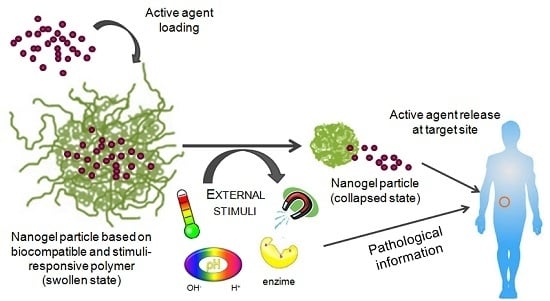
The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy
Abstract
:1. Introduction
2. Approaches for the Production of Stimuli-Responsive Nanogels
2.1. Stimuli-Responsive Nanogels
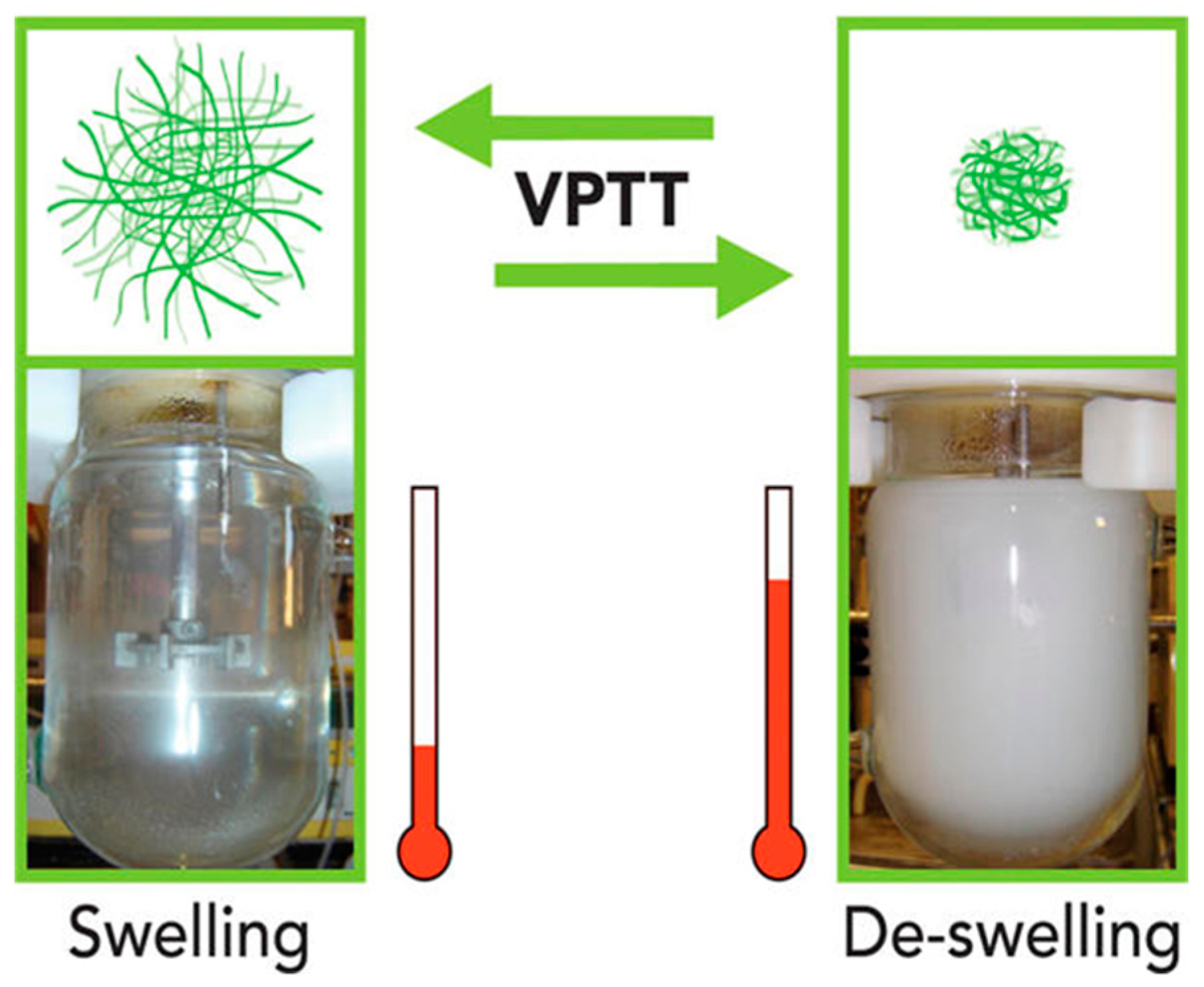
2.1.1. Thermo-Responsive Nanogels
2.1.2. pH-Responsive Nanogels
2.1.3. Light Responsive Nanogels
2.1.4. Magnetic Nanogels
2.2. Targeted Nanogels
2.3. Multi-Responsive Nanogels
3. Technological Aspects of Nanogels
3.1. Encapsulation Efficiency and Drug Loading
3.2.Sterilization of Nanogels
4. Biocompatibility and Biodegradability in Nanogels
4.1. Cytocompatibility Assays in Nanogels
4.2. Biocompatibility Studies in Animals
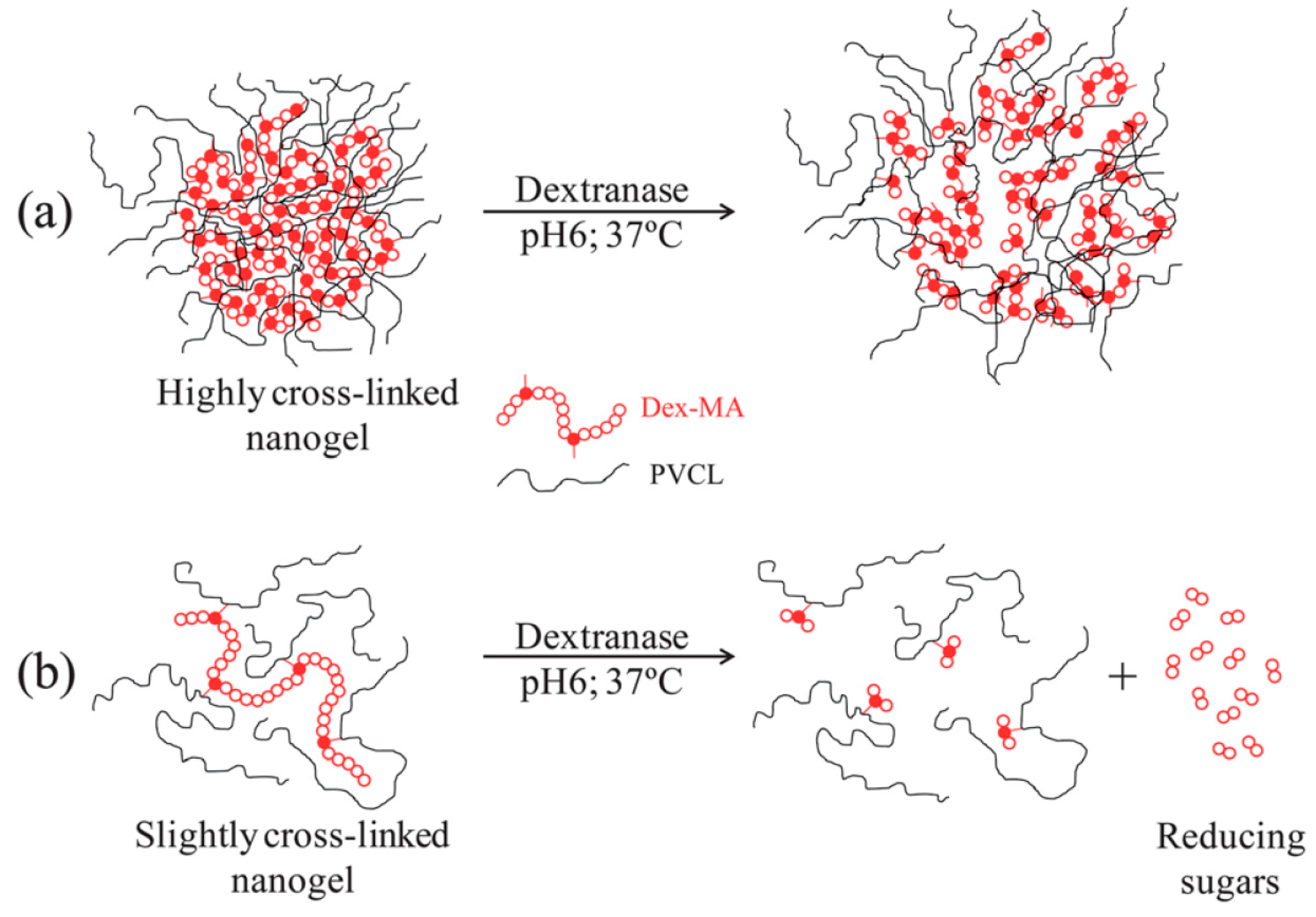
4.3. Biodegradation Mechanisms in Nanogels
5. Biotechnological Uses of Nanogels
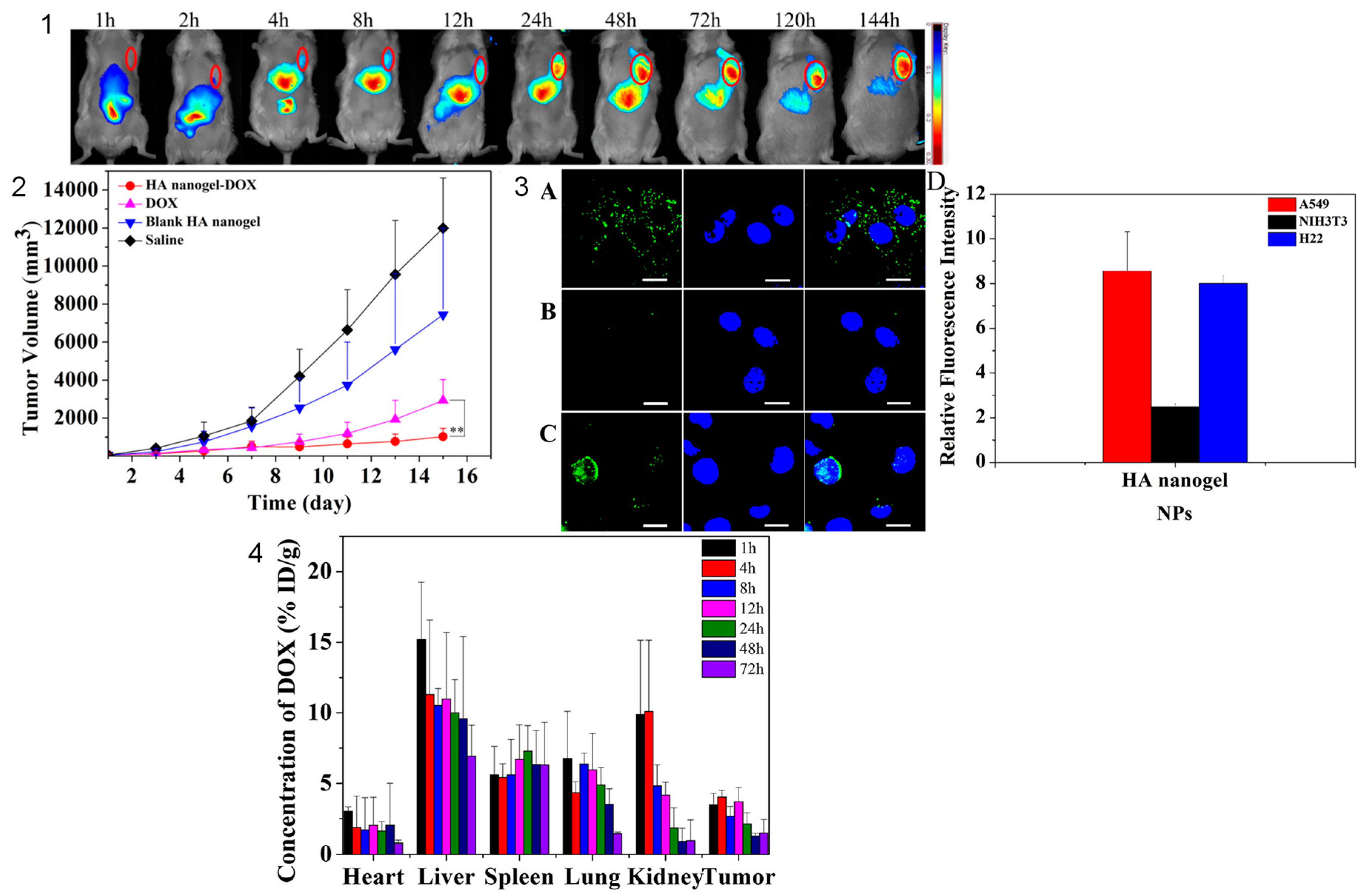
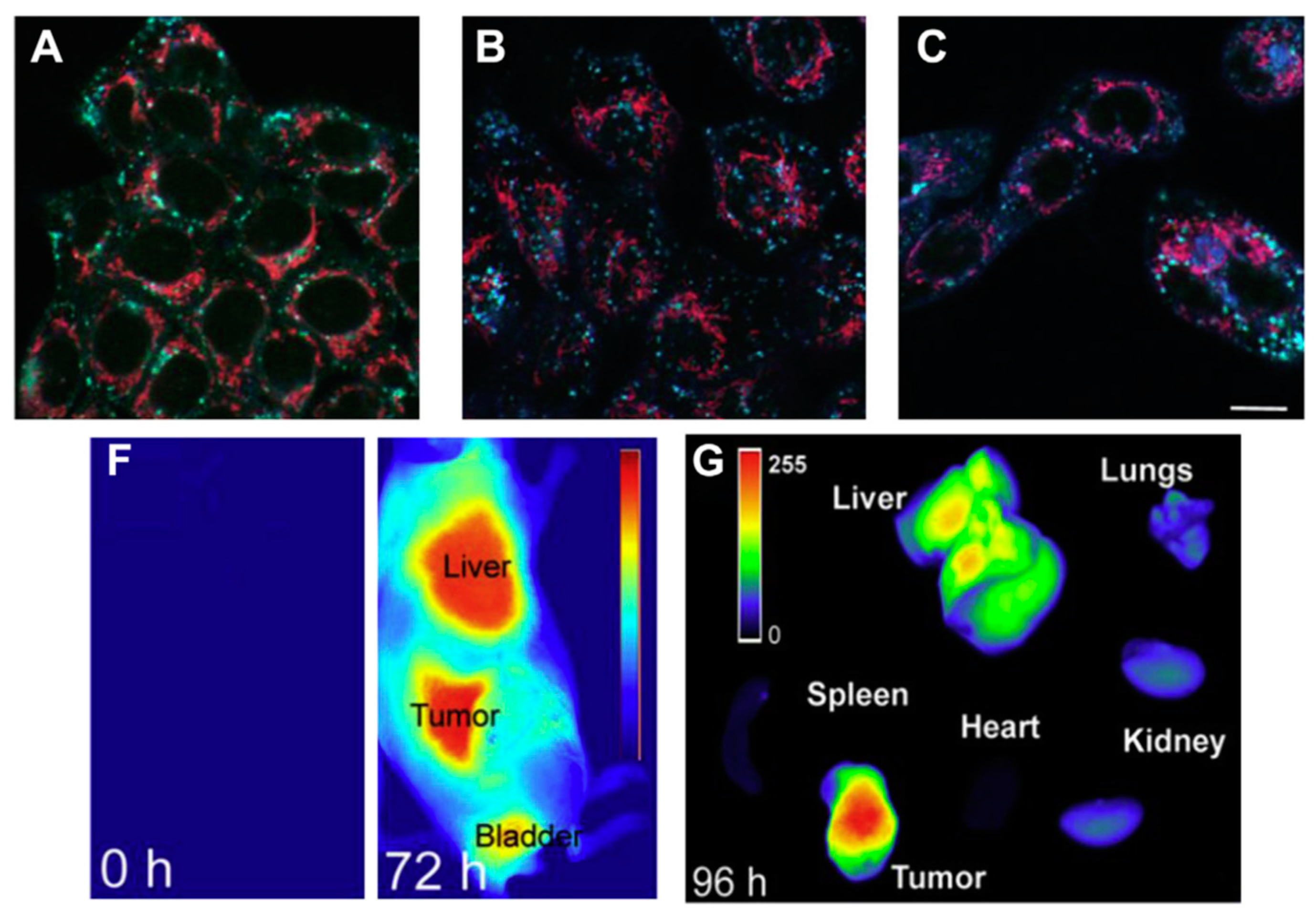
5.1. Nanogels for Cancer Therapy
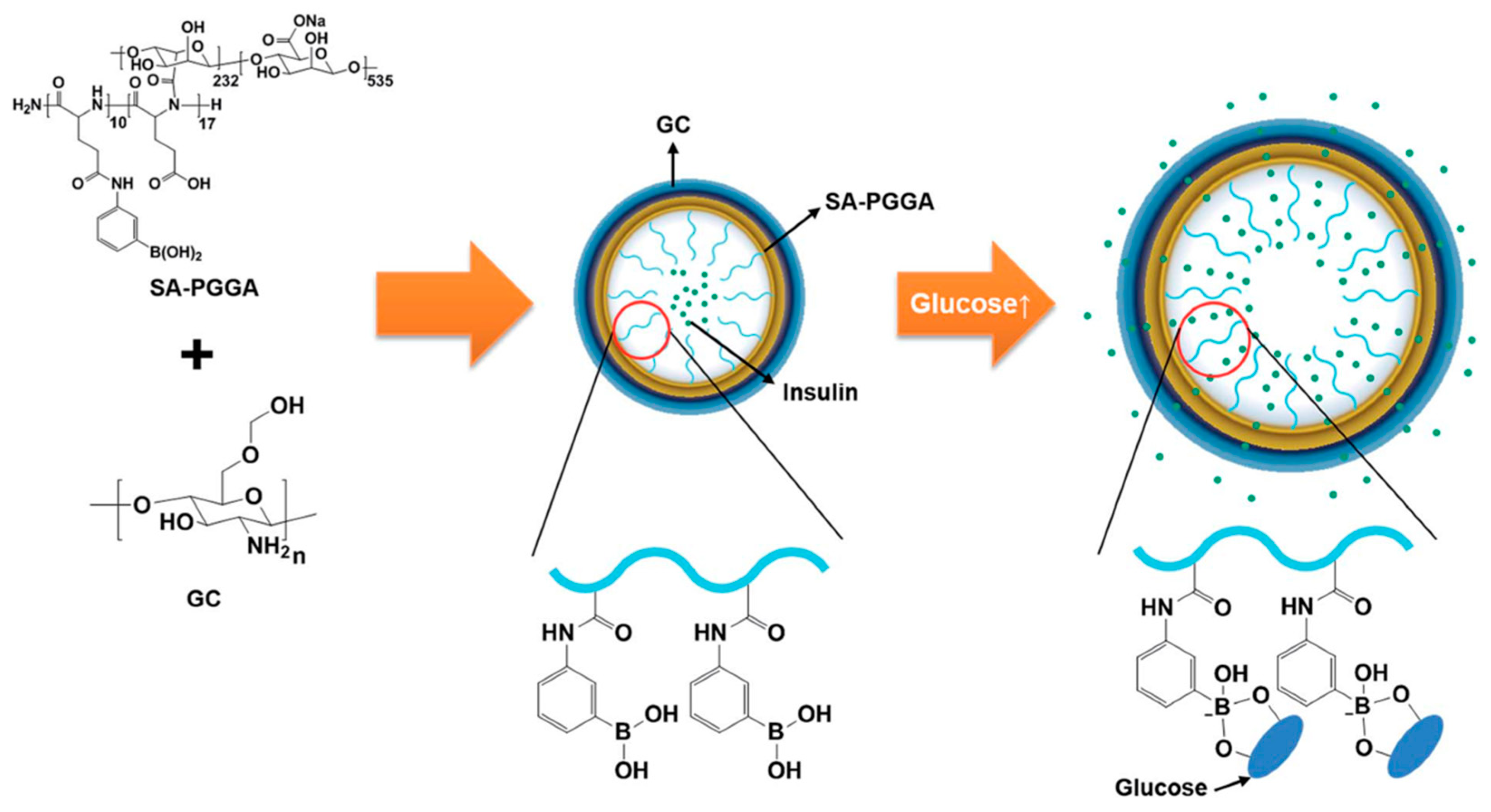
5.2. Nanogels for Chronic Diseases
5.3. Nanogels for Neurodegenerative Diseases
5.4. Nanogels and Tissue Engineering
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ramos, J.; Forcada, J.; Hidalgo-Alvarez, R. Cationic polymer nanoparticles and nanogels: From synthesis to biotechnological applications. Chem. Rev. 2014, 114, 367–428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2013, 5, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Controll. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® abi-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Controll. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Vinogradov, S.V. Colloidal microgels in drug delivery applications. Curr. Pharm. Des. 2006, 12, 4703–4712. [Google Scholar] [CrossRef] [PubMed]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Oishi, M.; Nagasaki, Y. Stimuli-responsive smart nanogels for cancer diagnostics and therapy. Nanomedicine 2010, 5, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Kitano, S.; Kageyama, S.; Nagata, Y.; Miyahara, Y.; Hiasa, A.; Naota, H.; Okumura, S.; Imai, H.; Shiraishi, T.; Masuya, M.; et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin. Cancer Res. 2006, 12, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Kitano, S.; Hirayama, M.; Nagata, Y.; Imai, H.; Shiraishi, T.; Akiyoshi, K.; Scott, A.M.; Murphy, R.; Hoffman, E.W.; et al. Humoral immune responses in patients vaccinated with 1–146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 2008, 99, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Nochi, T.; Yuki, Y.; Takahashi, H.; Sawada, S.-I.; Mejima, M.; Kohda, T.; Harada, N.; Kong, I.G.; Sato, A.; Kataoka, N.; et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010, 9, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V. Nanogels in the race for drug delivery. Nanomedicine 2010, 5, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Ichinohe, S.; Tamura, A.; Ikeda, Y.; Nagasaki, Y. In vitro and in vivo characteristics of core–shell type nanogel particles: Optimization of core cross-linking density and surface poly(ethylene glycol) density in PEGylated nanogels. Acta Biomater. 2011, 7, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Gran, M.L.; Peppas, N.A. Targeted nanodelivery of drugs and diagnostics. Nano Today 2010, 5, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Imaz, A.; Forcada, J. Temperature-sensitive nanogels: Poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide). Polym. Chem. 2012, 3, 852–856. [Google Scholar] [CrossRef]
