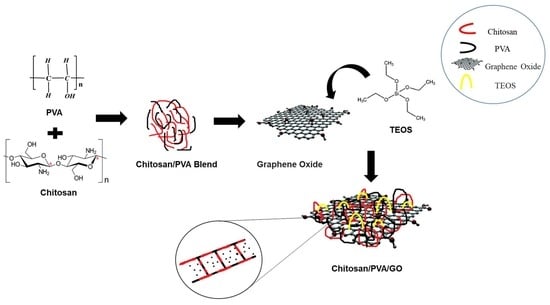
Development of pH-Responsive, Thermosensitive, Antibacterial, and Anticancer CS/PVA/Graphene Blended Hydrogels for Controlled Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR Analysis
2.2. Atomic Force Microscopy
2.3. Wetting Analysis
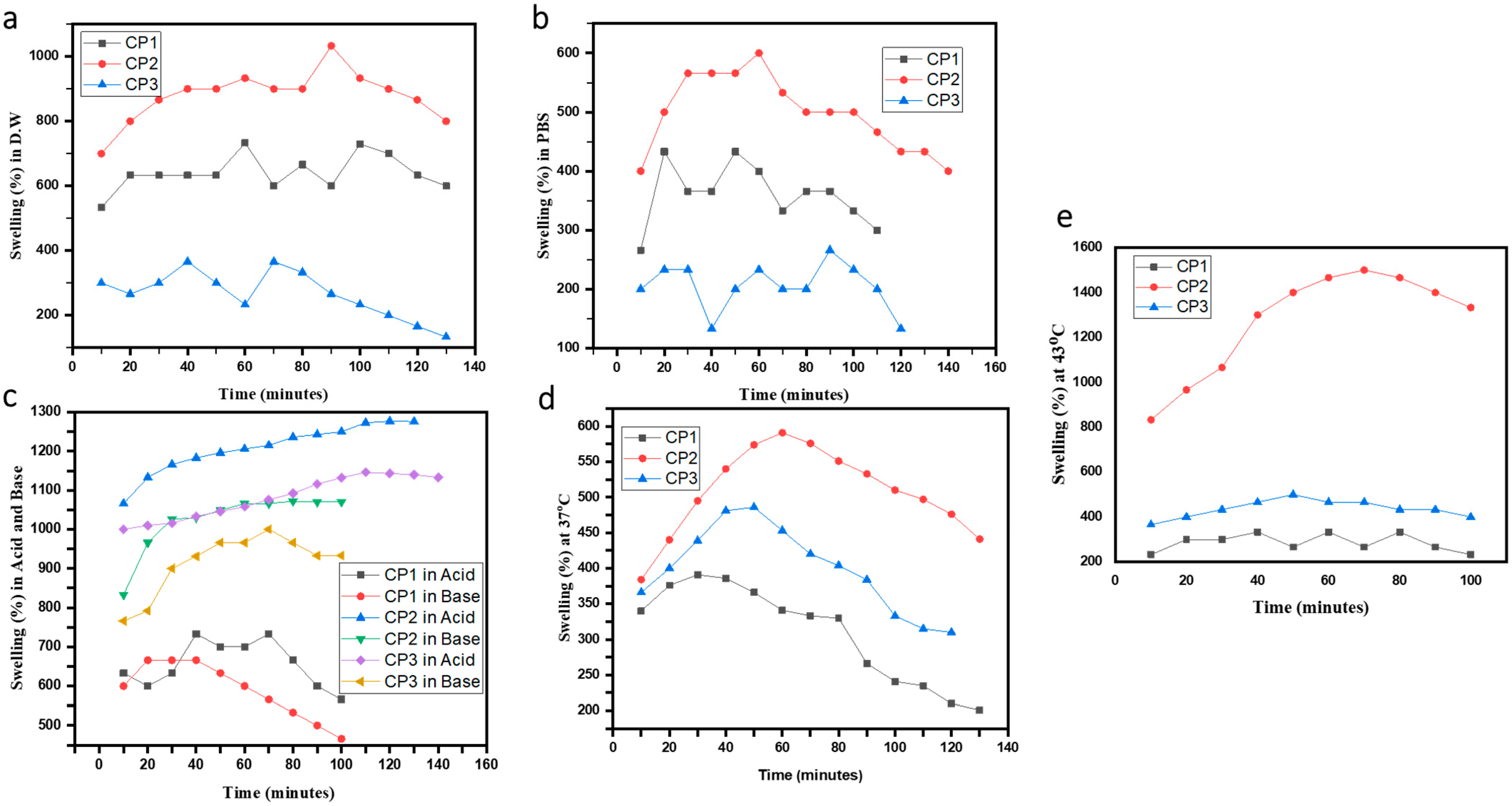
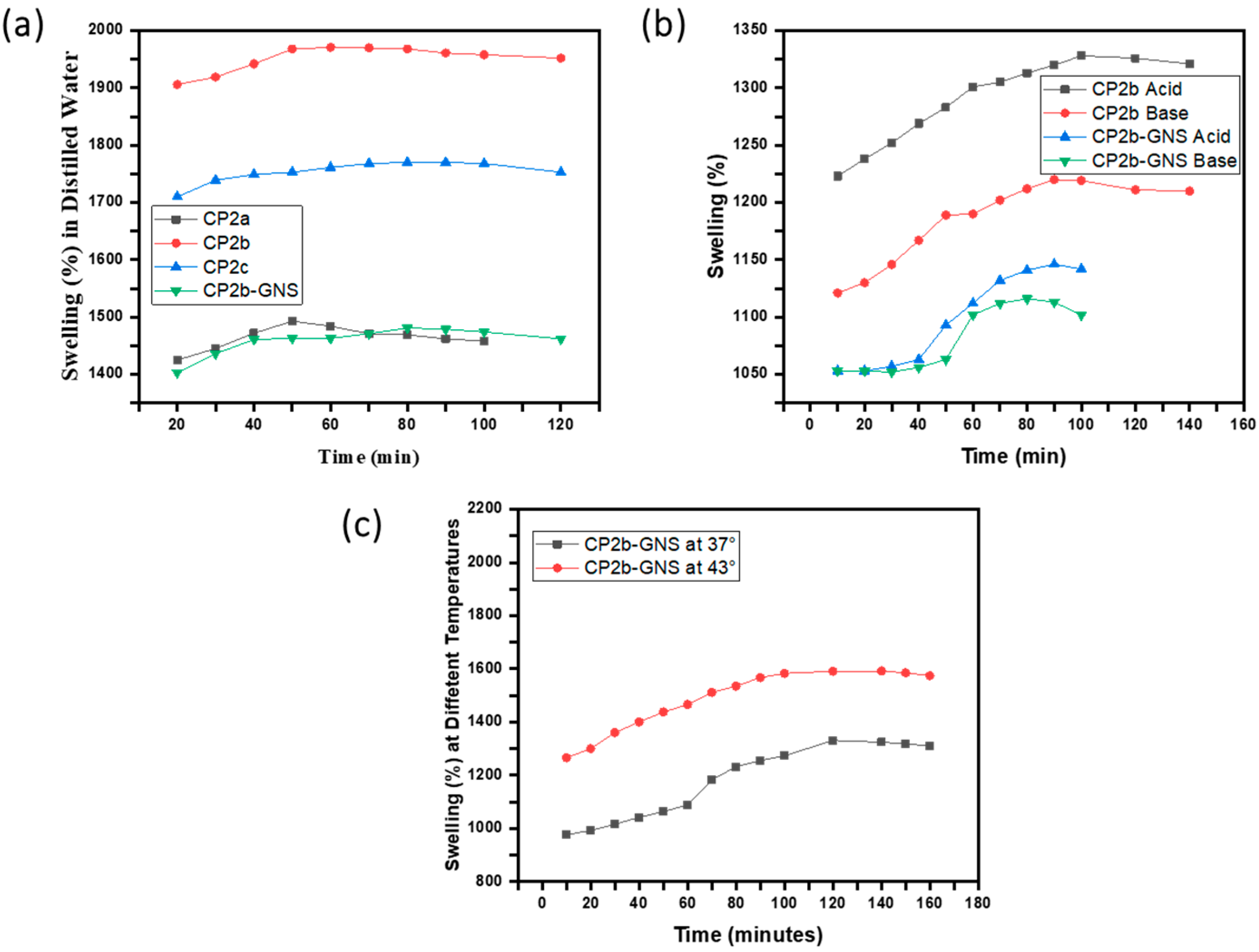
2.4. Swelling Studies
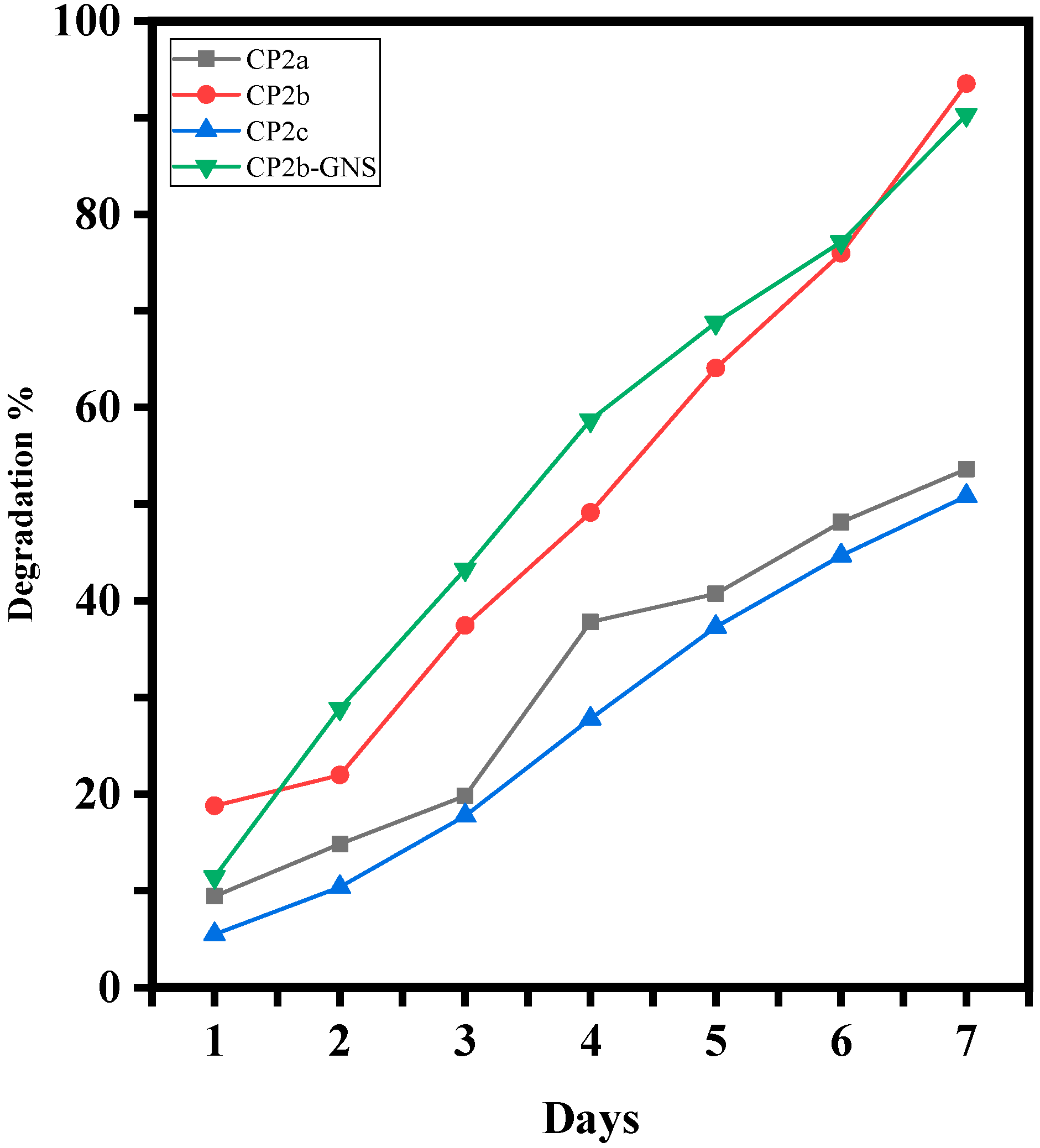
2.5. Degradation Analysis of Hydrogels
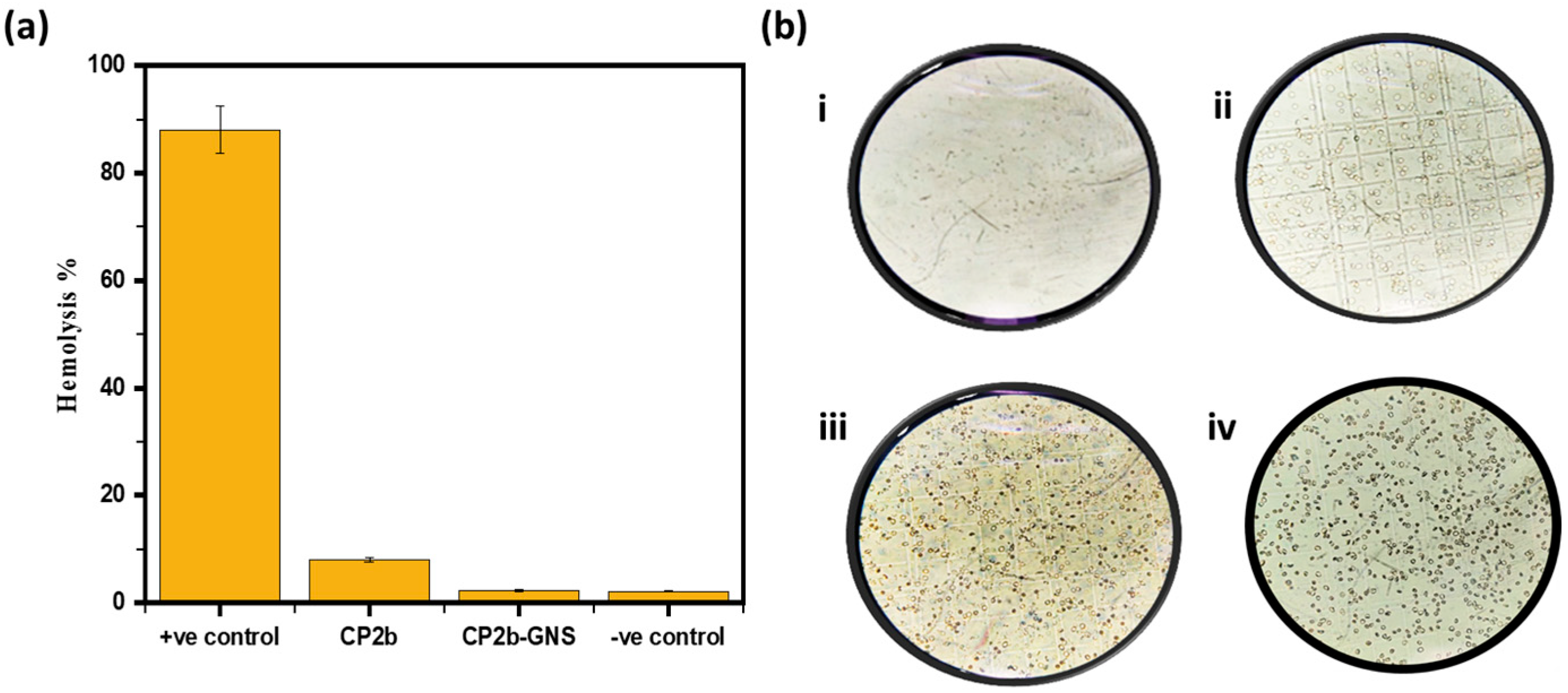
2.6. Hemolysis
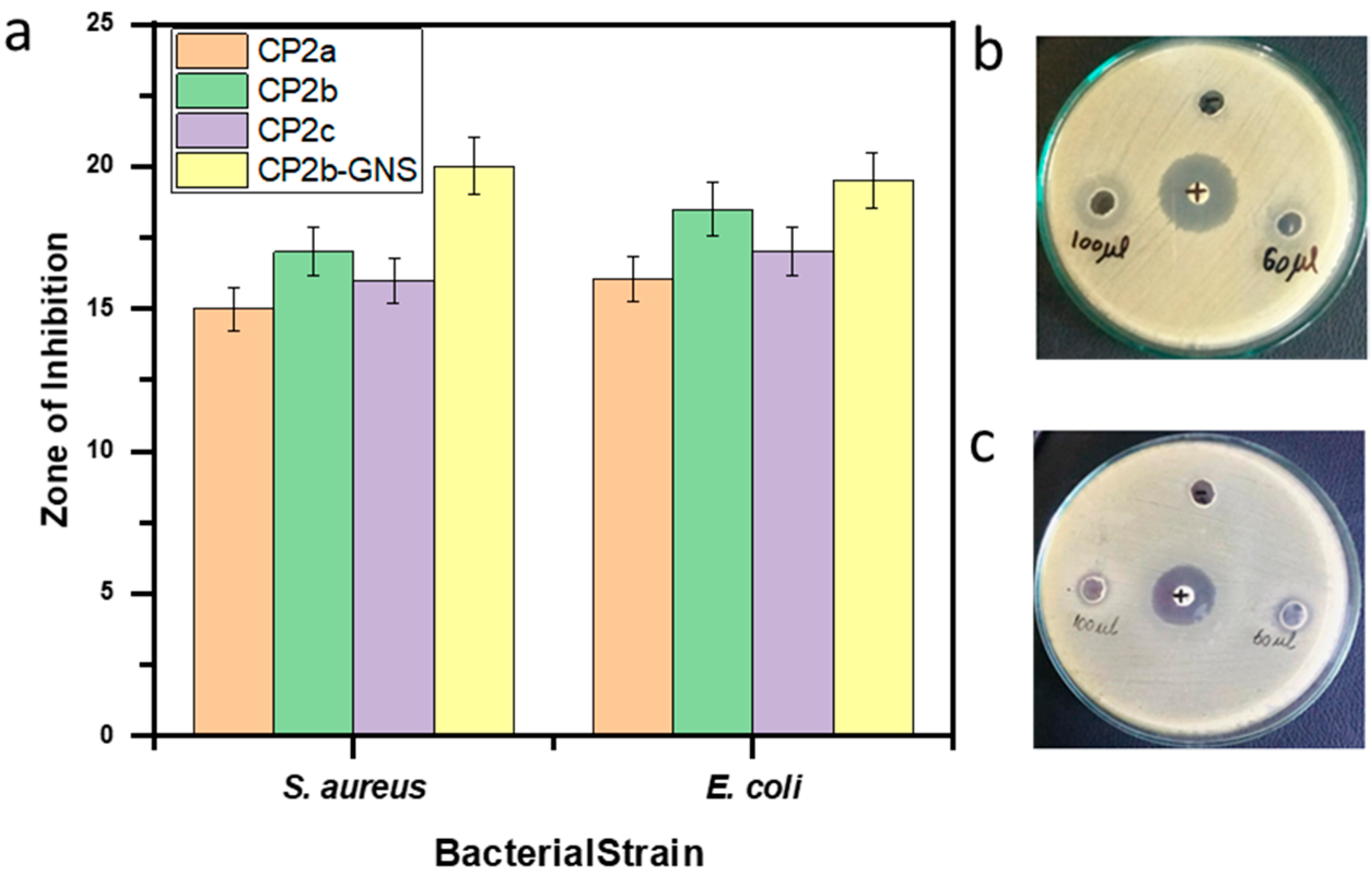
2.7. Antimicrobial Activity
2.8. In Vitro Drug Release Analysis
Drug Release Kinetics
2.9. Cell Viability Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of CS/PVA Hydrogels
4.2.2. Preparation of CS/PVA/GNS Hydrogels
4.3. Characterizations
4.3.1. FTIR Analysis
4.3.2. Atomic Force Microscopy
4.3.3. Contact Angle Measurement
4.4. Swelling Studies
4.5. Degradation Analysis
4.6. Loading and Release of Drug from Hydrogel
Drug Loading
4.7. In Vitro Release Test
4.8. Hemolysis
4.9. Antibacterial Activity Evaluation
4.9.1. Bacteria Culture
4.9.2. Cell Culture
4.10. MTT Cell Viability Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In Situ Gelling Biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching It Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2023, 2023, 202300217–202300218. [Google Scholar] [CrossRef]
