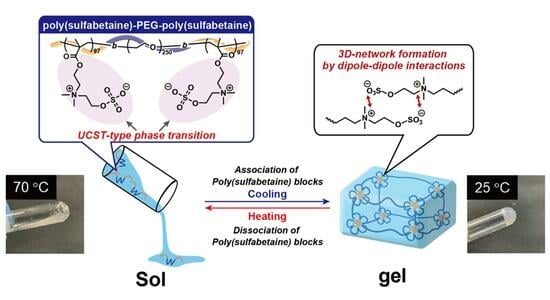
UCST-Type Thermoresponsive Sol–Gel Transition Triblock Copolymer Containing Zwitterionic Polymer Blocks
Abstract
:1. Introduction
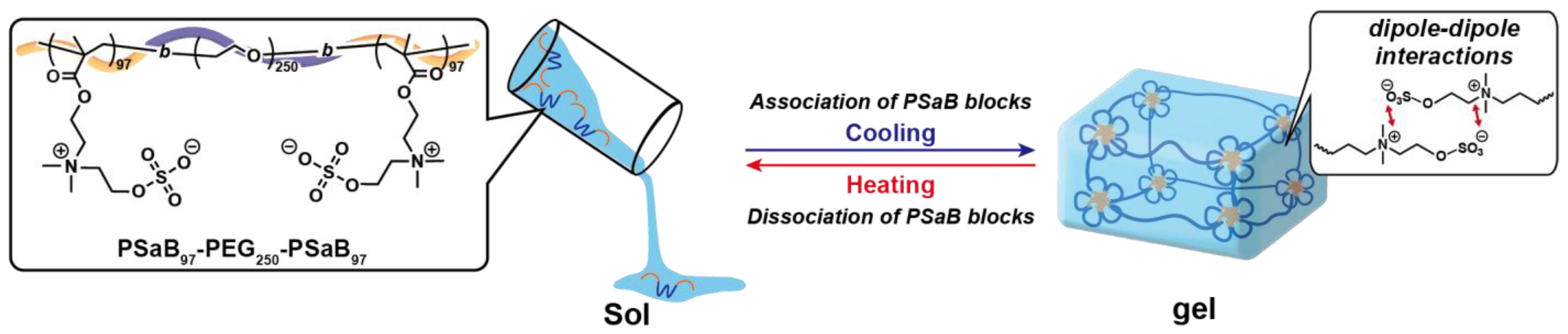
2. Results and Discussion
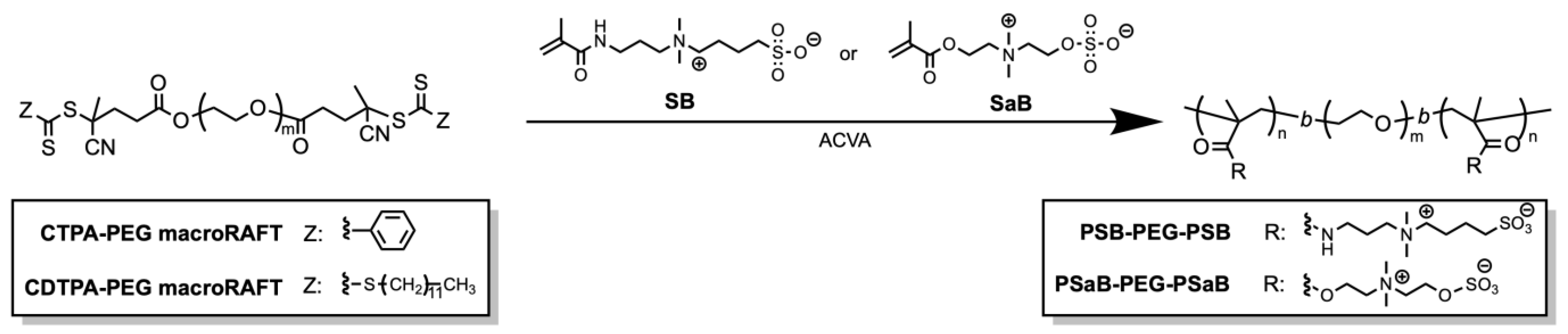
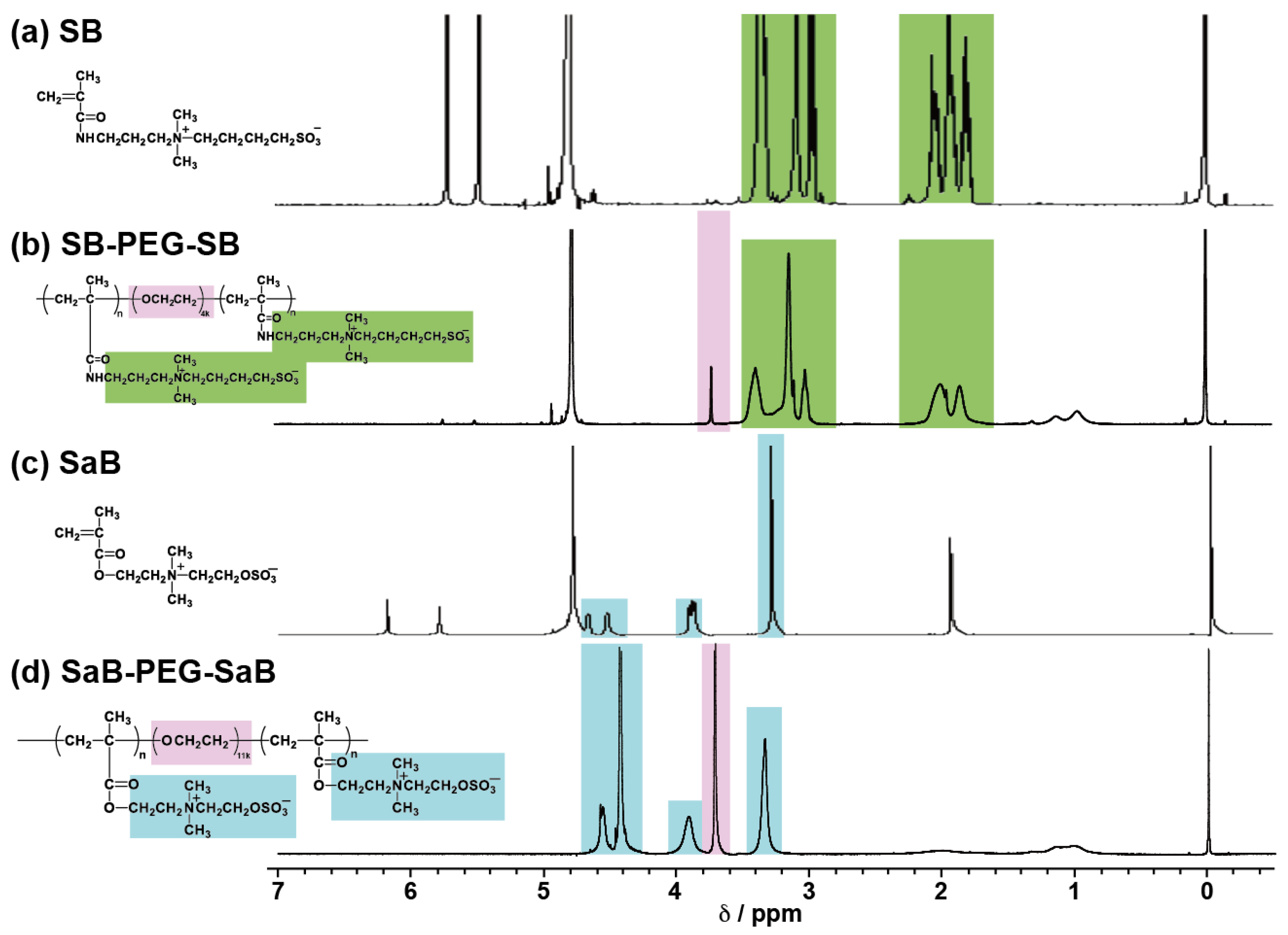
2.1. Synthesis of Triblock Copolymer Containing Zwitterionic Polymer Blocks
2.2. Thermoresponsive Behavior of Aqueous Triblock Copolymer Solutions under Dilute Conditions
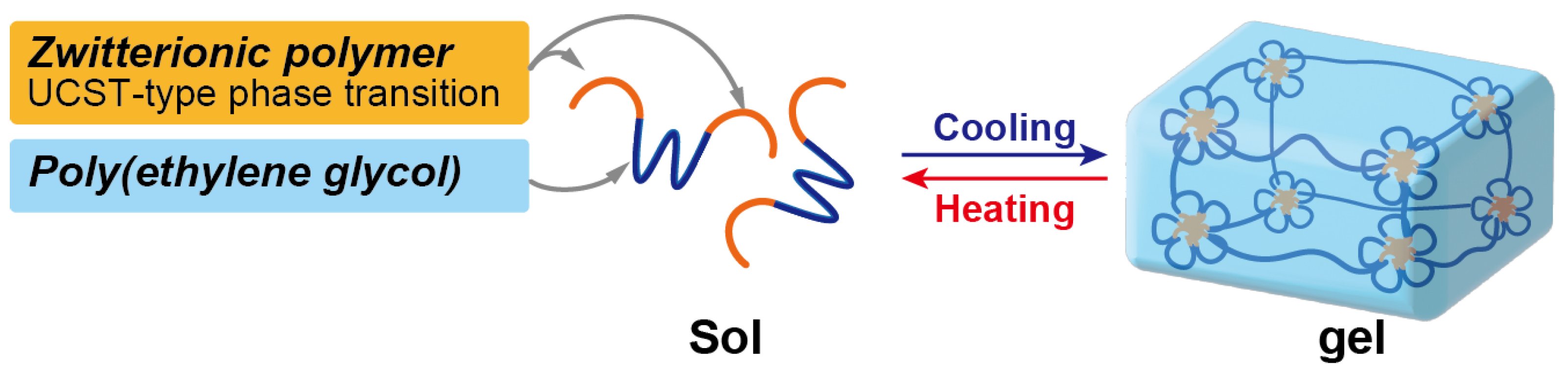
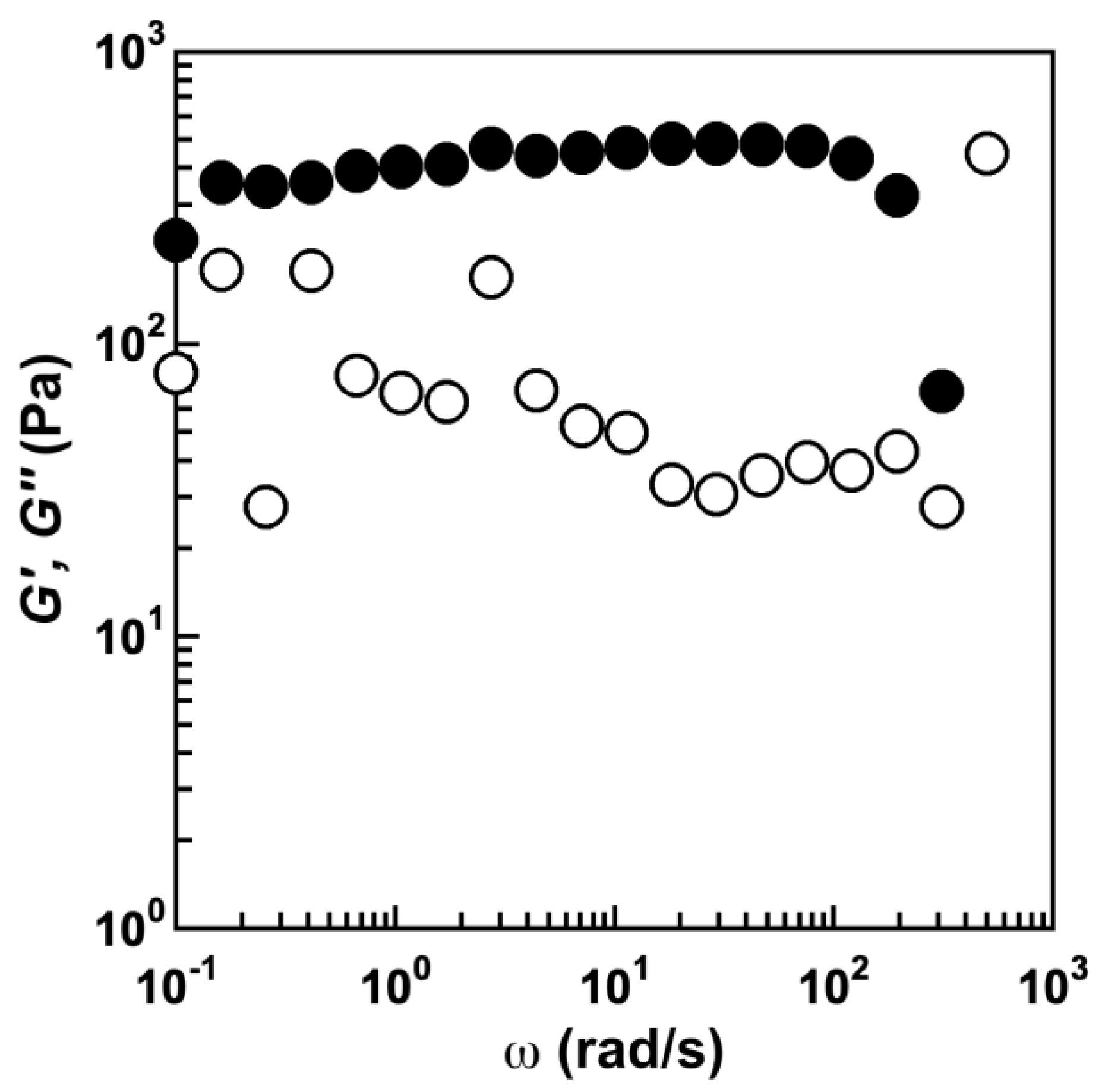
2.3. Thermoresponsive Behavior of Triblock Copolymers under Concentrated Conditions
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of PEG macroRAFT Agents
4.3. Synthesis of 4-((3-methacrylamidopropyl)dimethylammonio) butane-1-sulfonate (SB)
4.4. Synthesis of 3-{[2-(methacryloyloxy)ethyl]dimethylammonio}ethylene-1-sulfate (SaB)
4.5. Synthesis of Triblock Copolymers
4.6. Phase Transition Temperature Measurements
4.7. Rheological Measurement of the PSaB-PEG-PSaB Gel
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Moon, H.J.; Ko, D.Y.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.K.; Stevens, M.M. Tailoring Gelation Mechanisms for Advanced Hydrogel Applications. Adv. Funct. Mater. 2020, 30, 2002759. [Google Scholar] [CrossRef]
- Okihara, M.; Okuma, K.; Kawamura, A.; Miyata, T. Photoresponsive Gelation of Four-Armed Poly(ethylene glycol) with Photodimerizable Groups. Gels 2022, 8, 183. [Google Scholar] [CrossRef]