- Gao, W.-W.; Chan, J.M.; Farokhzad, O.C. pH-responsive nanoparticles for drug delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Banik, B.; Alexis, F. Stimulus responsive nanogels for drug delivery. Soft Matter 2011, 7, 5908–5916. [Google Scholar] [CrossRef]
- Imaz, A.; Forcada, J. Synthesis strategies to incorporate acrylic acid into N-vinylcaprolactam-based microgels. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3218–3227. [Google Scholar] [CrossRef]
- Ramos, J.; Imaz, A.; Callejas-Fernandez, J.; Barbosa-Barros, L.; Estelrich, J.; Quesada-Perez, M.; Forcada, J. Soft nanoparticles (thermo-responsive nanogels and bicelles) with biotechnological applications: From synthesis to simulation through colloidal characterization. Soft Matter 2011, 7, 5067–5082. [Google Scholar] [CrossRef]
- Pikabea, A.; Ramos, J.; Forcada, J. Production of cationic nanogels with potential use in controlled drug delivery. Part. Part. Syst. Charact. 2014, 31, 101–109. [Google Scholar] [CrossRef]
- Aguirre, G.; Villar-Alvarez, E.; González, A.; Ramos, J.; Taboada, P.; Forcada, J. Biocompatible stimuli-responsive nanogels for controlled antitumor drug delivery. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1694–1705. [Google Scholar] [CrossRef]
- Montanari, E.; De Rugeriis, M.; Di Meo, C.; Censi, R.; Coviello, T.; Alhaique, F.; Matricardi, P. One-step formation and sterilization of gellan and hyaluronan nanohydrogels using autoclave. J. Mater. Sci. Mater. Med. 2015, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Makuuchi, K.; Cheng, S. Radiation Processing of Polymer Materials and Its Industrial Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2012; p. 444. [Google Scholar]
- Ulański, P.; Janik, I.; Rosiak, J.M. Radiation formation of polymeric nanogels. Radiat. Phys. Chem. 1998, 52, 289–294. [Google Scholar] [CrossRef]
- Ulanski, P.; Rosiak, J.M. The use of radiation technique in the synthesis of polymeric nanogels. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 356–360. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.-Y.; Wei, H.; Sun, Y.-X.; Cheng, S.-X.; Shen, K.; Gu, Z.-W.; Zhang, X.-Z.; Zhuo, R.-X. Polyethyleneimine modified biocompatible poly(N-isopropylacrylamide)-based nanogels for drug delivery. J. Nanosci. Nanotechnol. 2008, 8, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Beija, M.; Marty, J.-D.; Destarac, M. Thermoresponsive poly(N-vinyl caprolactam)-coated gold nanoparticles: Sharp reversible response and easy tunability. Chem. Commun. 2011, 47, 2826–2828. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Cho, H.S.; Kim, J.; Kim, S.-C.; Cho, M.H.; Lee, J. Thermoresponsive oligomers reduce Escherichia coli O157:H7 biofouling and virulence. Biofouling 2014, 30, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Schwerdt, A.; Zintchenko, A.; Concia, M.; Roesen, N.; Fisher, K.; Lindner, L.H.; Issels, R.; Wagner, E.; Ogris, M. Hyperthermia-induced targeting of thermosensitive gene carriers to tumors. Hum. Gene Ther. 2008, 19, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Biodegradable and thermo-sensitive chitosan-g-poly(N-vinylcaprolactam) nanoparticles as a 5-Fluorouracil carrier. Carbohydr. Polym. 2011, 83, 776–786. [Google Scholar] [CrossRef]
- Stocke, N.A.; Arnold, S.M.; Zach Hilt, J. Responsive hydrogel nanoparticles for pulmonary delivery. J. Drug Deliv. Sci. Technol. 2015, 29, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Yusa, S.-I.; Sugahara, M.; Endo, T.; Morishima, Y. Preparation and characterization of a pH-responsive nanogel based on a photo-cross-linked micelle formed from block copolymers with controlled structure. Langmuir 2009, 25, 5258–5265. [Google Scholar] [CrossRef] [PubMed]