- Rawat, P.S.; Ravi, P.R.; Mir, S.I.; Khan, M.S.; Kathuria, H.; Katnapally, P.; Bhatnagar, U. Design, Characterization and Pharmacokinetic–Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma. Pharmaceutics 2023, 15, 405. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Mishra, S.; Rani, P.; Sen, G.; Dey, K.P. Preparation, Properties and Application of Hydrogels: A Review. In Hydrogels: Recent Advances; Springer: Berlin/Heidelberg, Germany, 2018; pp. 145–173. [Google Scholar]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local Drug Delivery Strategies for Cancer Treatment: Gels, Nanoparticles, Polymeric Films, Rods, and Wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Wang, Y.; Wang, Y.; Shi, S.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A Novel Composite Hydrogel Based on Chitosan and Inorganic Phosphate for Local Drug Delivery of Camptothecin Nanocolloids. J. Pharm. Sci. 2010, 100, 232–241. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Zhao, J.; Wu, C.; Ye, C.; Huang, M.; Wang, S. Preparation of Injectable Temperature-Sensitive Chitosan-Based Hydrogel for Combined Hyperthermia and Chemotherapy of Colon Cancer. Carbohydr. Polym. 2019, 222, 115039. [Google Scholar] [CrossRef] [PubMed]
- Oyetade, O.A.; Martincigh, B.S.; Skelton, A.A. Interplay between Electrostatic and Hydrophobic Interactions in the PH-Dependent Adsorption of Ibuprofen onto Acid-Functionalized Multiwalled Carbon Nanotubes. J. Phys. Chem. C 2018, 122, 22556–22568. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, X.; Pu, Y.; Yi, Y.; Zhang, T.; Wang, B. PH-Sensitive and Biocompatible Quercetin-Loaded GO–PEA–HA Carrier Improved Antitumour Efficiency and Specificity. Artif. Cells Nanomed. Biotechnol. 2018, 46, S28–S37. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, J.; Jiang, W.; Liu, R.; Li, Z.; Luan, Y. Amphiphilic Prodrug-Decorated Graphene Oxide as a Multi-Functional Drug Delivery System for Efficient Cancer Therapy. Mater. Sci. Eng. C 2018, 89, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Liau, J.J.; Li, Y.J. Characterization and Cell Culture of a Grafted Chitosan Scaffold for Tissue Engineering. Int. J. Polym. Sci. 2015, 2015, 935305. [Google Scholar] [CrossRef]
- Omidi, S.; Pirhayati, M.; Kakanejadifard, A. Co-Delivery of Doxorubicin and Curcumin by a PH-Sensitive, Injectable, and in Situ Hydrogel Composed of Chitosan, Graphene, and Cellulose Nanowhisker. Carbohydr. Polym. 2020, 231, 115745. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Shive, M.; Bichara, A.; Berrada, M.; Le Garrec, D.; Chenite, A.; Leroux, J.C. A Thermosensitive Chitosan-Based Hydrogel for the Local Delivery of Paclitaxel. Eur. J. Pharm. Biopharm. 2004, 57, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Kweon, D.K.; Song, S.B.; Park, Y.Y. Preparation of Water-Soluble Chitosan/Heparin Complex and Its Application as Wound Healing Accelerator. Biomaterials 2003, 24, 1595–1601. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Hydrogels as Smart Biomaterials. Polym. Int. 2007, 56, 1078–1098. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T.H. Thermal and Structural Studies of Poly (Vinyl Alcohol) and Hydroxypropyl Cellulose Blends. Nat. Sci. 2012, 4, 57–67. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef]
- Ghawanmeh, A.A.; Ali, G.A.M.; Algarni, H.; Sarkar, S.M.; Chong, K.F. Graphene Oxide-Based Hydrogels as a Nanocarrier for Anticancer Drug Delivery. Nano Res. 2019, 12, 973–990. [Google Scholar] [CrossRef]
- Feng, W.; Iscience, Z. Biomedical Applications of Chitosan-Graphene Oxide Nanocomposites. Iscience 2022, 25, 103629. [Google Scholar] [CrossRef]
- Xue, B.; Wang, Y.; Tian, J.; Zhang, W.; Zang, Z.; Cui, H.; Zhang, Y.; Jiang, Q.; Li, B.; Hai Liu, R. Effects of Chitooligosaccharide-Functionalized Graphene Oxide on Stability, Simulated Digestion, and Antioxidant Activity of Blueberry Anthocyanins. Food Chem. 2022, 368, 130684. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Yaqoob, Z.; Nainar, M.M.A.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Haider, S.; Busra, F.M. Chitosan/Poly Vinyl Alcohol/Graphene Oxide Based Ph-Responsive Composite Hydrogel Films: Drug Release, Anti-Microbial and Cell Viability Studies. Polymers 2021, 13, 3124. [Google Scholar] [CrossRef]
