A Review of High-Temperature Aerogels: Composition, Mechanisms, and Properties
Abstract
:1. Introduction
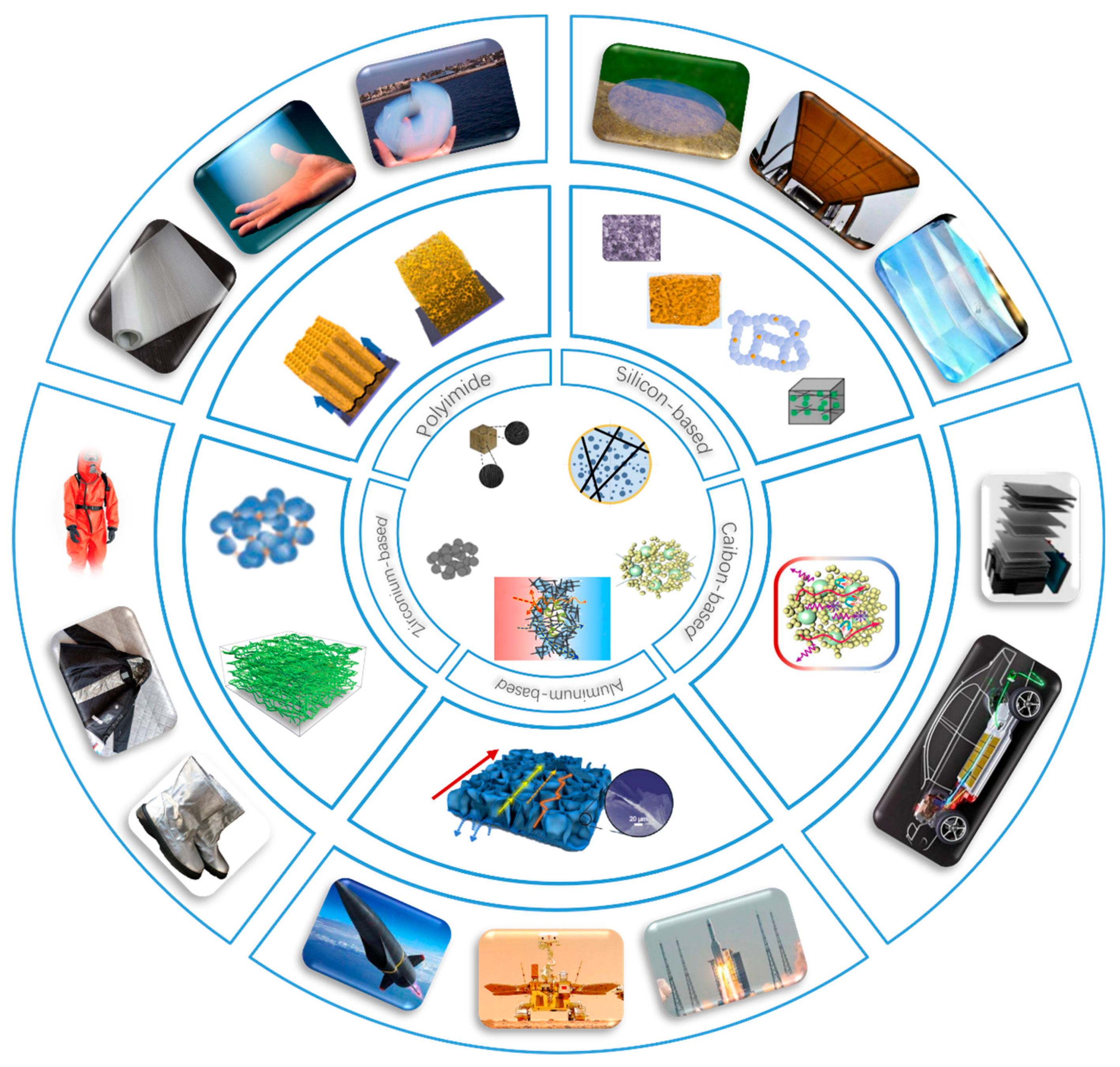
2. Classification and Characteristics of Thermal Insulation Materials
2.1. Silica-Based Aerogels
2.1.1. Silica Aerogels
2.1.2. Silica-Based Composite Aerogels
2.1.3. Nanofiber Composite Aerogels
2.2. Carbon-Based Aerogels
2.2.1. Carbon-Based Aerogels with Doping Elements
2.2.2. Carbon Fiber Aerogels
2.2.3. Graphene Aerogels
2.3. Alumina-Based Aerogels
2.3.1. Alumina Aerogels
2.3.2. Alumina–Silica Aerogels
2.3.3. Other Alumina-Based Aerogels
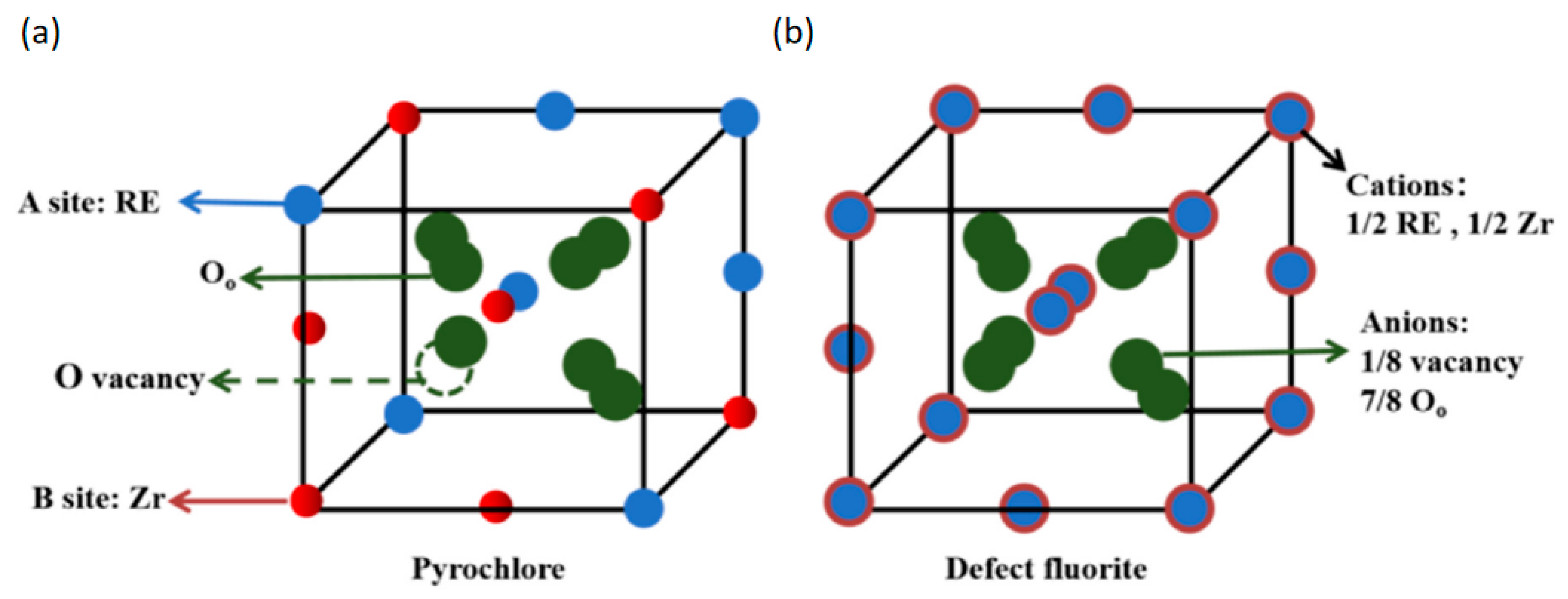
2.4. Zirconia-Based Aerogels
2.5. Polyimide Aerogels
3. Summary and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J. Advances on Dimensional Structure Designs and Functional Applications of Aerogels. Acta Chim. Sin. 2021, 79, 430–442. [Google Scholar] [CrossRef]
- Teresa, L. Silica aerogel composites with embedded fibres: A review on their preparation, properties and applications. J. Mater. Chem. A 2021, 79, 22768–22802. [Google Scholar]
- Sambucci, M.; Savoni, F.; Valente, M. Aerogel technology for thermal insulation of cryogenic tanks—Numerical analysis for comparison with traditional insulating materials. Gels 2023, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Jong-Hoon, L.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar]
- Feng, J. A facile method to fabricate monolithic alumina–silica aerogels with high surface areas and good mechanical properties. J. Eur. Ceram. Soc. 2020, 40, 2480–2488. [Google Scholar]
- Asim, N.; Badiei, M.; Alghoul, M.A. Biomass and industrial wastes as resource materials for aerogel preparation: Opportunities, challenges, and research directions. Ind. Eng. Chem. Res. 2019, 58, 17621–17645. [Google Scholar] [CrossRef]
- Schmidt, M.; Schwertfeger, F. Applications for silica aerogel products. J. Non-Cryst. Solids 1998, 225, 364–368. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Li, N. Heat insulating, fire retardant and flexible inorganic nanocomposite paper. Mater. Des. 2018, 144, 281–289. [Google Scholar] [CrossRef]
- Guo, J.; Fu, S.; Deng, Y. Hypocrystalline ceramic aerogels for thermal insulation at extreme conditions. Nature 2022, 606, 909–916. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Feng, J. Preparation of silica aerogels with high temperature resistance and low thermal conductivity by mono-dispersed silica sol. Mater. Des. 2020, 191, 108640. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhao, S.Y.; Yang, M. Structural characteristics and thermal conductivity of ambient pressure dried silica aerogels with one-step solvent exchange/surface modification. Mater. Chem. Phys. 2009, 113, 485–490. [Google Scholar] [CrossRef]
- Buscarino, G.; Ardizzone, V.; Vaccaro, G. Sintering process of amorphous SiO2 nanoparticles investigated by AFM, IR and Raman techniques. J. Non-Cryst. Solids 2011, 357, 1866–1870. [Google Scholar] [CrossRef]
