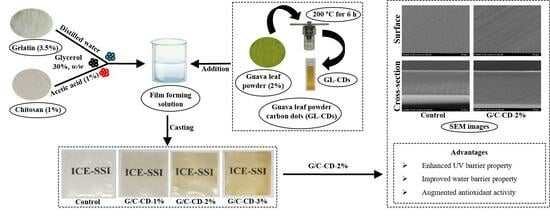
Active Fish Gelatin/Chitosan Blend Film Incorporated with Guava Leaf Powder Carbon Dots: Properties, Release and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties and Characteristics of G/C Blend Film Incorporated without and with GL-CDs at Varying Levels
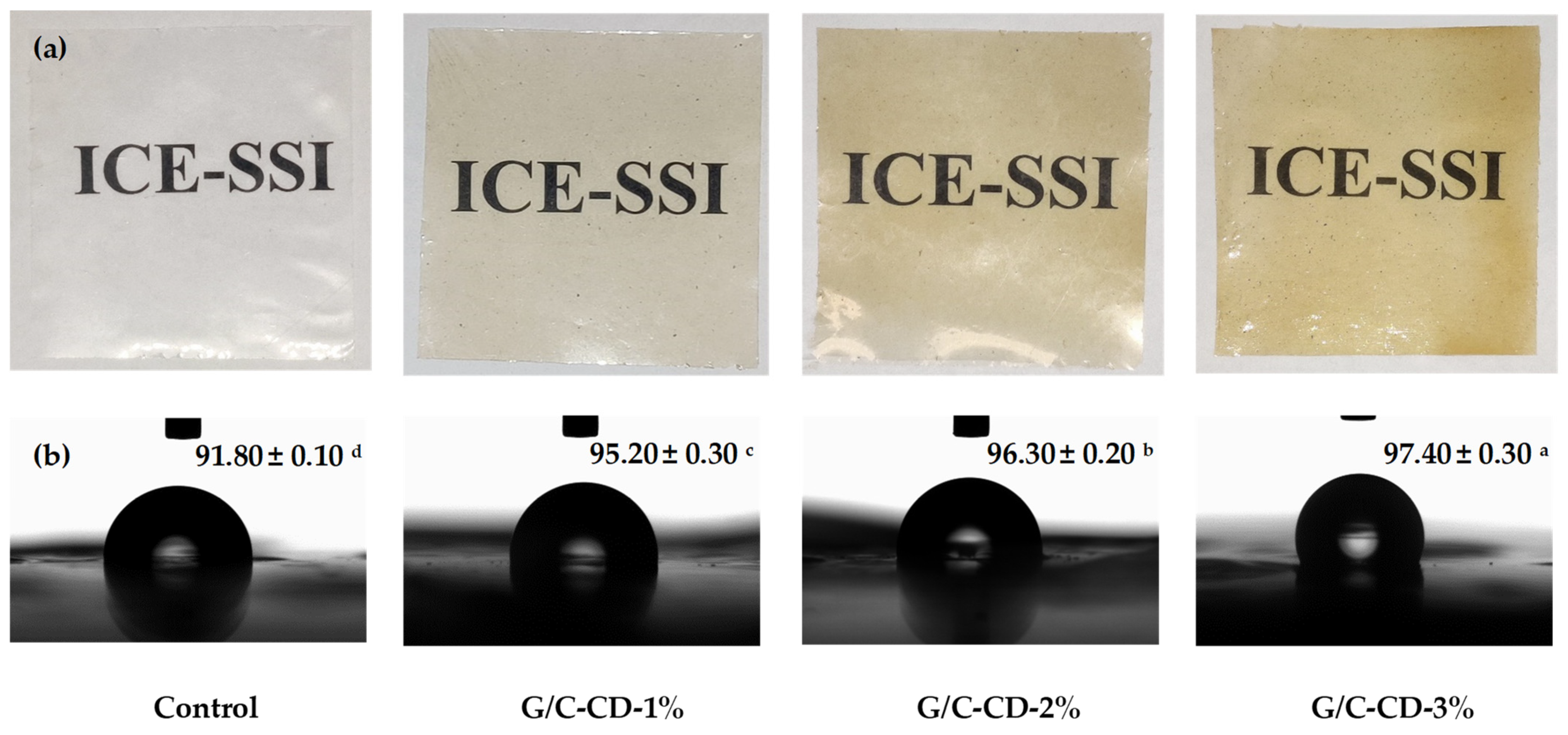
2.1.1. Overall Appearance and Water Contact Angle
2.1.2. Thickness
2.1.3. Mechanical Properties
2.1.4. Water Vapor Permeability (WVP)
2.1.5. Color
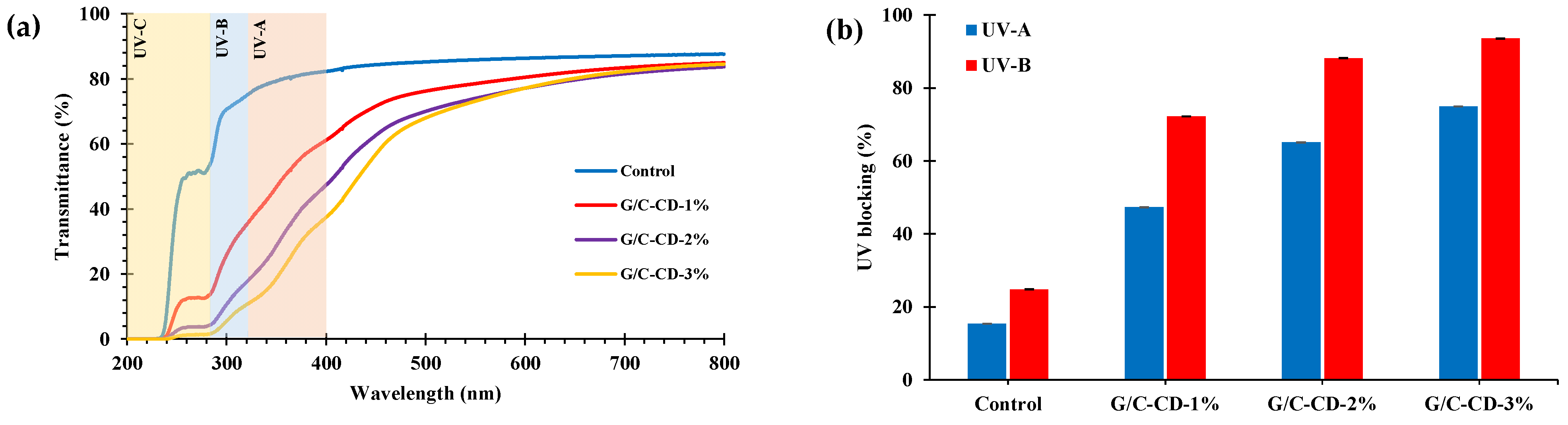
2.1.6. Light Transmittance, Opaqueness and UV-Blocking Properties
2.1.7. ATR-FTIR Spectra
2.1.8. Release Rate
2.1.9. Antioxidant Activities
2.2. Characteristics of the Selected G/C Blend Film without and with 2% GL-CD
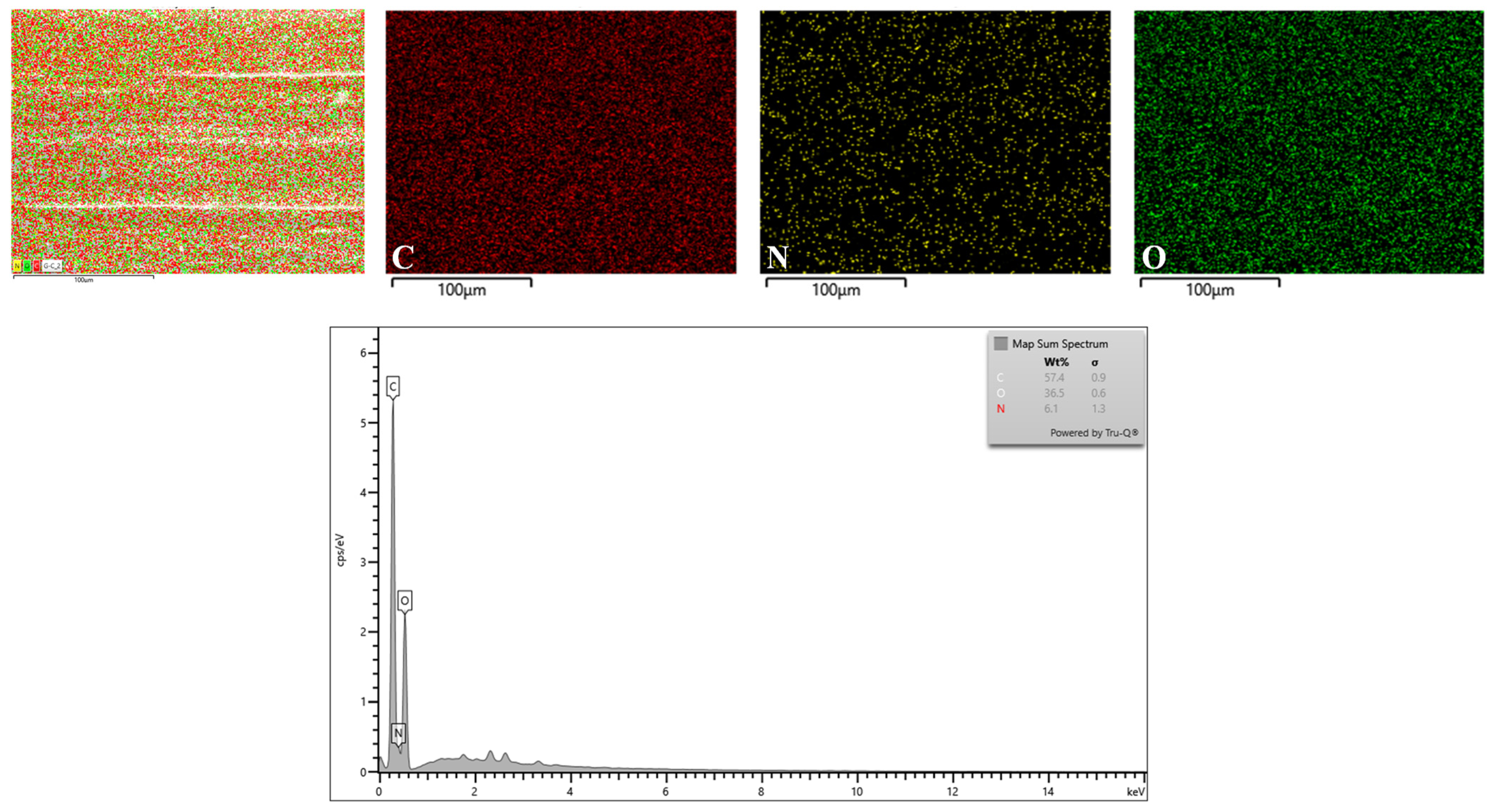
2.2.1. Microstructure, Elemental Mapping and Energy-Dispersive X-ray (EDX) Spectroscopy
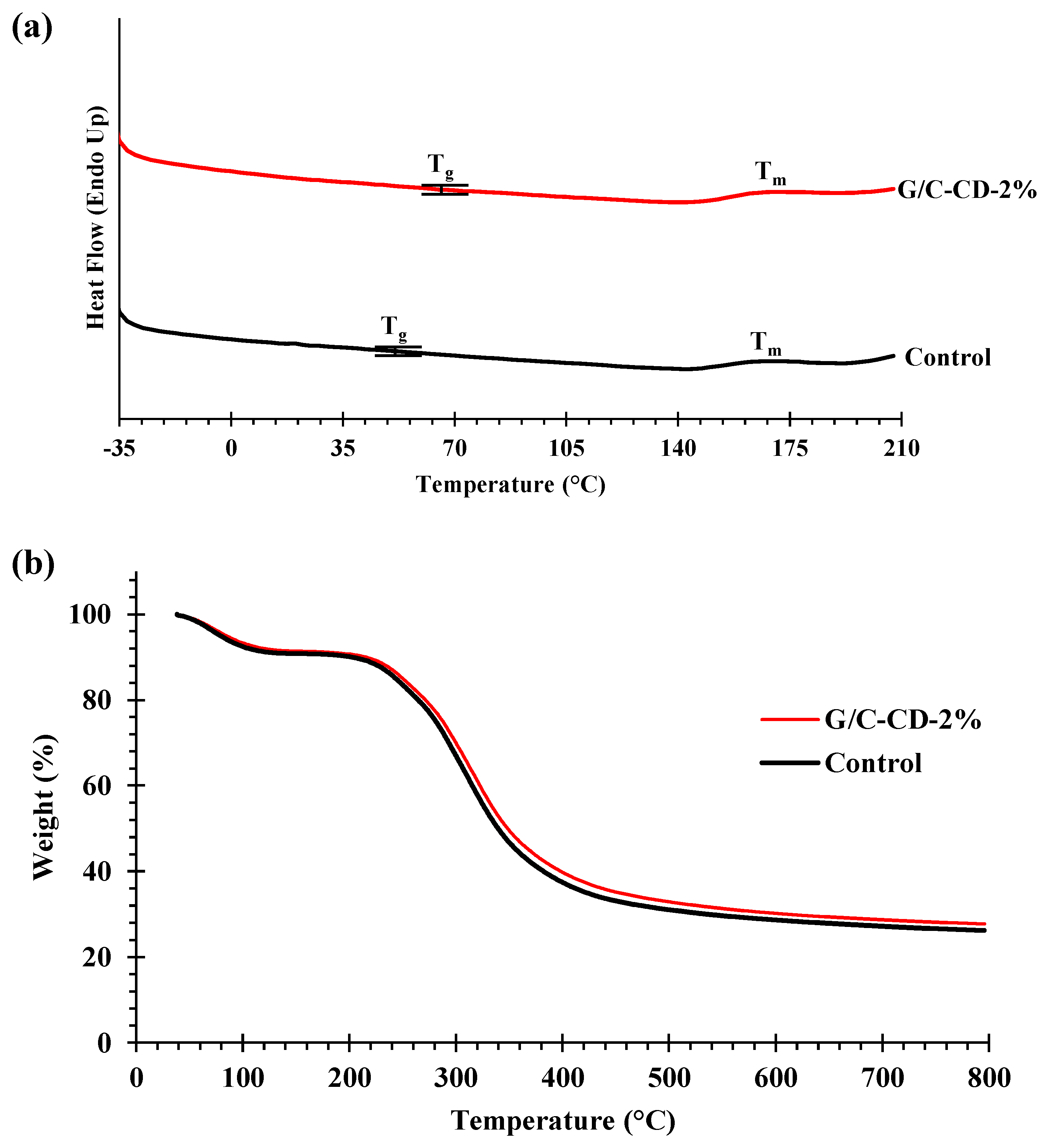
2.2.2. Thermal Properties
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Guava Leaf Powder (GLP) and Carbon Dots (GL-CDs)
3.3. Study on Properties of G/C Blend Film Added with GL-CDs
3.3.1. Appearance and Water Contact Angle
3.3.2. Thickness and Mechanical Properties
3.3.3. Water Vapor Permeability
3.3.4. Color, Light Transmittance, Opaqueness and UV-Blocking Properties
3.3.5. ATR-FTIR Spectra
3.3.6. Release Test
3.3.7. Antioxidant Activities
3.4. Characterization of the Selected G/C Blend Film without and with 2% GL-CDs
3.4.1. Microstructure and Elemental Mapping
3.4.2. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gowthaman, N.S.K.; Lim, H.N.; Sreeraj, T.R.; Amalraj, A.; Gopi, S. Advantages of Biopolymers over Synthetic Polymers. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar]
- Ong, H.-T.; Samsudin, H.; Soto-Valdez, H. Migration of Endocrine-Disrupting Chemicals into Food from Plastic Packaging Materials: An Overview of Chemical Risk Assessment, Techniques to Monitor Migration, and International Regulations. Crit. Rev. Food Sci. Nutr. 2022, 62, 957–979. [Google Scholar] [CrossRef]