- Norioka, C.; Okita, K.; Mukada, M.; Kawamura, A.; Miyata, T. Biomolecularly stimuli-responsive tetra-poly(ethylene glycol) that undergoes sol–gel transition in response to a target biomolecule. Polym. Chem. 2017, 8, 6378–6385. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Thermoreversible Gelation of PEG−PLGA−PEG Triblock Copolymer Aqueous Solutions. Macromolecules 1999, 32, 7064–7069. [Google Scholar] [CrossRef]
- Nagahama, K.; Takahashi, A.; Ohya, Y. Biodegradable polymers exhibiting temperature-responsive sol–gel transition as injectable biomedical materials. React. Funct. Polym. 2013, 73, 979–985. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef]
- He, Y.; Lodge, T.P. A thermoreversible ion gel by triblock copolymer self-assembly in an ionic liquid. Chem. Commun. 2007, 26, 2732–2734. [Google Scholar] [CrossRef]
- Halperin, A.; Kroger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Tymetska, S.; Shymborska, Y.; Stetsyshyn, Y.; Budkowski, A.; Bernasik, A.; Awsiuk, K.; Donchak, V.; Raczkowska, J. Thermoresponsive Smart Copolymer Coatings Based on P(NIPAM-co-HEMA) and P(OEGMA-co-HEMA) Brushes for Regenerative Medicine. ACS Biomater. Sci. Eng. 2023, 9, 6256–6272. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Contreras, B.A.; Schmalz, H.; Agarwal, S. pH dependent thermoresponsive behavior of acrylamide–acrylonitrile UCST-type copolymers in aqueous media. Polym. Chem. 2016, 7, 1979–1986. [Google Scholar] [CrossRef]
