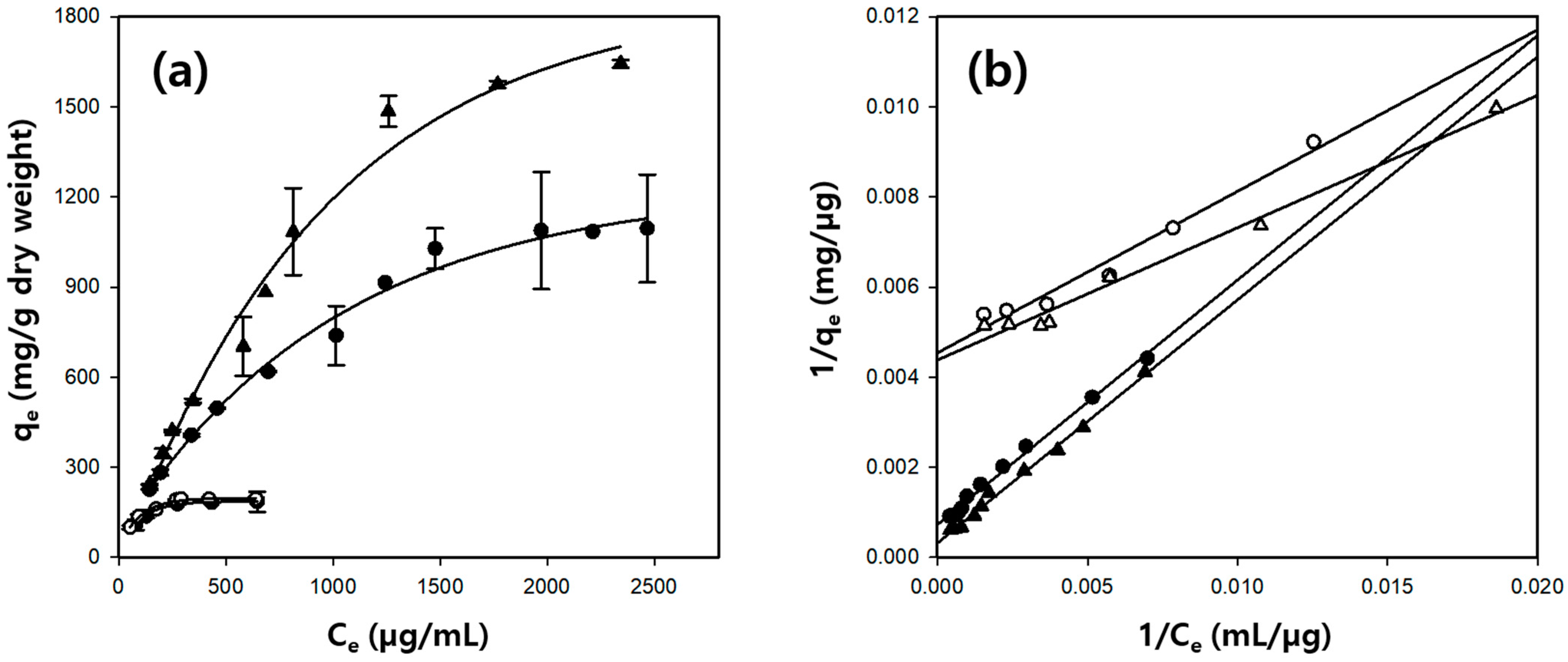
2.5. Isotherm Study of BSA Adsorption on Cellulose/Silk/Fe3O4 Hydrogel Microbeads
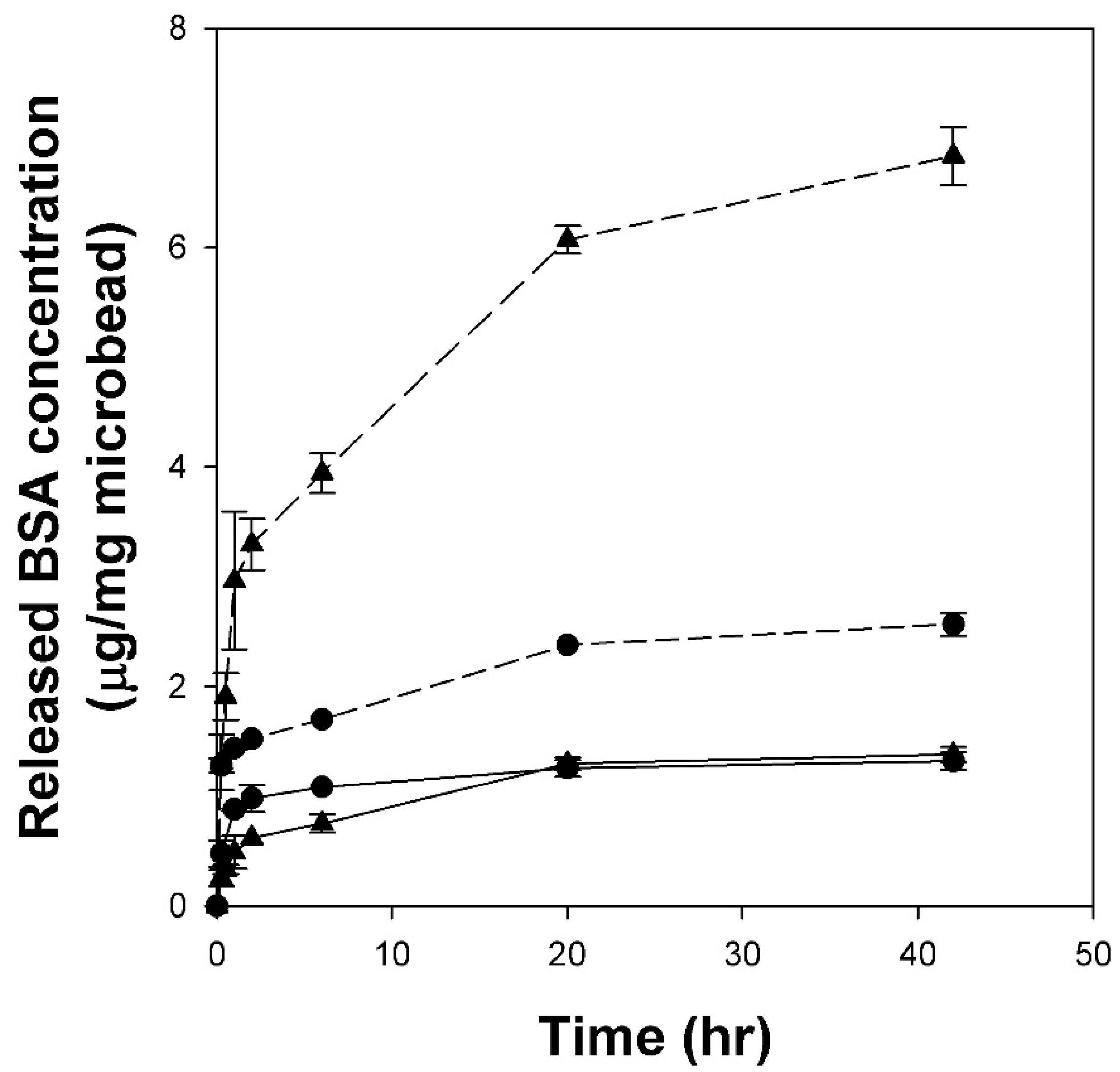
2.6. Release Profiles of BSA Adsorbed on Cellulose/Silk/Fe3O4 Microbeads
2.7. Cytotoxicity of Cellulose and Cellulose/Silk Composites
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Silk-Degumming Process
4.3. Preparation of Cellulose/Fe3O4 Hydrogel Microbeads
4.4. Preparation of Cellulose/Silk/Fe3O4 Hydrogel Microbeads
4.5. Characterization of Cellulose/Fe3O4-Based Hydrogel Microbeads
4.6. Dye Adsorption on Cellulose/Silk/Fe3O4 Hydrogel Microbeads
4.7. Protein Adsorption and Release Study on Cellulose/Silk/Fe3O4 Hydrogel Microbeads
4.8. Cytotoxicity of Regenerated Cellulose and Cellulose/Silk Film
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kabir, S.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Pathak, S.K.; Tran, V.T.; Cui, J.; Jeon, I. Hydrogels with superior mechanical properties from the synergistic effect in hydrophobic–hydrophilic copolymers. Chem. Eng. J. 2019, 362, 325–338. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent progress in biopolymer-based hydrogel materials for biomedical applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Shi, H.; Xia, Z.; Sahoo, J.K.; Yeo, J.; Kaplan, D.L. Fiber-based biopolymer processing as a route toward sustainability. Adv. Mater. 2022, 34, 2105196. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Yano, H. Cellulose nanofiber-based hydrogels with high mechanical strength. Cellulose 2012, 19, 1907–1912. [Google Scholar] [CrossRef]
- Jo, S.; Park, S.; Oh, Y.; Hong, J.; Kim, H.J.; Kim, K.J.; Oh, K.K.; Lee, S.H. Development of cellulose hydrogel microspheres for lipase immobilization. Biotechnol. Bioprocess Eng. 2019, 24, 145–154. [Google Scholar] [CrossRef]
- Deligkaris, K.; Tadele, T.S.; Olthuis, W.; van den Berg, A. Hydrogel-based devices for biomedical applications. Sens. Actuators B Chem. 2010, 147, 765–774. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Chirani, N.; Yahia, L.H.; Gritsch, L.; Motta, F.L.; Chirani, S.; Farè, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Kragl, U.; Michalik, D.; Ludwig, R.; Hollmann, D. The effect of additives on the viscosity and dissolution of cellulose in tetrabutylphosphonium hydroxide. ChemSusChem 2019, 12, 3458–3462. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Park, S.; Oh, Y.; Yun, J.; Yoo, E.; Jung, D.; Oh, K.K.; Lee, S.H. Cellulose/biopolymer/Fe3O4 hydrogel microbeads for dye and protein adsorption. Cellulose 2020, 27, 2757–2773. [Google Scholar] [CrossRef]