- Okafor, P.E.; Tang, G. Study of effective thermal conductivity of a novel SiO2 aerogel composite for high-temperature thermal in sulation. Int. J. Heat Mass Transf. 2023, 212, 124242. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Kong, Y. Facile preparation of nano-SiO2 composites with excellent high-temperature thermal insulation performance. Ceram. Int. 2022, 48, 27486–27492. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wang, Y. Flexible, high-temperature-resistant silica-polymer aerogel hybrids by templating polymthylsilsesquioxane microstructure with trace polyimide. Adv. Compos. Hybrid Mater. 2023, 6, 32. [Google Scholar] [CrossRef]
- Fan, Q.; Ou, R.; Hao, X. Water-induced self-assembly and in situ mineralization within plant phenolic glycol-gel toward ultras-trong and multifunctional thermal insulating aerogels. ACS Nano 2022, 16, 9062–9076. [Google Scholar] [CrossRef]
- Yao, J.; Gao, X.; Wu, Y. High-temperature resistant ambient pressure-dried aluminum doped silica aerogel from inorganic silicon and aluminum sources. Ceram. Int. 2022, 48, 15006–15016. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, H.; Hou, X. High-temperature resistant Y2SiO5-TiO2 aerogel composite for efficient thermal insulation. J. Porous Mater. 2020, 28, 57–64. [Google Scholar] [CrossRef]
- Gu, H.; Hou, X. Novel high-temperature-resistant Y2SiO5 aerogel with ultralow thermal conductivity. Int. J. Appl. Ceram. Technol. 2019, 16, 2393–2397. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, W.; Wang, H.; Yan, X. In situ formation of the TiCN phase in SiBCN ceramic aerogels enabling superior thermal and structural stability up to 1800 °C. ACS Appl. Mater. Interfaces 2023, 15, 12221–12231. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Si, Y. All-ceramic and elastic aerogels with nanofibrous-granular binary synergistic structure for thermal su-perinsulation. ACS Nano 2022, 16, 5487–5495. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, H.; Niu, M. Anisotropic and hierarchical SiC@SiO2 nanowire aerogel with exceptional stiffness and stability for thermal superinsulation. Sci. Adv. 2020, 6, eaay6689. [Google Scholar] [CrossRef]
- Su, L.; Wang, H.; Niu, M. Ultralight, recoverable, and high-temperature-resistant SiC nanowire aerogel. ACS Nano 2018, 12, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, F.; Chen, Y. Multifunctional SiC@SiO2 nanofiber aerogel with ultrabroadband electromagnetic wave absorption. Nanomicro. Lett. 2022, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, J.; Li, H. Directional fabricating of flexible and compressible cellulose nanofibril composite cryogel with excellent thermal insulation, flame-retardancy and radiative cooling for efficient thermal management. Cellulose 2022, 29, 9671–9691. [Google Scholar] [CrossRef]
- Zhou, T.; Cheng, X.; Pan, Y. Mechanical performance and thermal stability of glass fiber reinforced silica aerogel composites based on co-precursor method by freeze drying. Appl. Surf. Sci. 2018, 437, 321–328. [Google Scholar] [CrossRef]