- Kumar Rana, V.; Choi, M.-C.; Kong, J.-Y.; Yeon Kim, G.; Ju Kim, M.; Kim, S.-H.; Mishra, S.; Pal Singh, R.; Ha, C.-S. Synthesis and Drug-delivery Behavior of Chitosan-functionalized Graphene Oxide Hybrid Nanosheets. Macromol. Mater. Eng. 2011, 296, 131–140. [Google Scholar] [CrossRef]
- Yiang, G.T.; Chou, P.L.; Hung, Y.T.; Chen, J.N.; Chang, W.J.; Yu, Y.L.; Wei, C.W. Vitamin C Enhances Anticancer Activity in Methotrexate-Treated Hep3B Hepatocellular Carcinoma Cells. Oncol. Rep. 2014, 32, 1057–1063. [Google Scholar] [CrossRef]
- Mankotia, P.; Choudhary, S.; Sharma, K.; Kumar, V.; Kaur Bhatia, J.; Parmar, A.; Sharma, S.; Sharma, V. Neem Gum Based PH Responsive Hydrogel Matrix: A New Pharmaceutical Excipient for the Sustained Release of Anticancer Drug. Int. J. Biol. Macromol. 2020, 142, 742–755. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Raza, M.A.; Ghauri, F.A.; Rehman, Z.U.; Ilyas, M.T. Corrosion Study of Graphene Oxide Coatings on AZ31B Magnesium Alloy. J. Coat. Technol. Res. 2020, 17, 1321–1329. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Magne, T.M.; de Oliveira Vieira, T.; Alencar, L.M.R.; Junior, F.F.M.; Gemini-Piperni, S.; Carneiro, S.V.; Fechine, L.M.U.D.; Freire, R.M.; Golokhvast, K.; Metrangolo, P.; et al. Graphene and Its Derivatives: Understanding the Main Chemical and Medicinal Chemistry Roles for Biomedical Applications. J. Nanostruct. Chem. 2021, 12, 693–727. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Ortiz, A.G.; Barrera, E.V. Carbon Nano-Onions Reinforced Multilayered Thin Film System for Stimuli-Responsive Drug Release. Pharmaceutics 2020, 12, 1208. [Google Scholar] [CrossRef]
