Investigating Texture and Freeze–Thaw Stability of Cold-Set Gel Prepared by Soy Protein Isolate and Carrageenan Compounding
Abstract
:1. Introduction
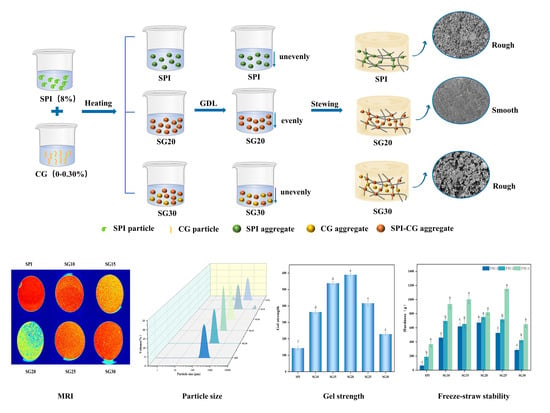
2. Results and Discussion
2.1. Water Distribution by LF-NMR and MRI
2.2. Frequency Sweep Rheological Property
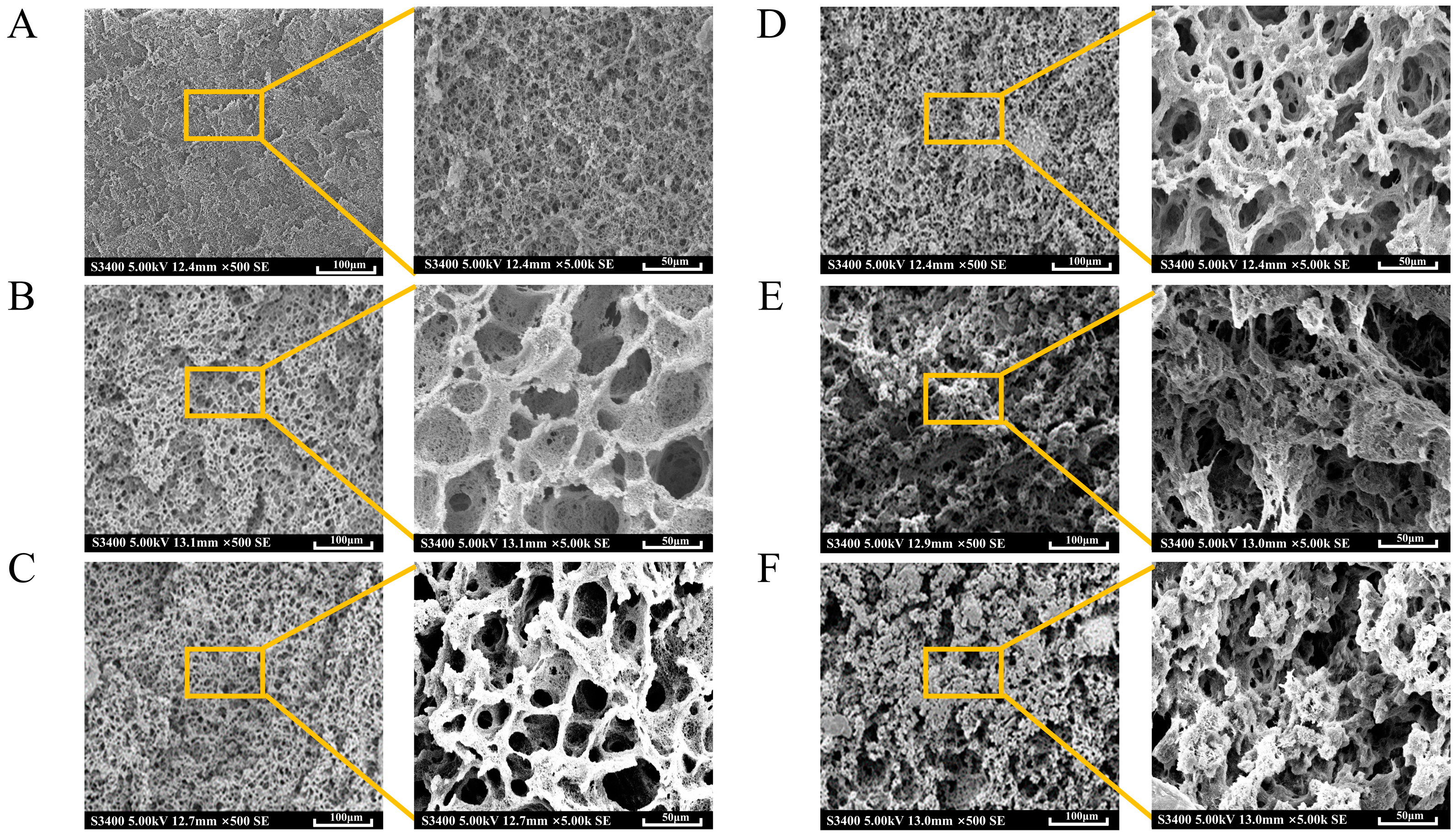
2.3. Microstructure
2.4. Intermolecular Forces
2.5. Particle Size of SPI-CG Mixed Solution
2.6. Zeta Potential of SPI-CG Mixed Solution
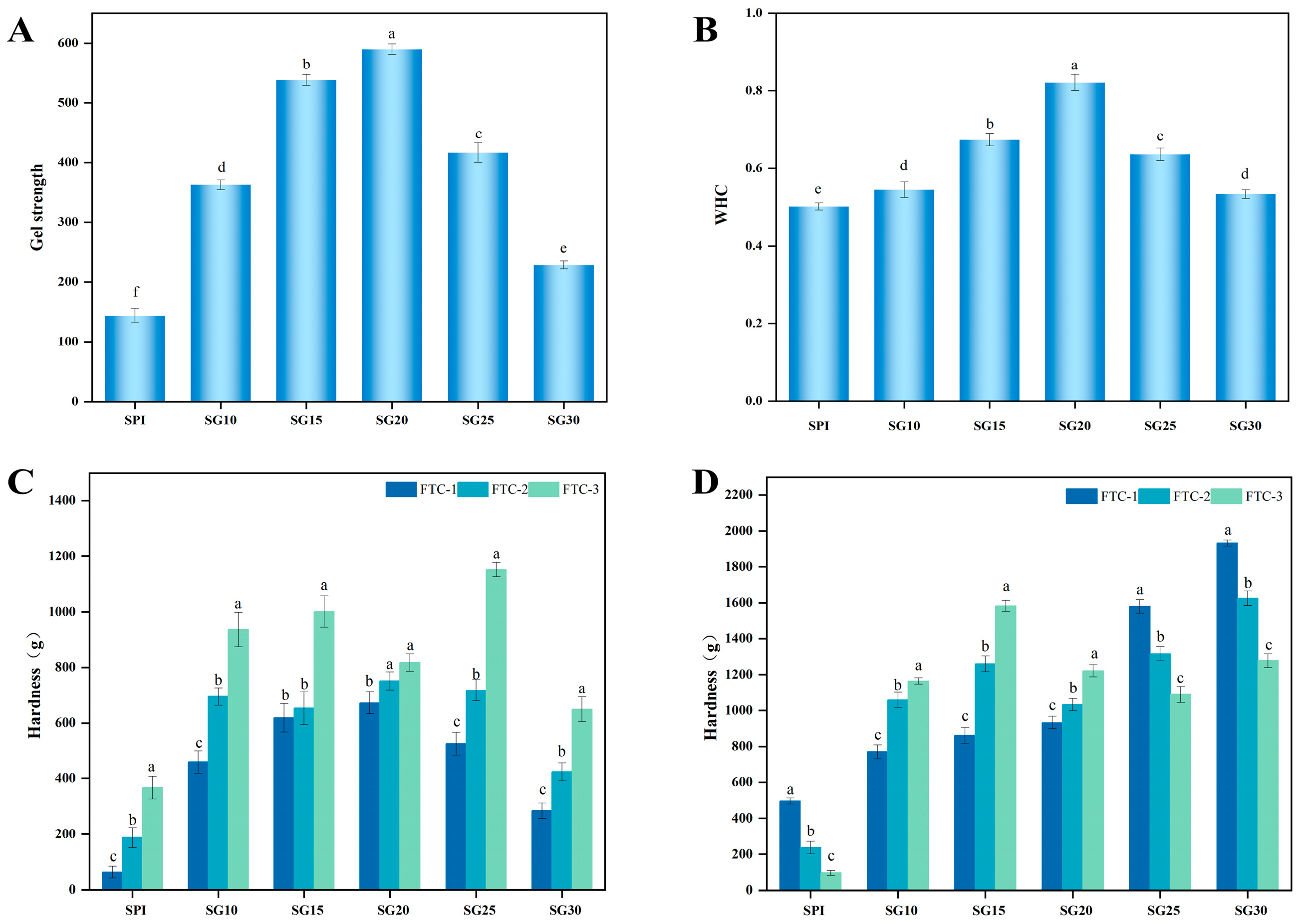
2.7. Gel Strength
2.8. Water-Holding Capacity
2.9. Freeze–Thaw Stability
2.10. Steaming Stability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. SPI-CG Composite Gels Preparation
4.3. SPI-CG Composite Gels Preparation Low-Field Nuclear Magnetic Resonance (LF-NMR)
4.4. Magnetic Resonance Imaging (MRI)
4.5. Rheological Properties
4.6. Scanning Electron Microscopy (SEM)
4.7. Intermolecular Forces
4.8. Particle Size and Zeta Potential
4.9. Gel Strength
4.10. SPI-CG Composite Gels Preparation Water-Holding Capacity (WHC)
4.11. Texture Profile Analysis (TPA)
4.12. Freeze–Thaw Stability
4.13. Steaming Stability
4.14. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.S.; Tang, C.H.; Li, B.S.; Yang, X.Q.; Li, L.; Ma, C.Y. Effects of High-Pressure Treatment on Some Physicochemical and Functional Properties of Soy Protein Isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Gao, X.Q.; Kang, Z.L.; Zhang, W.G.; Li, Y.P.; Zhou, G.H. Combination of Κ-Carrageenan and Soy Protein Isolate Effects on Functional Properties of Chopped Low-Fat Pork Batters During Heat-Induced Gelation. Food Bioprocess Technol. 2015, 8, 1524–1531. [Google Scholar] [CrossRef]
- Schreuders, F.K.G.; Dekkers, B.L.; Bodnár, I.; Erni, P.; MBoom, R.; van der Goot, A.J. Comparing Structuring Potential of Pea and Soy Protein with Gluten for Meat Analogue Preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.J.; Kong, Y.; Ma, Z.L.; Wu, C.L.; Regenstein, J.M.; Teng, F.; Li, Y. Different Commercial Soy Protein Isolates and the Characteristics of Chiba Tofu. Food Hydrocoll. 2021, 110, 106115. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Cazado, C.P.S.; Cavini, A.C.M.; Santos, L.M.F.; Moraes, I.C.F.; Pinho, S.C. Cold-Set Nacl-Induced Gels of Soy Protein Isolate and Locust Bean Gum: How the Ageing Process Affect Their Microstructure and the Stability of Incorporated Beta-Carotene. LWT-Food Sci. Technol. 2022, 154, 112667. [Google Scholar] [CrossRef]
- Lu, X.; Lu, Z.H.; Yin, L.J.; Cheng, Y.Q.; Li, L.T. Effect of Preheating Temperature and Calcium Ions on the Properties of Cold-Set Soybean Protein Gel. Food Res. Int. 2010, 43, 1673–1683. [Google Scholar] [CrossRef]
- Charoenrein, S.; Preechathammawong, N. Effect of Waxy Rice Flour and Cassava Starch on Freeze-Thaw Stability of Rice Starch Gels. Carbohydr. Polym. 2012, 90, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Chen, S.; Cui, Q.; Li, R.; Wang, X.B.; Jiang, L.Z. Effect of Ph on Freeze-Thaw Stability of Glycated Soy Protein Isolate. J. Oleo Sci. 2019, 68, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Zhang, A.Q.; Wang, X.B.; Xu, N.; Jiang, L.Z. The Radiation Assisted-Maillard Reaction Comprehensively Improves the Freeze-Thaw Stability of Soy Protein-Stabilized Oil-in-Water Emulsions. Food Hydrocoll. 2020, 103, 105684. [Google Scholar] [CrossRef]
- Yu, J.; Li, D.; Wang, L.J.; Wang, Y. Improving Freeze-Thaw Stability and 3d Printing Performance of Soy Protein Isolate Emulsion Gel Inks by Guar & Xanthan Gums. Food Hydrocoll. 2023, 136, 108293. [Google Scholar]
- Zhou, G.W.; Liu, J.N.; Wang, G.R.; Wang, L.; Zhang, A.Q.; Wang, Y.Y.; Wang, X.B. Effect of Ultrasonic Treatment on Freeze-Thaw Stability of Soy Protein Isolate Gel. J. Oleo Sci. 2019, 68, 1113–1123. [Google Scholar] [CrossRef]
- Lan, W.Q.; Zhao, Y.N.; Hu, X.Y.; Zhang, X.; Xie, J. Effects of Carrageenan Oligosaccharide on Lipid, Protein Oxidative Changes, and Moisture Migration of Litopenaeus vannamei During Freeze-Thaw Cycles. J. Food Process. Preserv. 2020, 44, e14675. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Liu, C.Z.; Sun, X.; Zhang, S.Z.; Yuan, Y.K.; Wang, D.F.; Xu, Y. Fabrication and Characterization of Cold-Gelation Whey Protein-Chitosan Complex Hydrogels for the Controlled Release of Curcumin. Food Hydrocoll. 