- Khan, A.; Ezati, P.; Rhim, J.-W. Chitosan/Gelatin-Based Multifunctional Film Integrated with Green Tea Carbon Dots to Extend the Shelf Life of Pork. Food Packag. Shelf Life 2023, 37, 101075. [Google Scholar] [CrossRef]
- Tagrida, M.; Nilsuwan, K.; Gulzar, S.; Prodpran, T.; Benjakul, S. Fish Gelatin/Chitosan Blend Films Incorporated with Betel (Piper betle L.) Leaf Ethanolic Extracts: Characteristics, Antioxidant and Antimicrobial Properties. Food Hydrocoll. 2023, 137, 108316. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Qi, P.; Sun, J.; Jiang, S.; Li, H.; Gu, X.; Zhang, S. Enhancing the Thermostability, UV Shielding and Antimicrobial Activity of Transparent Chitosan Film by Carbon Quantum Dots Containing N/P. Carbohydr. Polym. 2022, 278, 118957. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, Physicochemical and Biological Evaluation of Quercetin Based Chitosan-Gelatin Film for Food Packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular Interactions in Gelatin/Chitosan Composite Films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and Functional Properties of Fish Gelatin–Chitosan Blend Edible Films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of Plant Extracts on the Techno-Functional Properties of Biodegradable Packaging Films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.; Nur Hanani, Z.A. Storage Stability of Soy Protein Isolate Films Incorporated with Mango Kernel Extract at Different Temperature. Food Hydrocoll. 2019, 87, 541–549. [Google Scholar] [CrossRef]
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S. Advancements in Biodegradable Active Films for Food Packaging: Effects of Nano/Microcapsule Incorporation. Foods 2022, 11, 760. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinho, F.; Sanches Silva, A.