- Asadujjaman, A.; Kent, B.; Bertin, A. Phase transition and aggregation behaviour of an UCST-type copolymer poly(acrylamide-co-acrylonitrile) in water: Effect of acrylonitrile content, concentration in solution, copolymer chain length and presence of electrolyte. Soft Matter 2017, 13, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Käfer, F.; Lerch, A.; Agarwal, S. Tunable, concentration-independent, sharp, hysteresis-free UCST phase transition from poly(N-acryloyl glycinamide-acrylonitrile) system. J. Polym. Sci. Part A Polym. Chem. 2016, 55, 274–279. [Google Scholar] [CrossRef]
- Tian, Y.; Lai, J.; Li, C.; Sun, J.; Liu, K.; Zhao, C.; Zhang, M. Poly(N-acryloyl glycinamide-co-N-acryloxysuccinimide) Nanoparticles: Tunable Thermo-Responsiveness and Improved Bio-Interfacial Adhesion for Cell Function Regulation. ACS Appl. Mater. Interfaces 2023, 15, 7867–7877. [Google Scholar] [CrossRef]
- Shimada, N.; Ino, H.; Maie, K.; Nakayama, M.; Kano, A.; Maruyama, A. Ureido-derivatized polymers based on both poly(allylurea) and poly(L-citrulline) exhibit UCST-type phase transition behavior under physiologically relevant conditions. Biomacromolecules 2011, 12, 3418–3422. [Google Scholar] [CrossRef]
- Shimada, N.; Sasaki, T.; Kawano, T.; Maruyama, A. Rational Design of UCST-type Ureido Copolymers Based on a Hydrophobic Parameter. Biomacromolecules 2018, 19, 4133–4138. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Niu, C.H.; Sasaki, T.; Nomura, S.; Maruyama, A.; Shimada, N. Smart Protein Refolding System Based on UCST-Type Ureido Polymers. Biomacromolecules 2022, 23, 3860–3865. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.N.; Peiffer, D.G.; Agarwal, P.K.; Larabee, J.; Kaladas, J.J.; Soni, L.; Handwerker, B.; Garner, R.T. Phase behaviour and solution properties of sulphobetaine polymers. Polymer 1986, 27, 1734–1742. [Google Scholar] [CrossRef]