- Zarbab, A.; Sajjad, A.; Rasul, A.; Jabeen, F.; Javaid Iqbal, M. Synthesis and Characterization of Guar Gum Based Biopolymeric Hydrogels as Carrier Materials for Controlled Delivery of Methotrexate to Treat Colon Cancer. Saudi J. Biol. Sci. 2023, 30, 103731. [Google Scholar] [CrossRef]
- Jamal, A.; Shahzadi, L.; Ahtzaz, S.; Zahid, S.; Chaudhry, A.A.; ur Rehman, I.; Yar, M. Identification of Anti-Cancer Potential of Doxazocin: Loading into Chitosan Based Biodegradable Hydrogels for on-Site Delivery to Treat Cervical Cancer. Mater. Sci. Eng. C 2018, 82, 102–109. [Google Scholar] [CrossRef]
- Bet, M.R.; Goissis, G.; Vargas, S.; Selistre-De-Araujo, H.S. Cell Adhesion and Cytotoxicity Studies over Polyanionic Collagen Surfaces with Variable Negative Charge and Wettability. Biomaterials 2003, 24, 131–137. [Google Scholar] [CrossRef]
- Hahn, G.M.; Braun, J.; Har Kedar, I. Thermochemotherapy: Synergism between Hyperthermia (42–43°) and Adriamycin (or Bleomycin) in Mammalian Cell Inactivation. Proc. Natl. Acad. Sci. USA 1975, 72, 937–940. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Thermal Dose Determination in Cancer Therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Luo, J.; Yang, J.; Wang, W.; Kennedy, J.F. A Novel Biopolymer/Rectorite Nanocomposite with Antimicrobial Activity. Carbohydr. Polym. 2009, 77, 449–456. [Google Scholar] [CrossRef]