- Deepa, G.; Thulasidasan, A.K.T.; Anto, R.J.; Pillai, J.J.; Kumar, G.S.V. Cross-linked acrylic hydrogel for the controlled delivery of hydrophobic drugs in cancer therapy. Int. J. Nanomed. 2012, 7, 4077–4088. [Google Scholar]
- Kim, S.; Lee, D.J.; Kwag, D.S.; Lee, U.Y.; Youn, Y.S.; Lee, E.S. Acid pH-activated glycol chitosan/fullerene nanogels for efficient tumor therapy. Carbohydr. Polym. 2014, 101, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xiao, C.; Fan, J.; Chen, S.; Shen, J.; Wu, W.; Zhou, S. A nanogel of on-site tunable pH-response for efficient anticancer drug delivery. Acta Biomater. 2013, 9, 4546–4557. [Google Scholar] [CrossRef] [PubMed]
- Arunraj, T.R.; Sanoj Rejinold, N.; Ashwin Kumar, N.; Jayakumar, R. Bio-responsive chitin-poly(l-lactic acid) composite nanogels for liver cancer. Coll. Surf. B Biointerfaces 2014, 113, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Song, W.; Tang, Z.; Lv, S.; Lin, L.; Sun, H.; Li, Q.; Yang, Y.; Hong, H.; Chen, X. Nanoscaled poly(l-glutamic acid)/doxorubicin-amphiphile complex as pH-responsive drug delivery system for effective treatment of nonsmall cell lung cancer. ACS Appl. Mater. Interfaces 2013, 5, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Ashwani, K.S.; Gandhi, G.R.; Gupta, C.K. Photoregulation of drug release in azo-dextran nanogels. Int. J. Pharm. 2007, 342, 184–193. [Google Scholar] [CrossRef] [PubMed]
- American National Standards Institute. American National Standard for the Safe Use of Lasers; ANSI Z136.1-1993.v1993; Laser Institute Orlando: Orlando, FL, USA, 1993. [Google Scholar]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Del. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Niidome, Y.; Mori, T.; Katayama, Y.; Niidome, T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconj. Chem. 2009, 20, 209–212. [Google Scholar] [CrossRef] [PubMed]
- LaConte, L.; Nitin, N.; Bao, G. Magnetic nanoparticle probes. Mater. Today 2005, 8, 32–38. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Zhao, X.-Q.; Wang, T.-X.; Liu, W.; Wang, C.-D.; Wang, D.; Shang, T.; Shen, L.-H.; Ren, L. Multifunctional Au@IPN-pNIPAAM nanogels for cancer cell imaging and combined chemo-photothermal treatment. J. Mater. Chem. 2011, 21, 7240–7247. [Google Scholar] [CrossRef]
- Hasegawa, U.; Nomura, S.-I.M.; Kaul, S.C.; Hirano, T.; Akiyoshi, K. Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem. Biophys. Res. Commun. 2005, 331, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Pikabea, A.; Ramos, J.; Papachristos, N.; Stamopoulos, D.; Forcada, J. Synthesis and characterization of PDEAEMA-based magneto-nanogels: Preliminary results on the biocompatibility with cells of human peripheral blood. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1479–1494. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Bronich, T.K.; Kabanov, A.V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002, 54, 135–147. [Google Scholar] [CrossRef]
- Blackburn, W.H.; Dickerson, E.B.; Smith, M.H.; McDonald, J.F.; Lyon, L.A. Peptide-functionalized nanogels for targeted siRNA delivery. Bioconj. Chem. 2009, 20, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Bhuchar, N.; Sunasee, R.; Ishihara, K.; Thundat, T.; Narain, R. Degradable thermoresponsive nanogels for protein encapsulation and controlled release. Bioconj. Chem. 2012, 23, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Lee, J.H.; Kim, J.-Y.; Kim, J.-C.; Kim, Y.H.; Tae, G. Efficient skin permeation of soluble proteins via flexible and functional nano-carrier. J. Controll. Release 2012, 157, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Nukolova, N.V.; Yang, Z.; Kim, J.O.; Kabanov, A.V.; Bronich, T.K. Polyelectrolyte nanogels decorated with monoclonal antibody for targeted drug delivery. React. Funct. Polym. 