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active Polylactic Acid Film Incorporated with Green Tea Extract: Development, Characterization and Effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.B.d.; Costa, K.S.d.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G.d. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Somchaidee, P.; Tedsree, K. Green Synthesis of High Dispersion and Narrow Size Distribution of Zero-Valent Iron Nanoparticles Using Guava Leaf ( Psidium guajava L.) Extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035006. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef] [PubMed]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on Antioxidant Activity of Certain Plants in Thailand: Mechanism of Antioxidant Action of Guava Leaf Extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural Antimicrobial and Antioxidant Compounds for Active Food Packaging Applications. Biomass Convert. Biorefin. 2024, 14, 4419–4440. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Wang, H. Application of Carbon Dots in Food Preservation: A Critical Review for Packaging Enhancers and Food Preservatives. Crit. Rev. Food Sci. Nutr. 2023, 63, 6738–6756. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.-H.; Wei, Q.; Sun, D.-W. Carbon Dots: Principles and Their Applications in Food Quality and Safety Detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Riahi, Z.; Tae Kim, J.; Rhim, J.-W. Carrageenan-Based Multifunctional Packaging Films Containing Zn-Carbon Dots/Anthocyanin Derived from Kohlrabi Peel for Monitoring Quality and Extending the Shelf Life of Shrimps. Food Chem. 2024, 432, 137215. [Google Scholar] [CrossRef]
- Deepika; Kumar, L.; Gaikwad, K.K. Carbon Dots for Food Packaging Applications. Sustain. Food Technol. 2023, 1, 185–199. [Google Scholar] [CrossRef]