- Wei, L.; Cai, C.; Lin, J.; Chen, T. Dual-Drug Delivery System Based on Hydrogel/Micelle Composites. Biomaterials 2009, 30, 2606–2613. [Google Scholar] [CrossRef]
- Rasul, A.; Yu, B.; Zhong, L.; Khan, M.; Yang, H.; Ma, T. Cytotoxic Effect of Evodiamine in SGC-7901 Human Gastric Adenocarcinoma Cells via Simultaneous Induction of Apoptosis and Autophagy. Oncol. Rep. 2012, 27, 1481–1487. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Jian, Z.; Fei, G.; Qian, W.; Luo, G.; Wang, Z.; Xia, H. Novel Poly(Vinyl Alcohol)/Chitosan/Modified Graphene Oxide Biocomposite for Wound Dressing Application. Macromol. Biosci. 2020, 20, 1900385. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M.T.Z. Inflammation Targeted Chitosan-Based Hydrogel for Controlled Release of Diclofenac Sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef]
- Sankarganesh, P.; Parthasarathy, V.; Kumar, A.G.; Saraniya, S.R.M. Preparation of Cellulose—PVA Blended Hydrogels for Wound Healing Applications with Controlled Release of the Antibacterial Drug: An In Vitro Anticancer Activity. Biomass Convers. Biorefin. 2022, 14, 3385–3395. [Google Scholar] [CrossRef]







| Sample Name | Average Roughness (nm) | Root Mean Square Roughness (nm) |
|---|---|---|
| CP2b | 7.469 nm | 10.694 nm |
| CP2b-GNS | 2.803 nm | 3.552 nm |
| Models | Intercept | Slope | R2 |
|---|---|---|---|
| Zero Order | 2.9311 | 0.0119 | 0.9369 |
| First Order | 0.4926 | 0.0011 | 0.8927 |