- Gericke, M.; Trygg, J.; Fardim, P. Functional cellulose beads: Preparation, characterization, and applications. Chem. Rev. 2013, 113, 4812–4836. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L. Creation of regenerated cellulose microspheres with diameter ranging from micron to millimeter for chromatography applications. J. Chromatogr. A 2010, 1217, 5922–5929. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lu, X.; Wang, Z.; Lu, Q.; Lin, G.; Huang, B.; Lu, B. In situ polymerization approach to cellulose–polyacrylamide interpenetrating network hydrogel with high strength and pH-responsive properties. Cellulose 2019, 26, 1825–1839. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, B.; Liu, C.; Jiang, Y.; Yu, G.; Mu, X. Biocompatible magnetic cellulose–chitosan hybrid gel microspheres reconstituted from ionic liquids for enzyme immobilization. J. Mater. Chem. 2012, 22, 15085–15091. [Google Scholar] [CrossRef]
- Peng, S.; Meng, H.; Ouyang, Y.; Chang, J. Nanoporous magnetic cellulose–chitosan composite microspheres: Preparation, characterization, and application for Cu(II) adsorption. Ind. Eng. Chem. Res. 2014, 53, 2106–2113. [Google Scholar] [CrossRef]
- Weon, S.H.; Han, J.; Choi, Y.; Park, S.; Lee, S.H. Development of blended biopolymer-based photocatalytic hydrogel beads for adsorption and photodegradation of dyes. Gels 2023, 9, 630. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling behaviors of pH-and salt-responsive cellulose-based hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Rezaei, F.; Damoogh, S.; Reis, R.L.; Kundu, S.C.; Mottaghitalab, F.; Farokhi, M. Dual drug delivery system based on pH-sensitive silk fibroin/alginate nanoparticles entrapped in PNIPAM hydrogel for treating severe infected burn wound. Biofabrication 2020, 13, 015005. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiong, Y.; Yu, S.; Liu, S.; Liu, F.; Xie, C. Facile preparation for robust and freestanding silk fibroin films in a 1-butyl-3-methyl imidazolium acetate ionic liquid system. J. Appl. Polym. Sci. 2015, 132, 42822. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhang, Y.; Zheng, L.; Zhang, H.; Shi, H.H.; Zhang, X.C.; Liu, B. Mineralized self-assembled silk fibroin/cellulose interpenetrating network aerogel for bone tissue engineering. Biomater. Adv. 2022, 134, 112549. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose nanocrystals: A versatile nanoplatform for emerging biomedical applications. Expert Opin. Drug Deliv. 2016, 13, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Lukova, P.; Katsarov, P.; Pilicheva, B. Application of Starch, Cellulose, and Their Derivatives in the Development of Microparticle Drug-Delivery Systems. Polymers 2023, 15, 3615. [Google Scholar] [CrossRef]