- Hildebrand, V.; Laschewsky, A.; Wischerhoff, E. Modulating the solubility of zwitterionic poly((3-methacrylamidopropyl)ammonioalkane sulfonate)s in water and aqueous salt solutions via the spacer group separating the cationic and the anionic moieties. Polym. Chem. 2016, 7, 731–740. [Google Scholar] [CrossRef]
- Hildebrand, V.; Laschewsky, A.; Päch, M.; Müller-Buschbaum, P.; Papadakis, C.M. Effect of the zwitterion structure on the thermo-responsive behaviour of poly(sulfobetaine methacrylates). Polym. Chem. 2017, 8, 310–322. [Google Scholar] [CrossRef]
- Santa Chalarca, C.F.; Emrick, T. Reactive polymer zwitterions: Sulfonium sulfonates. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 83–92. [Google Scholar] [CrossRef]
- Wang, N.; Seymour, B.T.; Lewoczko, E.M.; Kent, E.W.; Chen, M.-L.; Wang, J.-H.; Zhao, B. Zwitterionic poly(sulfobetaine methacrylate)s in water: From upper critical solution temperature (UCST) to lower critical solution temperature (LCST) with increasing length of one alkyl substituent on the nitrogen atom. Polym. Chem. 2018, 9, 5257–5261. [Google Scholar] [CrossRef]
- Ishihara, K.; Fukazawa, K. 2-Methacryloyloxyethyl PhosphorylCholine (MPC) Polymers. In Phosphorus-Based Polymers: From Synthesis to Applications; Monge, S., David, G., Eds.; RSC Polymer Chemistry Series; The Royal Society of Chemistry: London, UK, 2014; pp. 68–96. [Google Scholar]