2011, 71, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Park, T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J. Controll. Release 2004, 100, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Kurisawa, M.; Chung, J.E.; Yang, Y.-Y. Synthesis and characterization of chitosan-g-poly(ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials 2007, 28, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.E.; Delgado, C.; Fisher, D.; Malik, F.; Agrawal, A.K. Polyethylene glycol modification: Relevance of improved methodology to tumour targeting. J. Drug Target. 1996, 3, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Nukolova, N.V.; Oberoi, H.S.; Cohen, S.M.; Kabanov, A.V.; Bronich, T.K. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 2011, 32, 5417–5426. [Google Scholar] [CrossRef] [PubMed]
- Peach, R.J.; Hollenbaugh, D.; Stamenkovic, I.; Aruffo, A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J. Cell Biol. 1993, 122, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Toole, B. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Saravanakumar, G.; Park, J.H.; Park, K. Hyaluronic acid-based nanocarriers for intracellular targeting: Interfacial interactions with proteins in cancer. Coll. Surf. B Biointerfaces 2012, 99, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Kim, K.S.; Bae, B.-C.; Kim, Y.-H.; Na, K. Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. Eur. J. Pharm. Sci. 2010, 40, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, C.; Wang, X.; Wang, W.; Huang, W.; Cha, R.; Wang, C.; Yuan, Z.; Liu, M.; Wan, H.; et al. Glycyrrhetinic acid-modified chitosan/poly(ethylene glycol) nanoparticles for liver-targeted delivery. Biomaterials 2010, 31, 4748–4756. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, M.; Farinha, D.; de Lima, M.; Faneca, H. Increased gene delivery efficiency and specificity of a lipid-based nanosystem incorporating a glycolipid. Int. J. Nanomed. 2014, 9, 4979–4989. [Google Scholar]
- Lou, S.; Gao, S.; Wang, W.; Zhang, M.; Zhang, J.; Wang, C.; Li, C.; Kong, D.; Zhao, Q. Galactose-functionalized multi-responsive nanogels for hepatoma-targeted drug delivery. Nanoscale 2015, 7, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.; Martínez, A.; Teijón, C.; Teijón, J.M.; Blanco, M.D. Bioresponsive nanohydrogels based on HEAA and NIPA for poorly soluble drugs delivery. Int. J. Pharm. 2014, 470, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-J.; Chen, Y.-Y.; Wang, D.-R.; Wei, C.; Guo, J.; Lu, D.-R.; Chu, C.-C.; Wang, C.-C. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 2012, 33, 6570–6579. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Z.; Sun, H.; Ding, J.; Song, W.; Chen, X. pH and reduction dual-responsive nanogel cross-linked by quaternization reaction for enhanced cellular internalization and intracellular drug delivery. Polym. Chem. 2013, 4, 1199–1207. [Google Scholar] [CrossRef]
- Lou, S.; Gao, S.; Wang, W.; Zhang, M.; Zhang, Q.; Wang, C.; Li, C.; Kong, D. Temperature/pH dual responsive microgels of crosslinked poly(N-vinylcaprolactam-co-undecenoic acid) as biocompatible materials for controlled release of doxorubicin. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Deka, S.R.; Quarta, A.; Di Corato, R.; Falqui, A.; Manna, L.; Cingolani, R.; Pellegrino, T. Acidic pH-responsive nanogels as smart cargo systems for the simultaneous loading and release of short oligonucleotides and magnetic nanoparticles. Langmuir 2010, 26, 10315–10324. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Core–shell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemo-photothermal treatment. Biomaterials 2010, 31, 7555–7566. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.; Ramos, J.; Forcada, J. Advanced design of dual-responsive core-shell nanogels for siRNA delivery. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3203–3217. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Controll. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm. J. 2016, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Pelaez-Fernandez, M.; Forcada, J.; Moncho-Jorda, A. Chapter 4 nanogels for drug delivery: The key role of nanogel-drug interactions. In Soft Nanoparticles for Biomedical Applications; The Royal Society of Chemistry: London, UK, 2014; pp. 133–156. [Google Scholar]
- Qiao, Z.-Y.; Zhang, R.; Du, F.-S.; Liang, D.-H.; Li, Z.-C. Multi-responsive nanogels containing motifs of ortho ester, oligo(ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. J. Controll. Release 2011, 152, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Naeye, B.; Deschout, H.; Caveliers, V.; Descamps, B.; Braeckmans, K.; Vanhove, C.; Demeester, J.; Lahoutte, T.; De Smedt, S.C.; Raemdonck, K. In vivo disassembly of IV administered sirna matrix nanoparticles at the renal filtration barrier. Biomaterials 2013, 34, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Naeye, B.; Deschout, H.; Röding, M.; Rudemo, M.; Delanghe, J.; Devreese, K.; Demeester, J.; Braeckmans, K.; de Smedt, S.C.; Raemdonck, K. Hemocompatibility of siRNA loaded dextran nanogels. Biomaterials 2011, 32, 9120–9127. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Martins, R.; Gamazo, C.; Irache, J.M. Design and influence of γ-irradiation on the biopharmaceutical properties of nanoparticles containing an antigenic complex from Brucella ovis. Eur. J. Pharm. Sci. 2009, 37, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Turker, S.; Özer, A.Y.; Kiliç, E.; Özalp, M.; Çolak, S.; Korkmaz, M. Gamma-irradiated liposome/noisome and lipogelosome/niogelosome formulations for the treatment of rheumatoid arthritis. Interv. Med. Appl. Sci. 2013, 5, 60–69. [Google Scholar] [PubMed]
- Andrés-Guerrero, V.; Zong, M.; Ramsay, E.; Rojas, B.; Sarkhel, S.; Gallego, B.; de Hoz, R.; Ramírez, A.I.; Salazar, J.J.; Triviño, A.; et al. Novel biodegradable polyesteramide microspheres for controlled drug delivery in ophthalmology. J. Controll. Release 2015, 211, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Song, C.X.; Labhasetwar, V.; Murphy, H.; Qu, X.; Humphrey, W.R.; Shebuski, R.J.; Levy, R.J. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J. Controll. Release 1997, 43, 197–212. [Google Scholar] [CrossRef]
- Allen LV, J.R. Remington: An Introduction to Pharmacy, 22nd ed.; Pharmaceutical Press: London, UK, 2012. [Google Scholar]
- Vinogradov, S.V.; Kohli, E.; Zeman, A.; Kabanov, A.V. Chemical engineering of nanogel drug carriers: Increased bioavailability and decreased cytotoxicity. Polym. Prep. 2006, 47, 27–28. [Google Scholar]
- Li, L.; Mak, K.Y.; Shi, J.; Leung, C.H.; Wong, C.M.; Leung, C.W.; Mak, C.S.K.; Chan, K.Y.; Chan, N.M.M.; Wu, E.X.; et al. Sterilization on dextran-coated iron oxide nanoparticles: Effects of autoclaving, filtration, UV irradiation, and ethanol treatment. Microelectron. Eng. 2013, 111, 310–313. [Google Scholar] [CrossRef]
- Konan, Y.N.; Gurny, R.; Allémann, E. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int. J. Pharm. 2002, 233, 239–252. [Google Scholar] [CrossRef]