- Gore, P.M.; Naebe, M.; Wang, X.; Kandasubramanian, B. Progress in silk materials for integrated water treatments: Fabrication, modification and applications. Chem. Eng. J. 2019, 374, 437–470. [Google Scholar] [CrossRef]
- Rastogi, S.; Kandasubramanian, B. Progressive trends in heavy metal ions and dyes adsorption using silk fibroin composites. Environ. Sci. Pollut. Res. 2020, 27, 210–237. [Google Scholar] [CrossRef]
- Gupta, S.; Nighojkar, A.; Mayilswamy, N.; Kandasubramanian, B. Recent trends in the application of silk-based composites for remediation of toxic contaminants from wastewater. J. Polym. Environ. 2023, 31, 2243–2272. [Google Scholar] [CrossRef]
- Love, S.A.; Popov, E.; Rybacki, K.; Hu, X.; Salas-de la Cruz, D. Facile treatment to fine-tune cellulose crystals in cellulose-silk biocomposites through hydrogen peroxide. Int. J. Biol. Macromol. 2020, 147, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, H. Preparation and characterization of cellulose composite hydrogels from tea residue and carbohydrate additives. Carbohydr. Polym. 2016, 147, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Li, T.; Zhang, R.; Wu, Q.; Chen, T.; Sun, P.; Ramamoorthy, A. Conformations and intermolecular interactions in cellulose/silk fibroin blend films: A solid-state NMR perspective. J. Phys. Chem. B 2017, 121, 6108–6116. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, Y.; Yun, J.; Yoo, E.; Jung, D.; Park, K.S.; Oh, K.K.; Lee, S.H. Characterization of blended cellulose/biopolymer films prepared using ionic liquid. Cellulose 2020, 27, 5101–5119. [Google Scholar] [CrossRef]
- Shefa, A.A.; Amirian, J.; Kang, H.J.; Bae, S.H.; Jung, H.I.; Choi, H.J.; Lee, S.Y.; Lee, B.T. In vitro and in vivo evaluation of effectiveness of a novel TEMPO-oxidized cellulose nanofiber-silk fibroin scaffold in wound healing. Carbohydr. Polym. 2017, 177, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Freddi, G.; Romanò, M.; Massafra, M.R.; Tsukada, M. Silk fibroin/cellulose blend films: Preparation, structure, and physical properties. J. Appl. Polym. Sci. 1995, 56, 1537–1545. [Google Scholar] [CrossRef]