2020, 103, 105619. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, T.; Zhang, X.; Xu, J.T.; Guo, S.T. Enhancing the Anti-Freezing Properties of Glucono-6-Lactone Induced Tofu through the Incorporation of Curdlan. Food Hydrocoll. 2024, 150, 109656. [Google Scholar] [CrossRef]
- Huang, J.J.; Bakry, A.M.; Zeng, S.W.; Xiong, S.B.; Yin, T.; You, J.; Fan, M.C.; Huang, Q.L. Effect of Phosphates on Gelling Characteristics and Water Mobility of Myofibrillar Protein from Grass Carp (Ctenopharyngodon idellus). Food Chem. 2019, 272, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Dong, S.Z.; Zhong, L.; Zhan, Q.P.; Hu, Q.H.; Zhao, L.Y. Effects of Carboxymethyl Chitosan on the Gelling Properties, Microstructure, and Molecular Forces of Pleurotus eryngii Protein Gels. Food Hydrocoll. 2023, 145, 109158. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wang, D.N.; Xu, J.X.; Tu, D.K.; Zhuang, W.J.; Tian, Y.T. Effect of Polysaccharide Concentration on Heat-Induced Tremella Fuciformis Polysaccharide-Soy Protein Isolation Gels: Gel Properties and Interactions. Int. J. Biol. Macromol. 2024, 262, 129782. [Google Scholar] [CrossRef] [PubMed]
- Laneuville, S.I.; Turgeon, S.L. Microstructure and Stability of Skim Milk Acid Gels Containing an Anionic Bacterial Exopolysaccharide and Commercial Polysaccharides. Int. Dairy J. 2014, 37, 5–15. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Li, J.H.; Su, Y.J.; Chang, C.H.; Li, X.; Yang, Y.J.; Gu, L.P. Preparation and Characterization of Hen Egg Proteins-Soybean Protein Isolate Composite Gels. Food Hydrocoll. 2019, 97, 105191. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Fan, Z.W.; Zhang, X.; Zhong, G. Effect of Soybean Soluble Polysaccharide on the Pasting, Gels, and Rheological Properties of Kudzu and Lotus Starches. Food Hydrocoll. 2019, 89, 443–452. [Google Scholar] [CrossRef]
- Deng, C.N.; Liu, Y.; Li, J.L.; Yadav, M.P.; Yin, L.J. Diverse Rheological Properties, Mechanical Characteristics and Microstructures of Corn Fiber Gum/Soy Protein Isolate Hydrogels Prepared by Laccase and Heat Treatment. Food Hydrocoll. 2018, 76, 113–122. [Google Scholar] [CrossRef]