- Thilakarathne, V.; Briand, V.A.; Zhou, Y.; Kasi, R.M.; Kumar, C.V. Protein polymer conjugates: Improving the stability of hemoglobin with poly(acrylic acid). Langmuir 2011, 27, 7663–7671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dou, H.; Zhang, Z.; Sun, K.; Jin, Y.; Dai, T.; Zhou, G.; Shen, Z. Fluorescent dextran-based nanogels: Efficient imaging nanoprobes for adipose-derived stem cells. Polym. Chem. 2013, 4, 4103–4112. [Google Scholar] [CrossRef]
- Ivanov, A.E.; Thammakhet, C.; Kuzimenkova, M.V.; Thavarungkul, P.; Kanatharana, P.; Mikhalovska, L.I.; Mikhalovsky, S.V.; Galaev, I.Y.; Mattiasson, B. Thin semitransparent gels containing phenylboronic acid: Porosity, optical response and permeability for sugars. J. Mol. Recognit. 2008, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.P. A review of the effects of gamma radiation on pharmaceutical materials. J. Biomater. Appl. 1995, 10, 59–96. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, C.; Sabatino, M.A.; Grimaldi, N.; Bulone, D.; Bondì, M.L.; Casaletto, M.P.; Rigogliuso, S.; Adamo, G.; Ghersi, G. Minimalism in radiation synthesis of biomedical functional nanogels. Biomacromolecules 2012, 13, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- An, J.-C.; Weaver, A.; Kim, B.; Barkatt, A.; Poster, D.; Vreeland, W.N.; Silverman, J.; Al-Sheikhly, M. Radiation-induced synthesis of poly(vinylpyrrolidone) nanogel. Polymer 2011, 52, 5746–5755. [Google Scholar] [CrossRef]
- Kadlubowski, S. Radiation-induced synthesis of nanogels based on poly(N-vinyl-2-pyrrolidone)—A review. Radiat. Phys. Chem. 2014, 102, 29–39. [Google Scholar] [CrossRef]
- Dispenza, C.; Adamo, G.; Sabatino, M.A.; Grimaldi, N.; Bulone, D.; Bondì, M.L.; Rigogliuso, S.; Ghersi, G. Oligonucleotides-decorated-poly(N-vinyl pyrrolidone) nanogels for gene delivery. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Abd El-Rehim, H.A.; Hegazy, E.-S.A.; Hamed, A.A.; Swilem, A.E. Controlling the size and swellability of stimuli-responsive polyvinylpyrrolidone–poly(acrylic acid) nanogels synthesized by gamma radiation-induced template polymerization. Eur. Polym. J. 2013, 49, 601–612. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Chacko, R.T.; Jiwpanich, S.; Bickerton, S.; Babu, R.P.; Thayumanavan, S. Self-cross-linked polymer nanogels: A versatile nanoscopic drug delivery platform. J. Am. Chem. Soc. 2010, 132, 17227–17235. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lee, E.S.; Bae, Y.H. Self-organized nanogels responding to tumor extracellular pH: pH-dependent drug release and in vitro cytotoxicity against MCF-7 cells. Bioconj. Chem. 2007, 18, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Mortisen, D.; Peroglio, M.; Alini, M.; Eglin, D. Tailoring thermoreversible hyaluronan hydrogels by “click” chemistry and raft polymerization for cell and drug therapy. Biomacromolecules 2010, 11, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Oberoi, H.S.; Desale, S.; Kabanov, A.V.; Bronich, T.K. Polypeptide nanogels with hydrophobic moieties in the cross-linked ionic cores: Synthesis, characterization and implications for anticancer drug delivery. J. Drug Target. 2013, 21, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chang, Y.; Liu, G.; Wang, H.; Cao, A.; An, Z. Biocompatible, antifouling, and thermosensitive core-shell nanogels synthesized by raft aqueous dispersion polymerization. Macromolecules 2011, 44, 2524–2530. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Rangasamy, J. Multifunctional chitin nanogels for simultaneous drug delivery, bioimaging, and biosensing. ACS Appl. Mater. Interfaces 2011, 3, 3654–3665. [Google Scholar] [CrossRef] [PubMed]