- Shang, S.; Zhu, L.; Fan, J. Physical properties of silk fibroin/cellulose blend films regenerated from the hydrophilic ionic liquid. Carbohydr. Polym. 2011, 86, 462–468. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Chen, J.; Feng, J.; Yan, W. Influence of metal oxides on the adsorption characteristics of PPy/metal oxides for Methylene Blue. J. Colloid Interface Sci. 2016, 475, 26–35. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Bazmizeynabad, F.; Seyyedi, B. kappa-Carrageenan beads as new adsorbent to remove crystal violet dye from water: Adsorption kinetics and isotherm. Desalination Water Treat. 2015, 53, 2529–2539. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Li, S.; Du, K. Fabrication and characterization of porous cellulose beads with high strength and specific surface area via preliminary chemical cross-linking reaction for protein separation. Biochem. Eng. J. 2020, 153, 107412. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Liang, C.; Qiao, L.; Du, K. High-strength and low-crystallinity cellulose/agarose composite microspheres: Fabrication, characterization and protein adsorption. Biochem. Eng. J. 2021, 166, 107826. [Google Scholar] [CrossRef]
- Qiao, L.; Lei, S.; Du, K. High-surface-area interconnected macroporous nanofibrous cellulose microspheres: A versatile platform for large capacity and high-throughput protein separation. Cellulose 2021, 28, 2125–2136. [Google Scholar] [CrossRef]
- Kim, J.W.; Hwang, I.J. Separation of valuables from spent selective catalytic reduction catalyst leaching solution by fabricated anion extraction resins. J. Environ. Chem. Eng. 2018, 6, 1100–1108. [Google Scholar] [CrossRef]
- García-Zubiri, I.X.; González-Gaitano, G.; Isasi, J.R. Sorption models in cyclodextrin polymers: Langmuir, Freundlich, and a dual-mode approach. J. Colloid Interface Sci. 2009, 337, 11–18. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Gianak, O.; Pavlidou, E.; Sarafidis, C.; Karageorgiou, V.; Deliyanni, E. Silk fibroin nanoparticles for drug delivery: Effect of bovine serum albumin and magnetic nanoparticles addition on drug encapsulation and release. Separation 2018, 5, 25. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Salama, A.; Sarhan, A.A. Ionotropically cross-linked pH-sensitive IPN hydrogel matrices as potential carriers for intestine-specific oral delivery of protein drugs. Drug Dev. Ind. Pharm. 2011, 37, 121–130. [Google Scholar] [CrossRef]
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.L.; Miao, J.C.; Sheng, W.H.; Xie, Y.F.; Huang, Q.; Shan, Y.B.; Yang, J.C. Cytocompatibility of regenerated silk fibroin film: A medical biomaterial applicable to wound healing. J. Zhejiang Univ. Sci. B 2010, 11, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Ghosh, S.K.; Kundu, S.C. Silk fibroin protein from mulberry and non-mulberry silkworms: Cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. J. Mater. Sci. Mater. Med. 2008, 19, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano Life 2012, 2, 1241006. [Google Scholar] [CrossRef]