- Yang, X.Y.; Ren, Y.M.; Liu, H.F.; Huo, C.Y.; Li, L. Differences in the Physicochemical, Digestion and Microstructural Characteristics of Soy Protein Gel Acidified with Lactic Acid Bacteria, Glucono-6-Lactone and Organic Acid. Int. J. Biol. Macromol. 2021, 185, 462–470. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Liu, Y.N.; Wang, Y.; Jin, Y.; Guo, X.Y.; Liu, Y.D.; Qi, X.M.; Lei, H.J.; Xu, H.D. Heat-Induced Whey Protein Isolate Gels Improved by Cellulose Nanocrystals: Gelling Properties and Microstructure. Carbohydr. Polym. 2020, 231, 115749. [Google Scholar] [CrossRef]
- Çakir, E.; Daubert, C.R.; Drake, M.A.; Vinyard, C.J.; Essick, G.; Foegeding, E.A. The Effect of Microstructure on the Sensory Perception and Textural Characteristics of Whey Protein/Κ-Carrageenan Mixed Gels. Food Hydrocoll. 2012, 26, 33–43. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Li, J.M.; Liu, Y.N.; Peng, F.; Wang, X.J.; Wang, C.; Li, M.; Xu, H.D. Gel Properties and Formation Mechanism of Soy Protein Isolate Gels Improved by Wheat Bran Cellulose. Food Chem. 2020, 324, 126876. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, K.; Xie, Y.; Nie, W.; Li, P.J.; Zhou, H.; Xu, B.C. Glutathione-Mediated Formation of Disulfide Bonds Modulates the Properties of Myofibrillar Protein Gels at Different Temperatures. Food Chem. 2021, 364, 130356. [Google Scholar] [CrossRef]
- Lu, Z.; Lee, P.R.; Yang, H.S. Kappa-Carrageenan Improves the Gelation and Structures of Soy Protein Isolate through the Formation of Hydrogen Bonding and Electrostatic Interactions. Food Hydrocoll. 2023, 140, 108585. [Google Scholar] [CrossRef]
- Wang, W.J.; Shen, M.Y.; Liu, S.C.; Jiang, L.; Song, Q.Q.; Xie, J.H. Gel Properties and Interactions of Mesona blumes Polysaccharide-Soy Protein Isolates Mixed Gel: The Effect of Salt Addition. Carbohydr. Polym. 2018, 192, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Shan, S.; Gao, X.; Shi, Y.D.; Lu, W.H. The Effect of Sweet Tea Polysaccharide on the Physicochemical and Structural Properties of Whey Protein Isolate Gels. Int. J. Biol. Macromol. 2023, 240, 124344. [Google Scholar] [CrossRef]