- Markevicius, G.; Ladj, R.; Niemeyer, P. Ambient-dried thermal superinsulating monolithic silica-based aerogels with short cellulo-sic fibers. J. Mater. Sci. 2016, 52, 2210–2221. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, L.; Yang, M. Optimization of ultralight SiO2/TiO2 nanofibrous aerogel for high-temperature application. Ceram. Int. 2023, 49, 38058–38069. [Google Scholar] [CrossRef]
- Yang, M. Flexible electrospun strawberry-like structure SiO2 aerogel nanofibers for thermal insulation. Ceram. Int. 2023, 49, 9165–9172. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, C. Carbon Aerogel Composites Prepared by Ambient Drying and Using Oxidized Polyacrylonitrile Fibers as Rein-forcements. ACS Appl. Mater. Interfaces 2011, 3, 4796–4803. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Huang, Y.-S. The mechanical properties and delamination of carbon fiber-reinforced polymer laminates modified with carbon aerogel. J. Mater. Sci. 2016, 52, 3520–3534. [Google Scholar] [CrossRef]
- Li, X.; Feng, J. Preparation and properties of PAN-based carbon fiber-reinforced SiCO aerogel composites. Ceram. Int. 2019, 45, 17064–17072. [Google Scholar] [CrossRef]
- Yun, Y.; Tang, B.; Wang, X. Ultratough cellular films from graphene oxide hydrogel: A way to exploit rigidity and flexibility of two-dimensional honeycomb carbon. Carbon 2016, 107, 548–556. [Google Scholar]
- Cheng, H.; Fan, Z.; Hong, C.; Zhang, X. Lightweight multiscale hybrid carbon-quartz fiber fabric reinforced phenolic-silica aerogel nanocomposite for high temperature thermal protection. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106313. [Google Scholar] [CrossRef]
- Su, R.; Wang, X.; Wang, D.; Li, L.; Liang, G.; Zheng, Z.; Li, K. Preparation of carbon foam-reinforced carbon aerogels and their copyrolysis mechanism. Microporous Mesoporous Mater. 2021, 319, 111059–111067. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Jiang, Y. Anti-oxidation performance of carbon aerogel composites with SiCO ceramic inner coating. Ceram. Int. 2019, 45, 9704–9711. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Pan, R.; Wu, C.; Yan, X.; Wang, H.; Zhang, X. Multiscale, elastic, and low-density carbon fibre/siliconoxycar-bide-phenolic interpenetrating aerogel nanocomposite for ablative thermal protection. Compos. Part B Eng. 2022, 245, 110212–110223. [Google Scholar]
- Li, J.; Guo, P.; Hu, C.; Pang, S.; Ma, J.; Zhao, R.; Tang, S. Fabrication of Large Aerogel-Like Carbon/Carbon Composites with Ex-cellent Load-Bearing Capacity and Thermal-Insulating Performance at 1800 °C. ACS Nano 2022, 16, 6565–6577. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, C.; Jiang, Y.; Yang, Y.; Peng, F.; Liu, L.; Men, J.; Feng, J.; Li, L.; Tang, G.; et al. Carbon layer encapsulation strategy for designing multifunctional core-shell nanorod aerogels as high-temperature thermal superinsulators. Chem. Eng. J. 2023, 455, 140502–140515. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Liu, H.; Wang, T. “Finger coral-like” ceramic fiber aerogels with enhanced high-temperature thermal insulation, anti-oxidation, and mechanical performance. Compos. Sci. Technol. 2022, 225, 109515–109523. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Liu, H.; Feng, Z.; Zhang, B.; Wei, D.; Liao, X.; Han, R. A novel carbon-based fiber aerogel with interfacial ther-mal resistance: Temperature insulation, oxidation resistance, and mechanical performance. Ceram. Int. 2023, 49, 13698–13707. [Google Scholar] [CrossRef]