- Patil, A.S.; Waghmare, R.D.; Pawar, S.P.; Salunkhe, S.T.; Kolekar, G.B.; Sohn, D.; Gore, A.H. Photophysical Insights of Highly Transparent, Flexible and Re-Emissive PVA @ WTR-CDs Composite Thin Films: A next Generation Food Packaging Material for UV Blocking Applications. J. Photochem. Photobiol. A Chem. 2020, 400, 112647. [Google Scholar] [CrossRef]
- Kousheh, S.A.; Moradi, M.; Tajik, H.; Molaei, R. Preparation of Antimicrobial/Ultraviolet Protective Bacterial Nanocellulose Film with Carbon Dots Synthesized from Lactic Acid Bacteria. Int. J. Biol. Macromol. 2020, 155, 216–225. [Google Scholar] [CrossRef]
- Fu, B.; Liu, Q.; Liu, M.; Chen, X.; Lin, H.; Zheng, Z.; Zhu, J.; Dai, C.; Dong, X.; Yang, D.-P. Carbon Dots Enhanced Gelatin/Chitosan Bio-Nanocomposite Packaging Film for Perishable Foods. Chin. Chem. Lett. 2022, 33, 4577–4582. [Google Scholar] [CrossRef]
- Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Rhim, J.-W. Carrageenan/Alginate-Based Functional Films Incorporated with Allium Sativum Carbon Dots for UV-Barrier Food Packaging. Food Bioprocess Technol. 2023, 16, 2001–2015. [Google Scholar] [CrossRef]
- Khan, A.; Ezati, P.; Rhim, J.-W. Chitosan/Starch-Based Active Packaging Film with N, P-Doped Carbon Dots for Meat Packaging. ACS Appl. Bio Mater. 2023, 6, 1294–1305. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and Water Vapour Transport Properties in Yeast Biomass Based Films: A Study of Plasticizer Content and Thickness Effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.-W. Gelatin/Carrageenan-Based Functional Films with Carbon Dots from Enoki Mushroom for Active Food Packaging Applications. ACS Appl. Polym. Mater. 2021, 3, 6437–6445. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Kerry, J.P. Gelatin Films: Study Review of Barrier Properties and Implications for Future Studies Employing Biopolymer Films. Food Packag. Shelf Life 2021, 29, 100688. [Google Scholar] [CrossRef]
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green Chitosan–Carbon Dots Nanocomposite Hydrogel Film with Superior Properties. Carbohydr. Polym. 2015, 115, 238–245. [Google Scholar] [CrossRef]