- Ishihara, K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J. Biomed. Mater. Res. A 2019, 107, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Fukazawa, K. Cell-membrane-inspired polymers for constructing biointerfaces with efficient molecular recognition. J. Mater. Chem. B 2022, 10, 3397–3419. [Google Scholar] [CrossRef]
- Miyata, T.; Namera, T.; Liu, Y.; Kawamura, A.; Yamaoka, T. Photoresponsive behaviour of zwitterionic polymer particles with photodimerizable groups on their surfaces. J. Mater. Chem. B 2022, 10, 2637–2648. [Google Scholar] [CrossRef]
- Nakaura, H.; Kawamura, A.; Miyata, T. Reductively Responsive Gel Capsules Prepared Using a Water-Soluble Zwitterionic Block Copolymer Emulsifier. Langmuir 2019, 35, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Sasaoka, M.; Kawamura, A.; Miyata, T. Core–shell microgels having zwitterionic hydrogel core and temperature-responsive shell prepared via inverse miniemulsion RAFT polymerization. Polym. Chem. 2022, 13, 3489–3497. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, B. Thermoreversible physically crosslinked hydrogels from UCST-type thermosensitive ABA linear triblock copolymers. Polym. Chem. 2016, 7, 6980–6991. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Vasantha, V.A.; Jana, S.; Parthiban, A.; Vancso, J.G. Halophilic polysulfabetaines—Synthesis and study of gelation and thermoresponsive behavior. RSC Adv. 2014, 4, 22596–22600. [Google Scholar] [CrossRef]