- Wang, W.J.; Shen, M.Y.; Jiang, L.; Song, Q.Q.; Liu, S.C.; Xie, J.H. Influence of Mesona blumes Polysaccharide on the Gel Properties and Microstructure of Acid-Induced Soy Protein Isolate Gels. Food Chem. 2020, 313, 126125. [Google Scholar] [CrossRef]
- Everett, D.W.; McLeod, R.E. Interactions of Polysaccharide Stabilisers with Casein Aggregates in Stirred Skim-Milk Yoghurt. Int. Dairy J. 2005, 15, 1175–1183. [Google Scholar] [CrossRef]
- de Jong, S.; van de Velde, F. Charge Density of Polysaccharide Controls Microstructure and Large Deformation Properties of Mixed Gels. Food Hydrocoll. 2007, 21, 1172–1187. [Google Scholar] [CrossRef]
- Zhang, K.; Tian, X.J.; Shen, R.X.; Zhao, K.X.; Zhang, Y.F.; Wang, W.H. Delaying in Vitro Gastric Digestion of Myofibrillar Protein Gel Using Carboxymethylated Cellulose Nanofibrils: Forming a Compact and Uniform Microstructure. Food Hydrocoll. 2023, 140, 108661. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; Zhou, Z.; Xu, X.Y.; Fan, G.; Wang, L.F.; Huang, X.J.; Pan, S.Y.; Zhu, L. Acid-Induced Gelation Behavior of Soybean Protein Isolate with High Intensity Ultrasonic Pre-Treatments. Ultrason. Sonochem. 2013, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.R.; Ledward, D.A.; Bardsley, R.G.; Poulter, R.G. Species Dependence of Fish Myosin Stability to Heat and Frozen Storage. Int. J. Food Sci. Technol. 1994, 29, 287–301. [Google Scholar] [CrossRef]
- Shiroodi, S.G.; Rasco, B.A.; Lo, Y.M. Influence of Xanthan-Curdlan Hydrogel Complex on Freeze-Thaw Stability and Rheological Properties of Whey Protein Isolate Gel over Multiple Freeze-Thaw Cycle. J. Food Sci. 2015, 80, E1498–E1505. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Gao, Y.T.; Gao, T.; Geng, M.J.; Liu, Y.; Teng, F.; Li, Y. Fabrication of Water-in-Oil-in-Gel Emulsion Gel Based on Ph-Shifting Soybean Lipophilic Protein and Carboxymethyl Chitosan: Gel Performance, Physicochemical Properties and Digestive Characteristics. Food Hydrocoll. 2024, 147, 109385. [Google Scholar] [CrossRef]