- Li, K.-K.; Yin, S.-W.; Yang, X.-Q.; Tang, C.-H.; Wei, Z.-H. Fabrication and Characterization of Novel Antimicrobial Films Derived from Thymol-Loaded Zein–Sodium Caseinate (SC) Nanoparticles. J. Agric. Food Chem. 2012, 60, 11592–11600. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Y.; Li, Y. Development of Tea Extracts and Chitosan Composite Films for Active Packaging Materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Sanyang, M.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Chen, J.; Long, Z.; Wang, S.; Meng, Y.; Zhang, G.; Nie, S. Biodegradable Blends of Graphene Quantum Dots and Thermoplastic Starch with Solid-State Photoluminescent and Conductive Properties. Int. J. Biol. Macromol. 2019, 139, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Mathew, J.; Radhakrishnan, E.K. Polyvinyl Alcohol/Silver Nanocomposite Films Fabricated under the Influence of Solar Radiation as Effective Antimicrobial Food Packaging Material. J. Polym. Res. 2019, 26, 223. [Google Scholar] [CrossRef]
- Duncan, S.E.; Chang, H.-H. Implications of Light Energy on Food Quality and Packaging Selection. Adv. Food Nutr. Res. 2012, 67, 25–73. [Google Scholar]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the Physical Properties and Biological Activity of Chitosan Films Grafted with Gallic Acid and Caffeic Acid: A Comparison Study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, Structural and Antimicrobial Properties of Poly Vinyl Alcohol–Chitosan Biodegradable Films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Iahnke, A.O.e.S.; Stoll, L.; Bellé, A.S.; Hertz, P.F.; Rios, A.d.O.; Rahier, H.; Flôres, S.H. Gelatin Capsule Residue-based Films Crosslinked with the Natural Agent Genipin. Packag. Technol. Sci. 2020, 33, 15–26. [Google Scholar] [CrossRef]