| Higuchi model | 39.281 | 6.5261 | 0.9312 |
| Korsmeyer-Peppas model | 0.0602 | 0.3491 | 0.9659 |
| Formulations | CS | PVA | Cross Linker (TEOS) |
|---|---|---|---|
| CP1 | 0.2 g | 0.8 g | -- |
| CP2 | 0.4 g | 0.6 g | -- |
| CP3 | 0.6 g | 0.4 g | -- |
| CP2a | 0.6 g | 0.4 g | 50 µL |
| CP2b | 0.6 g | 0.4 g | 100 µL |
| CP2c | 0.6 g | 0.4 g | 150 µL |
| Formulations | CS | PVA | GNS | TEOS |
|---|---|---|---|---|
| CP2b-GNS | 0.6 g | 0.4 g | 0.5% | 100 µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansha, S.; Sajjad, A.; Zarbab, A.; Afzal, T.; Kanwal, Z.; Iqbal, M.J.; Raza, M.A.; Ali, S. Development of pH-Responsive, Thermosensitive, Antibacterial, and Anticancer CS/PVA/Graphene Blended Hydrogels for Controlled Drug Delivery. Gels 2024, 10, 205. https://doi.org/10.3390/gels10030205
Mansha S, Sajjad A, Zarbab A, Afzal T, Kanwal Z, Iqbal MJ, Raza MA, Ali S. Development of pH-Responsive, Thermosensitive, Antibacterial, and Anticancer CS/PVA/Graphene Blended Hydrogels for Controlled Drug Delivery. Gels. 2024; 10(3):205. https://doi.org/10.3390/gels10030205
Chicago/Turabian StyleMansha, Saira, Amna Sajjad, Aneeqa Zarbab, Tahmina Afzal, Zakia Kanwal, Muhammad Javaid Iqbal, Mohsin Ali Raza, and Sharafat Ali. 2024. "Development of pH-Responsive, Thermosensitive, Antibacterial, and Anticancer CS/PVA/Graphene Blended Hydrogels for Controlled Drug Delivery" Gels 10, no. 3: 205. https://doi.org/10.3390/gels10030205