- Du, J.; Zhang, H.; Ming, W.; He, W.; Ma, J.; Cao, Y.; Liu, K. A review on machining of carbon fiber reinforced ceramic matrix composites. Ceram. Int. 2019, 45, 18155–18166. [Google Scholar] [CrossRef]
- Li, J.; Zhu, C.; Zhao, Z.; Liu, X. Fire properties of carbon fiber reinforced polymer improved by coating nonwoven flame retardant mat for aerospace application. J. Appl. Polym. Sci. 2019, 136, 47801–47815. [Google Scholar] [CrossRef]
- Wada, T. Application of Glass Fiber and Carbon Fiber-Reinforced Thermoplastics in Face Guards. Polymers 2020, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, T.; Liu, S.; Liu, K.; Cao, Z.; Pang, W.; Jiang, H. Preparation of high-temperature resistant aluminum-doped silica aerogel from aluminum sol source by ambient pressure drying. J. Sol-Gel Sci. Technol. 2023, 109, 162–173. [Google Scholar] [CrossRef]
- Wu, C.; Wang, H.; Pan, Y.; Huang, H.; Fan, J.; Zhang, X. Synergistic reinforcement and multiscaled design of lightweight heat pro-tection and insulation integrated composite with outstanding high-temperature resistance up to 2500 °C. Compos. Sci. Technol. 2023, 232, 109878–109888. [Google Scholar]
- Tan, C.; Cao, J.; Yang, G. High-performance tin oxide-nitrogen doped graphene aerogel hybrids as anode materials for lithium-ion batteries. J. Power Sources 2014, 270, 28–33. [Google Scholar] [CrossRef]
- Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar]
- Xie, Y.; Han, M.; Wang, R.; Deng, X.; Zhang, P.; Wang, X. Graphene Aerogel Based Bolometer for Ultrasensitive Sensing from Ul-traviolet to Far-Infrared. ACS Nano 2019, 13, 5385–5396. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, S.; Hu, P.; Zhao, G.; Li, Y.; Zhang, X.; Han, W. Enhanced mechanical, thermal, and electric properties of gra-phene aerogels via supercritical ethanol drying and high-temperature thermal reduction. Sci. Rep. 2017, 7, 1439–1450. [Google Scholar] [CrossRef]
- Feng, J. Hydrothermal assisted synthesis of heat resistant, well-crystallized aerogels constructed by boehmite nano rods. Ceram. Int. 2022, 48, 16232–16240. [Google Scholar]
- Shen, J. Highly thermally stable alumina-based aerogels modified by partially hydrolyzed aluminum tri-sec-butoxide. J. Sol-Gel Sci. Technol. 2017, 84, 507–514. [Google Scholar]
- Artem, E.L. Investigation of alumina aerogel structural characteristics at different «precursor-water-ethanol» ratio. J. Non-Cryst. Solids 2021, 553, 120475. [Google Scholar]
- Shen, J. Preparation and characterization of monolithic alumina aerogels. J. Non-Cryst. Solids 2011, 357, 2903–2906. [Google Scholar]