- Imaz, A.; Forcada, J. N-vinylcaprolactam-based microgels for biomedical applications. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1173–1181. [Google Scholar] [CrossRef]
- Hoare, T.; Sivakumaran, D.; Stefanescu, C.F.; Lawlor, M.W.; Kohane, D.S. Nanogel scavengers for drugs: Local anesthetic uptake by thermoresponsive nanogels. Acta Biomater. 2012, 8, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Kutscher, H.L.; Gao, D.; Sunil, V.R.; Malaviya, R.; Vayas, K.; Stein, S.; Laskin, J.D.; Laskin, D.L.; Sinko, P.J. Biodistribution and renal clearance of biocompatible lung targeted poly(ethylene glycol) (PEG) nanogel aggregates. J. Controll. Release 2012, 164, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Manchun, S.; Dass, C.R.; Cheewatanakornkool, K.; Sriamornsak, P. Enhanced anti-tumor effect of pH-responsive dextrin nanogels delivering doxorubicin on colorectal cancer. Carbohydr. Polym. 2015, 126, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Jain, N.K.; Bedi, P.M.S. Nanostructured lipid carriers based nanogel for meloxicam delivery: Mechanistic, in vivo and stability evaluation. Drug Dev. Ind. Pharm. 2015, 41, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, B.; Lin, C.; Zhuang, Z. Fluorescence tomographic imaging of sentinel lymph node using near-infrared emitting bioreducible dextran nanogels. Int. J. Nanomed. 2014, 9, 5667–5682. [Google Scholar]
- Dai, T.; Zhou, S.; Yin, C.; Li, S.; Cao, W.; Liu, W.; Sun, K.; Dou, H.; Cao, Y.; Zhou, G. Dextran-based fluorescent nanoprobes for sentinel lymph node mapping. Biomaterials 2014, 35, 8227–8235. [Google Scholar] [CrossRef] [PubMed]
- Brugués, A.P.; Naveros, B.C.; Calpena Campmany, A.C.; Pastor, P.H.; Saladrigas, R.F.; Lizandra, C.R. Developing cutaneous applications of paromomycin entrapped in stimuli-sensitive block copolymer nanogel dispersions. Nanomedicine 2015, 10, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Misra, G.P.; Vaish, A.; Flanagan, J.M.; Sutermaster, B.; Lowe, T.L. Novel nanogels with both thermoresponsive and hydrolytically degradable properties. Macromolecules 2008, 41, 8339–8345. [Google Scholar] [CrossRef]
- Klinger, D.; Aschenbrenner, E.M.; Weiss, C.K.; Landfester, K. Enzymatically degradable nanogels by inverse miniemulsion copolymerization of acrylamide with dextran methacrylates as crosslinkers. Polym. Chem. 2012, 3, 204–216. [Google Scholar] [CrossRef]
- Aguirre, G.; Ramos, J.; Forcada, J. Synthesis of new enzymatically degradable thermo-responsive nanogels. Soft Matter 2013, 9, 261–270. [Google Scholar] [CrossRef]
- Steinhilber, D.; Sisson, A.L.; Mangoldt, D.; Welker, P.; Licha, K.; Haag, R. Synthesis, reductive cleavage, and cellular interaction studies of biodegradable, polyglycerol nanogels. Adv. Funct. Mater. 2010, 20, 4133–4138. [Google Scholar] [CrossRef]
- Klinger, D.; Landfester, K. Enzymatic- and light-degradable hybrid nanogels: Crosslinking of polyacrylamide with acrylate-functionalized dextrans containing photocleavable linkers. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1062–1075. [Google Scholar] [CrossRef]
- Morimoto, N.; Hirano, S.; Takahashi, H.; Loethen, S.; Thompson, D.H.; Akiyoshi, K. Self-assembled pH-sensitive cholesteryl pullulan nanogel as a protein delivery vehicle. Biomacromolecules 2013, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 2012, 90, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Chitosan-based responsive hybrid nanogels for integration of optical pH-sensing, tumor cell imaging and controlled drug delivery. Biomaterials 2010, 31, 8371–8381. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, C.-S.; Jung, Y.-S.; Na, K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly(l-lactide) copolymers. Carbohydr. Polym. 2012, 87, 1105–1111. [Google Scholar] [CrossRef]