- Vasantha, V.A.; Junhui, C.; Ying, T.B.; Parthiban, A. Salt-Responsive Polysulfabetaines from Acrylate and Acrylamide Precursors: Robust Stabilization of Metal Nanoparticles in Hyposalinity and Hypersalinity. Langmuir 2015, 31, 11124–11134. [Google Scholar] [CrossRef] [PubMed]
- Nizardo, N.M.; Schanzenbach, D.; Schonemann, E.; Laschewsky, A. Exploring Poly(ethylene glycol)-Polyzwitterion Diblock Copolymers as Biocompatible Smart Macrosurfactants Featuring UCST-Phase Behavior in Normal Saline Solution. Polymers 2018, 10, 325. [Google Scholar] [CrossRef]
- Morimoto, N.; Yamamoto, M. Design of an LCST-UCST-Like Thermoresponsive Zwitterionic Copolymer. Langmuir 2021, 37, 3261–3269. [Google Scholar] [CrossRef]




| PEG Block | Zwitterionic Polymer Block | Triblock Copolymer | |||
|---|---|---|---|---|---|
| Mn (×103) a) | Dp a) | Mn (×103) b) | Dp b) | Mn (×103) b) | |
| PSB140-PEG91-PSB140 | 3.9 | 91 | 42.9 | 140 | 89.8 |
| PSB96-PEG250-PSB96 | 11.2 | 250 | 29.4 | 96 | 69.8 |
| PSaB148-PEG91-PSaB148 | 3.9 | 91 | 45.4 | 148 | 94.7 |
| PSaB97-PEG250-PSaB97 | 11.2 | 250 | 29.7 | 97 | 70.4 |
| In Water | In PBS(−) | |||
|---|---|---|---|---|
| CPLCST (°C) | CPUCST (°C) | CPLCST (°C) | CPUCST (°C) | |
| PSB140-PEG91-PSB140 | – | 43.2 | n.d. a) | n.d. a) |
| PSB96-PEG250-PSB96 | 29.4 | 44.0 | n.d. a) | n.d. a) |
| PSaB148-PEG91-PSaB148 | n.d. b) | n.d. b) | – | 73.8 |
| PSaB97-PEG250-PSaB97 | n.d. a) | n.d. a) | 53.4 | 73.1 |
| Dp of PEG | PEG | RAFT Agent | DCC | DMAP | DCM | Temp. | ||
|---|---|---|---|---|---|---|---|---|
| CTPA | CDTPA | |||||||