- Han, M.Y.; Wang, P.; Xu, X.L.; Zhou, G.H. Low-Field Nmr Study of Heat-Induced Gelation of Pork Myofibrillar Proteins and Its Relationship with Microstructural Characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhang, B.; Fan, J.L.; Yang, Q.; Chen, H.Q. Effects of Sodium Tripolyphosphate Modification on the Structural, Functional, and Rheological Properties of Rice Glutelin. Food Chem. 2019, 281, 18–27. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yuan, C.; Liu, Y.W.; Xu, D.Y.; Cui, B. The Influence of a Hydroxypropyl-Beta-Cyclodextrin Composite on the Gelation of Kappa-Carrageenan. Food Hydrocoll. 2019, 90, 276–284. [Google Scholar] [CrossRef]
- Lv, Y.Q.; Xu, L.L.; Tang, T.T.; Li, J.H.; Gu, L.P.; Chang, C.H.; Zhang, M.; Yang, Y.J.; Su, Y.J. Gel Properties of Soy Protein Isolate-Potato Protein-Egg White Composite Gel: Study on Rheological Properties, Microstructure, and Digestibility. Food Hydrocoll. 2023, 135, 108223. [Google Scholar] [CrossRef]
- Huang, M.Y.; Xu, Y.J.; Xu, L.N.; Bai, Y.; Zeng, X.M.; Zheng, R.; Xu, X.L. Conformation Changes and Emulsifying Properties of Myofibrillar Proteins in Water: Effects of Electrostatic Interaction with Chitosan. Food Res. Int. 2023, 163, 112154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.B.; Miao, Z.C.; Qi, Q.; Zheng, Q.H.; Mao, Y.X.; Chu, Z.J.; Zhang, H.; Xu, X.Y.; Zheng, M.Z.; Liu, J.S. Interactions of Soy Protein Isolate with Common and Waxy Corn Starches and Their Effects on Acid-Induced Cold Gelation Properties of Complexes. Food Chem.-X 2023, 18, 106671. [Google Scholar] [CrossRef] [PubMed]
- Kocher, P.N.; Foegeding, E.A. Microcentrifuge-Based Method for Measuring Water-Holding of Protein Gels. J. Food Sci. 1993, 58, 1040–1046. [Google Scholar] [CrossRef]
- Jin, I.H.; Kim, J.E.; Seo, J.H.; Lee, S.P. Physicochemical Properties of Soy Protein Isolate Gels Emulsified with Various Oils Using a Microbial Transglutaminase. Food Sci. Biotechnol. 2013, 22, 129–136. [Google Scholar] [CrossRef]