- Son, M.H.; Park, S.W.; Jung, Y.K. Antioxidant and Anti-Aging Carbon Quantum Dots Using Tannic Acid. Nanotechnology 2021, 32, 415102. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of Antioxidant Chitosan Film Incorporated with Zataria Multiflora Boiss Essential Oil and Grape Seed Extract. LWT-Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Murru, C.; Badía-Laíño, R.; Díaz-García, M.E. Synthesis and Characterization of Green Carbon Dots for Scavenging Radical Oxygen Species in Aqueous and Oil Samples. Antioxidants 2020, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Okazaki, S. All-Atomistic Molecular Dynamics Study of the Glass Transition of Amorphous Polymers. Polymer 2022, 254, 125044. [Google Scholar] [CrossRef]
- Nagarajan, M.; Prodpran, T.; Benjakul, S.; Songtipya, P. Properties and Characteristics of Multi-Layered Films from Tilapia Skin Gelatin and Poly(Lactic Acid). Food Biophys. 2017, 12, 222–233. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qian, C.; Kan, J.; Jin, C. Effect of Grafting Method on the Physical Property and Antioxidant Potential of Chitosan Film Functionalized with Gallic Acid. Food Hydrocoll. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-Tocopherol on Physicochemical Properties of Chitosan-Based Films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tajik, S.; Shojaee-Aliabadi, S.; Ghasemlou, M.; Moayyed, H.; Khaksar, R.; Noghabi, M.S. Development of New Active Packaging Film Made from a Soluble Soybean Polysaccharide Incorporated Zataria Multiflora Boiss and Mentha Pulegium Essential Oils. Food Chem. 2014, 146, 614–622. [Google Scholar] [CrossRef]
- Koutchma, T.; Popović, V.; Ros-Polski, V.; Popielarz, A. Effects of Ultraviolet Light and High-Pressure Processing on Quality and Health-Related Constituents of Fresh Juice Products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 844–867. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Three-layer Sheets Based on Gelatin and Poly(Lactic Acid), Part 1: Preparation and Properties. J. Appl. Polym. Sci. 2010, 118, 3102–3110. [Google Scholar] [CrossRef]