- Paramo, L.; Jiménez-Chávez, A.; Medina-Ramirez, I.E.; Böhnel, H.N.; Escobar-Alarcón, L.; Esquivel, K. Biocompatibility Evaluation of TiO2, Fe3O4, and TiO2/Fe3O4 Nanomaterials: Insights into Potential Toxic Effects in Erythrocytes and HepG2 Cells. Nanomaterials 2023, 13, 2824. [Google Scholar] [CrossRef]
- Wei, Y.; Yin, G.; Ma, C.; Huang, Z.; Chen, X.; Liao, X.; Yao, Y.; Yin, H. Synthesis and cellular compatibility of biomineralized Fe3O4 nanoparticles in tumor cells targeting peptides. Colloids Surf. B Biointerfaces 2013, 107, 180–188. [Google Scholar] [CrossRef]




| Dissolution Conditions | Average Diameter of Cellulose/Fe3O4 Microbeads (µm) | ||
|---|---|---|---|
| Solvent | Temperature (°C) | Time (min) | |
| [Emim][Ac] | 100 | 120 | 48.8 |
| LiBr (60%) | 100 | 60 | 140.3 |
| ZnCl2 (68%) | 80 | 60 | 58.3 |
| TBAH (40%) | RT * | 90 | 14.6 |
| TBPH (40%) | RT | 90 | 17.5 |
| NaOH/thiourea (9.3%/7.4%) | 4 | 20 | 80.7 |
| Solvent | Silk Content (%) | Pseudo-Second-Order Model | qe, exp. (mg/g) | ||
|---|---|---|---|---|---|
| k2 (×10−3 g/mg·h) | qe, cal. (mg/g) | R2 | |||
| TBAH | 0 | 9.46 | 201.8 | 0.999 | 184.0 |
| 1 | 25.33 | 524.8 | 0.999 | 507.6 | |
| TBPH | 0 | 8.11 | 225.9 | 0.996 | 207.9 |
| 1 | 9.82 | 611.4 | 0.997 | 569.7 | |
| Solvent | Silk Content (%) | Langmuir Model | qe, exp. (mg/g) | ||
|---|---|---|---|---|---|
| b (×10−3 L/mg) | qm (mg/g) | R2 | |||
| TBAH | 0 | 12.65 | 220.4 | 0.973 | 185.4 |
| 1 | 1.34 | 1378.0 | 0.993 | 1095.2 | |
| TBPH | 0 | 14.88 | 228.5 | 0.985 | 194.3 |
| 1 | 0.59 | 3142.0 | 0.996 | 1643.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weon, S.H.; Na, Y.; Han, J.; Lee, J.W.; Kim, H.J.; Park, S.; Lee, S.H. pH-Responsive Cellulose/Silk/Fe3O4 Hydrogel Microbeads Designed for Biomedical Applications. Gels 2024, 10, 200. https://doi.org/10.3390/gels10030200
Weon SH, Na Y, Han J, Lee JW, Kim HJ, Park S, Lee SH. pH-Responsive Cellulose/Silk/Fe3O4 Hydrogel Microbeads Designed for Biomedical Applications. Gels. 2024; 10(3):200. https://doi.org/10.3390/gels10030200
Chicago/Turabian StyleWeon, Seung Hyeon, Yuhyeon Na, Jiwoo Han, Jeong Woo Lee, Hyung Joo Kim, Saerom Park, and Sang Hyun Lee. 2024. "pH-Responsive Cellulose/Silk/Fe3O4 Hydrogel Microbeads Designed for Biomedical Applications" Gels 10, no. 3: 200. https://doi.org/10.3390/gels10030200