- Feng, J. Dual template strategy to prepare ultralight and high-temperature resistant ceramic nanorod aerogels for efficient thermal insulation. Ceram. Int. 2023, 49, 22677–22689. [Google Scholar]
- Chen, D.R. Alpha Al2O3 Nanosheet-Based Biphasic Aerogels with High-Temperature Resistance up to 1600 °C. ACS Appl. Mate-Rials Interfaces 2023, 15, 6848–6858. [Google Scholar]
- Zhang, H. Insulating and Robust Ceramic Nanorod Aerogels with High-Temperature Resistance over 1400 °C. ACS Appl. Mater. Interfaces 2021, 13, 20548–20558. [Google Scholar] [CrossRef]
- Guo, A.R. Ultralight, thermal insulating, and high-temperature-resistant mullite-based nanofibrous aerogels. Chem. Eng. J. 2019, 360, 464–472. [Google Scholar]
- Feng, J. Thermally insulating, fiber-reinforced alumina–silica aerogel composites with ultra-low shrinkage up to 1500 °C. Chem. Eng. J. 2021, 411, 6684–6702. [Google Scholar]
- Feng, J. Novel silica-modified boehmite aerogels and fiber-reinforced insulation composites with ultra-high thermal stability and low thermal conductivity. J. Eur. Ceram. Soc. 2022, 42, 6684–6702. [Google Scholar]
- Shen, J. Opacifier embedded and fiber reinforced alumina-based aerogel composites for ultra-high temperature thermal insulation. Ceram. Int. 2019, 45, 644–650. [Google Scholar]
- Fan, J.P. Synthesis of Al2O3-SiO2 aerogels with low thermal conductivity and high strength by methyltriethoxysilane as a silica pre-cursor. J. Sol-Gel Sci. Technol. 2023, 108, 35–46. [Google Scholar]
- Shen, J. A Facile Method to Fabricate Al2O3-SiO2 Aerogels with Low Shrinkage up to 1200 °C. Molecules 2023, 28, 2743. [Google Scholar] [CrossRef] [PubMed]
- Hou, F. Super-insulated, flexible, and high resilient mullite fiber reinforced silica aerogel composites by interfacial modification with nanoscale mullite whisker. Compos. Part B Eng. 2022, 230, 109549. [Google Scholar]
- Shen, J. A Facile Method for Fabricating a Monolithic Mullite Fiber-Reinforced Alumina Aerogel with Excellent Mechanical and Thermal Properties. Gels 2022, 8, 380. [Google Scholar] [CrossRef]
- Miao, Y. Synthesis of Al2O3-SiO2 aerogel from water glass with high thermal stability and low thermal conductivity. J. Sol-Gel Sci. Technol. 2023, 106, 561–571. [Google Scholar]
- Xu, X.B. Facile synthesis of mullite fiber/alumina composite aerogel with enhanced bending strength and controllable thermal insu-lation performance. J. Sol-Gel Sci. Technol. 2022, 104, 311–318. [Google Scholar]
- Ma, C. Al2O3-SiO2 aerogel reinforced with aluminum silicate nanofibers: A strategy to preserve the properties of Al2O3-SiO2 aerogel. J. Sol-Gel Sci. Technol. 2023, 109, 523–533. [Google Scholar]
- Feng, J. Fiber-reinforced alumina-carbon core-shell aerogel composite with heat-induced gradient structure for thermal protection up to 1800 °C. Chem. Eng. J. 2023, 461, 141721. [Google Scholar]
- Wang, B. Improvement of thermal insulation and compressive performance of Al2O3–SiO2 aerogel by doping carbon nanotubes. Ceram. Int. 2022, 48, 16290–16299. [Google Scholar]
- Shen, J. Organic/inorganic double-precursor cross-linked alumina aerogel with high specific surface area and high-temperature re-sistance. Ceram. Int. 2022, 48, 17261–17269. [Google Scholar]