- Benjakul, S.; Karnjanapratum, S. Characteristics and Nutritional Value of Whole Wheat Cracker Fortified with Tuna Bone Bio-Calcium Powder. Food Chem. 2018, 259, 181–187. [Google Scholar] [CrossRef]



| Film Samples | Thickness (mm) | YM (MPa) | TS (MPa) | EAB (%) |
|---|---|---|---|---|
| Control | 0.033 ± 0.001 d | 485.67 ± 14.22 c | 26.92 ± 0.73 a | 14.89 ± 0.09 a |
| G/C-CD-1% | 0.037 ± 0.002 c | 542.00 ± 61.61 bc | 22.16 ± 1.41 b | 14.06 ± 1.36 a |
| G/C-CD-2% | 0.039 ± 0.001 b | 586.00 ± 9.54 b | 20.25 ± 0.39 c | 12.08 ± 1.30 b |
| G/C-CD-3% | 0.041 ± 0.002 a | 759.00 ± 2.00 a | 17.77 ± 0.14 d | 5.48 ± 0.73 c |
| Film Samples | WVP ** (×10−11 g·m·m−2·s−1·Pa−1) | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|
| Control | 1.01 ± 0.03 a | 89.34 ± 0.12 a | −1.95 ± 0.04 b | −0.34 ± 0.07 d | 2.38 ± 0.12 d |
| G/C-CD-1% | 0.72 ± 0.11 b | 82.27 ± 1.64 b | −1.87 ± 0.24 b | 10.11 ± 0.97 c | 14.65 ± 1.79 c |
| G/C-CD-2% | 0.67 ± 0.03 b | 79.18 ± 0.90 c | −1.46 ± 0.11 a | 17.33 ± 0.87 b | 22.3 ± 1.21 b |
| G/C-CD-3% | 0.65 ± 0.09 b | 76.11 ± 0.80 d | −1.39 ± 0.29 a | 26.48 ± 1.82 a | 31.7 ± 1.98 a |
| Film Samples | Light Transmittance (%) at Different Wavelengths (nm) | Opaqueness Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 700 | 800 | ||
| Control | 0.04 ± 0.01 a | 50.34 ± 0.79 a | 77.46 ± 2.07 a | 80.50 ± 1.85 a | 83.91 ± 1.51 a | 85.3 ± 1.27 a | 86.25 ± 1.08 a | 86.95 ± 0.90 a | 2.11 ± 0.16 b |
| G/C-CD-1% | 0.02 ± 0.02 ab | 12.88 ± 0.54 b | 46.98 ± 1.72 b | 61.78 ± 2.20 b | 77.08 ± 2.22 b | 81.42 ± 2.05 b | 84.38 ± 1.75 a | 85.84 ± 1.55 a | 2.45 ± 0.28 b |
| G/C-CD-2% | 0.01 ± 0.01 b | 4.10 ± 0.07 c | 28.87 ± 0.03 c | 47.42 ± 0.06 c | 69.58 ± 0.30 c | 76.77 ± 0.30 c | 81.26 ± 0.30 b | 83.48 ± 0.29 b | 2.99 ± 0.05 a |