- Lai, R.; Liu, Y.W.; Liu, J. Properties of the Konjac Glucomannan and Zein Composite Gel with or without Freeze-Thaw Treatment. Food Hydrocoll. 2021, 117, 106700. [Google Scholar] [CrossRef]


| Simple Name | T2 | Time of Relaxation (ms) | Water Proportions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T2b (ms) | T2b−1 (ms) | T21 (ms) | T22 (ms) | P2b (%) | P2b−1 (%) | P21 (%) | P22 (%) | ||
| SPI | 16,899.61 ± 77.86 a | 0.52 ± 0.09 cd | N.D | 109.70 ± 5.01 a | 1762.91 ± 28.94 a | 2.18 ± 0.10 b | N.D | 97.10 ± 0.60 a | 0.72 ± 0.08 d |
| SG10 | 13,841.79 ± 71.68 b | 0.74 ± 0.09 bc | N.D | 89.07 ± 2.93 b | 1773.75 ± 10.01 a | 1.85 ± 0.10 c | N.D | 97.06 ± 0.10 ab | 0.45 ± 0.11 d |
| SG15 | 12,903.93 ± 60.83 c | 0.69 ± 0.12 bcd | N.D | 83.10 ± 5.17 bc | 1162.32 ± 12.12 b | 2.49 ± 0.10 a | N.D | 96.69 ± 0.26 b | 1.33 ± 0.21 c |
| SG20 | 11,768.12 ± 126.89 e | 0.79 ± 0.14 b | 1.70 ± 0.11 b | 77.53 ± 4.35 c | 943.79 ± 49.42 d | 1.98 ± 0.12 c | 0.90 ± 0.06 b | 94.00 ± 0.20 d | 3.84 ± 0.16 a |
| SG25 | 12,856.64 ± 118.82 c | 1.12 ± 0.10 a | 2.77 ± 0.06 a | 89.07 ± 6.45 b | 1084.37 ± 54.99 c | 1.27 ± 0.11 d | 1.67 ± 0.75 a | 95.26 ± 0.14 c | 2.47 ± 0.22 b |
| SG30 | 12,371.15 ± 106.05 d | 0.52 ± 0.07 d | N.D | 83.10 ± 6.90 bc | 1080.48 ± 43.66 c | 0.61 ± 0.06 e | N.D | 95.44 ± 0.15 c | 2.79 ± 0.15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yu, Z.; Ren, S.; Liu, J.; Xu, J.; Guo, Z.; Wang, Z. Investigating Texture and Freeze–Thaw Stability of Cold-Set Gel Prepared by Soy Protein Isolate and Carrageenan Compounding. Gels 2024, 10, 204. https://doi.org/10.3390/gels10030204
Wang Z, Yu Z, Ren S, Liu J, Xu J, Guo Z, Wang Z. Investigating Texture and Freeze–Thaw Stability of Cold-Set Gel Prepared by Soy Protein Isolate and Carrageenan Compounding. Gels. 2024; 10(3):204. https://doi.org/10.3390/gels10030204
Chicago/Turabian StyleWang, Zhuying, Zhenhai Yu, Shuanghe Ren, Jun Liu, Jing Xu, Zengwang Guo, and Zhongjiang Wang. 2024. "Investigating Texture and Freeze–Thaw Stability of Cold-Set Gel Prepared by Soy Protein Isolate and Carrageenan Compounding" Gels 10, no. 3: 204. https://doi.org/10.3390/gels10030204