- Ding, B. Multiphase ceramic nanofibers with super-elasticity from −196–1600 °C. Nano Today 2022, 44, 101455–101465. [Google Scholar]
- Zhang, L. Improved catalytic activity on the thermal decomposition of ammonium perchlorate and efficient adsorption of uranium using a novel ultra-low density Al2O3-based aerogels. J. Hazard. Mater. 2020, 387, 122015–122027. [Google Scholar]
- Zhao, C. Ultra-small sepiolite fiber toughened alumina aerogel with enhanced thermal stability and machinability. J. Porous Mater. 2020, 27, 1535–1546. [Google Scholar]
- Ding, B. Ultrastrong, Superelastic, and Lamellar Multiarch Structured ZrO2–Al2O3 Nanofibrous Aerogels with High-Temperature Resistance over 1300 °C. ACS Nano 2020, 14, 15616–15625. [Google Scholar]
- Liu, S. Preparation and thermal insulation performance of Al2O3-Y2O3-SiO2 ternary composite aerogels with high specific surface area and low density. SSRN 2021, 630, 122872. [Google Scholar]
- Cui, S. Rational design of a novel mullite aerogel with extremely high mechanical strength and anti-oxidation behavior for ad-vanced thermal protection in extreme environments. J. Eur. Ceram. Soc. 2024, 44, 1761–1771. [Google Scholar]
- Wang, J. Design of Economical and Achievable Aluminum Carbon Composite Aerogel for Efficient Thermal Protection of Aero-space. Gels 2022, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liu, D.; Deng, Y. Carbonaceous ceramic nanofibrous aerogels for high-temperature thermal superinsulation. Nano Res. 2022, 16, 5047–5055. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Zhou, F. A novel high-entropy (Sm0.2Eu0.2Tb0.2Dy0.2Lu0.2)2Zr2O7 ceramic aerogel with ultralow thermal conduc-tivity. Ceram. Int. 2021, 47, 29960–29968. [Google Scholar] [CrossRef]
- Ren, S.; Liu, K.; Wang, K. ZrC/C aerogel with high compressive strength by a carbothermic process. J. Eur. Ceram. Soc. 2021, 41, 4710–4719. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z. Synthesis of high-temperature resistant monolithic zirconia-based aerogel via facile water glass assisted sol–gel method. J. Sol-Gel Sci. Technol. 2018, 85, 567–573. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X. Elastic and highly fatigue resistant ZrO2-SiO2 nanofibrous aerogel with low energy dissipation for thermal in-sulation. Chem. Eng. J. 2022, 433, 133628. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y. A three-dimensional network modifier (dimethyldiethoxysilane) makes ZrO2-SiO2 aerogel with excellent thermal insulation performance and high-temperature stability. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131716. [Google Scholar] [CrossRef]
- Mao, L.; Sun, X. A new strategy to obtain thin ZrO2–Al2O3 composite aerogel coating with prominent high–temperature resistance and rapid heat dissipation. J. Solid State Chem. 2022, 314, 123384. [Google Scholar]