| G/C-CD-3% | 0.01 ± 0.01 b | 0.82 ± 0.44 d | 14.41 ± 2.70 d | 32.01 ± 3.29 d | 63.65 ± 1.80 d | 73.84 ± 0.92 d | 79.70 ± 0.40 b | 82.40 ± 0.31 b | 3.20 ± 0.19 a |
| Film Samples | DPPH-RS-A (µmol TE/100 g Sample) | ABTS-RS-A (µmol TE/100 g Sample) | FRA-P (µmol TE/100 g Sample) | MC-A (µmol EE/100 g Sample) |
|---|---|---|---|---|
| Control | 14.42 ± 1.54 d | 62.00 ± 0.50 d | ND | ND |
| G/C-CD-1% | 87.67 ± 2.14 c | 196.50 ± 14.73 c | 60.41 ± 8.86 c | 29.08 ± 0.34 c |
| G/C-CD-2% | 95.95 ± 0.93 b | 358.50 ± 25.86 b | 186.97 ± 11.59 b | 33.96 ± 2.52 b |
| G/C-CD-3% | 99.87 ± 0.46 a | 567.50 ± 2.00 a | 312.03 ± 2.96 a | 51.34 ± 2.69 a |
| Film Samples | Tg | Tm | Δ1 | Δ2 | Δ3 | Δ4 | Residue | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Td1, Onset | Δw1 | Td2, Onset | Δw2 | Td3, Onset | Δw3 | Td4, Onset | Δw4 | ||||
| Control | 52.47 | 168.17 | 54.99 | 9.20 | 223.33 | 9.77 | 312.09 | 52.24 | 622.14 | 2.61 | 26.18 |
| G/C-CD-2% | 64.97 | 170.17 | 51.54 | 8.60 | 221.49 | 9.06 | 308.69 | 51.34 | 613.29 | 3.31 | 27.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugan, G.; Nilsuwan, K.; Prodpran, T.; Ponnusamy, A.; Rhim, J.-W.; Kim, J.T.; Benjakul, S. Active Fish Gelatin/Chitosan Blend Film Incorporated with Guava Leaf Powder Carbon Dots: Properties, Release and Antioxidant Activity. Gels 2024, 10, 281. https://doi.org/10.3390/gels10040281
Murugan G, Nilsuwan K, Prodpran T, Ponnusamy A, Rhim J-W, Kim JT, Benjakul S. Active Fish Gelatin/Chitosan Blend Film Incorporated with Guava Leaf Powder Carbon Dots: Properties, Release and Antioxidant Activity. Gels. 2024; 10(4):281. https://doi.org/10.3390/gels10040281
Chicago/Turabian StyleMurugan, Gokulprasanth, Krisana Nilsuwan, Thummanoon Prodpran, Arunachalasivamani Ponnusamy, Jong-Whan Rhim, Jun Tae Kim, and Soottawat Benjakul. 2024. "Active Fish Gelatin/Chitosan Blend Film Incorporated with Guava Leaf Powder Carbon Dots: Properties, Release and Antioxidant Activity" Gels 10, no. 4: 281. https://doi.org/10.3390/gels10040281