| CDTPA-PEG macroRAFT | 91 | 3.00 g (0.77 mmol) | 0.49 g (1.2 mmol) | 0.32 g (1.5 mmol) | 0.04 g (0.32 mmol) | 25 mL | r.t. | |
| CTPA-PEG macroRAFT | 250 | 2.50 g (0.22 mmol) | 0.28 g (1.0 mmol) | 0.21 g (1.0 mmol) | 0.02 g (0.16 mmol) | 13 mL | 40 °C | |
| Zwitterionic Monomer | macroRAFT Agent | ACVA | TFE | Time | |||
|---|---|---|---|---|---|---|---|
| SB | SaB | ||||||
| PSB140-PEG91-PSB140 | 2.16 g (7.0 mmol) | – | 0.12 g (25 μmol) | (CDTPA-PEG91 macroRAFT) | 2.0 mg (7.1 μmol) | 3.6 mL | 8 h |
| PSB96-PEG250-PSB96 | 0.58 g (1.9 mmol) | – | 0.050 g (4.1 μmol) | (CTPA-PEG250 macroRAFT) | 0.46 mg (1.6 μmol) | 1.5 mL | 8 h |
| PSaB148-PEG91-PSaB148 | – | 1.28 g (4.6 mmol) | 0.30 g (64 μmol) | (CDTPA-PEG91 macroRAFT) | 5.2 mg (18 μmol) | 2.2 mL | 6 h |
| PSaB97-PEG250-PSaB97 | – | 1.07 g (3.8 mmol) | 0.20 g (16 μmol) | (CTPA-PEG250 macroRAFT) | 2.1 mg (7.5 μmol) | 3.0 mL | 6 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamura, A.; Takahashi, R.; Miyata, T. UCST-Type Thermoresponsive Sol–Gel Transition Triblock Copolymer Containing Zwitterionic Polymer Blocks. Gels 2024, 10, 288. https://doi.org/10.3390/gels10050288
Kawamura A, Takahashi R, Miyata T. UCST-Type Thermoresponsive Sol–Gel Transition Triblock Copolymer Containing Zwitterionic Polymer Blocks. Gels. 2024; 10(5):288. https://doi.org/10.3390/gels10050288
Chicago/Turabian StyleKawamura, Akifumi, Ryogo Takahashi, and Takashi Miyata. 2024. "UCST-Type Thermoresponsive Sol–Gel Transition Triblock Copolymer Containing Zwitterionic Polymer Blocks" Gels 10, no. 5: 288. https://doi.org/10.3390/gels10050288