- Jia, C.; Liu, Y. A foldable all-ceramic air filter paper with high efficiency and high-temperature resistance. Nano Lett. 2020, 20, 4993–5000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Y.; Shao, Z.; Gao, W.; Fan, W.; Liu, T.; Bai, H. Multifunctional polyimide aerogel textile inspired by polar bear hair for thermoregulation in extreme environments. Chem. Eng. J. 2020, 390, 124623–124631. [Google Scholar] [CrossRef]
- Lee, D.H.; Jo, M.J.; Han, S.W.; Yu, S.; Park, H. Polyimide aerogel with controlled porosity: Solvent-induced synergistic pore development during solvent exchange process. Polymer 2020, 205, 122879. [Google Scholar] [CrossRef]
- Wu, S.; Du, A.; Xiang, Y.; Liu, M.; Li, T.; Shen, J.; Zhang, Z.; Li, C.; Zhou, B. Silica-aerogel-powders “jammed” polyimide aero-gels with excellent hydrophobicity and conversion to ultra-light polyimide aerogel. RSC Adv. 2016, 6, 58268–58278. [Google Scholar] [CrossRef]
- Wu, T.; Dong, J.; Xu, G.; Zhao, X.; Zhang, Q. Attapulgite-reinforced polyimide hybrid aerogels with high dimensional stability and excellent thermal insulation property. Polymer 2019, 176, 196–205. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Y.; Zhang, L.; Gao, W.; Huang, Y.; Liu, T. Graphene/montmorillonite hybrid synergistically reinforced polyimide composite aerogels with enhanced flame-retardant performance. Compos. Sci. Technol. 2017, 139, 57–63. [Google Scholar]
- Kotek, R. A review on aerogel: 3D nanoporous structured fillers in polymer-based nanocomposites. Polym. Compos. 2017, 39, 3383–3408. [Google Scholar]
- Saadatnia, Z. A High Performance Triboelectric Nanogenerator Using Porous Polyimide Aerogel Film. Sci. Rep. 2019, 9, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Ding, Y.; Lin, Z.; Wang, C.; Li, Y.; Xu, F. Lightweight, Superelastic and Mechanically Flexible Graphene/Polyimide Nanocomposite Foam for Strain Sensor Application. ACS Nano 2023, 9, 8933–8941. [Google Scholar]
- Wang, K.L. Advanced polyimide materials: Syntheses, physical properties and applications. Progress Polym. Sci. 2012, 37, 907–974. [Google Scholar]
- Kantor, Z. Heterogeneous silica-polyimide aerogel-in-aerogel nanocomposites. Chem. Eng. J. 2022, 443, 136401–136412. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Cong, B.; Zhou, H.; Zhao, X.; Chen, C.; Wang, D.; Liu, C.; Qu, C. Anisotropic all-aromatic polyimide aerogels with robust and high-temperature stable properties for flexible thermal protection. Chem. Eng. J. 2022, 431, 134047–134057. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Bai, L.; Xu, H.; Qin, S.; Li, Y.; Zhang, G. A Review of High-Temperature Aerogels: Composition, Mechanisms, and Properties. Gels 2024, 10, 286. https://doi.org/10.3390/gels10050286
Wang C, Bai L, Xu H, Qin S, Li Y, Zhang G. A Review of High-Temperature Aerogels: Composition, Mechanisms, and Properties. Gels. 2024; 10(5):286. https://doi.org/10.3390/gels10050286
Chicago/Turabian StyleWang, Conghui, Letian Bai, Hongxin Xu, Shengjian Qin, Yanfang Li, and Guanglei Zhang. 2024. "A Review of High-Temperature Aerogels: Composition, Mechanisms, and Properties" Gels 10, no. 5: 286. https://doi.org/10.3390/gels10050286