- Sunasee, R.; Wattanaarsakit, P.; Ahmed, M.; Lollmahomed, F.B.; Narain, R. Biodegradable and nontoxic nanogels as nonviral gene delivery systems. Bioconj. Chem. 2012, 23, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Maciel, D.; Figueira, P.; Xiao, S.; Hu, D.; Shi, X.; Rodrigues, J.; Tomás, H.; Li, Y. Redox-responsive alginate nanogels with enhanced anticancer cytotoxicity. Biomacromolecules 2013, 14, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, M.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z. In situ forming reduction-sensitive degradable nanogels for facile loading and triggered intracellular release of proteins. Biomacromolecules 2013, 14, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Arunraj, T.R.; Sanoj Rejinold, N.; Ashwin Kumar, N.; Jayakumar, R. Doxorubicin-chitin-poly(caprolactone) composite nanogel for drug delivery. Int. J. Biol. Macromol. 2013, 62, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Men, K.; Zhang, J.; Li, Y.; Song, J.; Luo, S.; Shi, H.; Wen, Y.; Guo, G.; Huang, M.; et al. Efficient inhibition of C-26 colon carcinoma by VSVMP gene delivered by biodegradable cationic nanogel derived from polyethyleneimine. ACS Nano 2010, 4, 5573–5584. [Google Scholar] [CrossRef] [PubMed]
- Kadlubowski, S.; Grobelny, J.; Olejniczak, W.; Cichomski, M.; Ulanski, P. Pulses of Fast Electrons as a Tool To Synthesize Poly(acrylic acid) Nanogels. Intramolecular Cross-Linking of Linear Polymer Chains in Additive-Free Aqueous Solution. Macromolecules 2003, 36, 2484–2492. [Google Scholar] [CrossRef]
- Makridakis, M.; Vlahou, A. Secretome proteomics for discovery of cancer biomarkers. J. Proteom. 2010, 73, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; Kleifeld, O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Demeester, J.; De Smedt, S. Advanced nanogel engineering for drug delivery. Soft Matter 2009, 5, 707–715. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Gao, J.; Zhang, D.; Jia, L.; Liu, Y.; Zheng, D.; Liu, G.; Tian, X.; Wang, F.; Zhang, Q. Galactose-decorated pH-responsive nanogels for hepatoma-targeted delivery of oridonin. Biomacromolecules 2011, 12, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Zagar, T.M.; Vujaskovic, Z.; Formenti, S.; Rugo, H.; Muggia, F.; O’Connor, B.; Myerson, R.; Stauffer, P.; Hsu, I.C.; Diederich, C.; et al. Two phase i dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int. J. Hyperth. 2014, 30, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.P.; Borys, N. Lyso-thermosensitive liposomal doxorubicin: An adjuvant to increase the cure rate of radiofrequency ablation in liver cancer. Future Oncol. 2011, 7, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Achazi, K.; Schade, B.; Haag, R. Charge-conversional and reduction-sensitive poly(vinyl alcohol) nanogels for enhanced cell uptake and efficient intracellular doxorubicin release. J. Controll. Release 2015, 205, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, W.; Wang, Y.; Zhao, Y.; Chen, H.; Xu, H.; Yang, X. Dual temperature/pH-sensitive drug delivery of poly(N-isopropylacrylamide-co-acrylic acid) nanogels conjugated with doxorubicin for potential application in tumor hyperthermia therapy. Coll. Surf. B Biointerfaces 2011, 84, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Ding, J.; Xiao, C.; Zhuang, X.; He, C.; Chen, L.; Chen, X. Intracellular microenvironment responsive pegylated polypeptide nanogels with ionizable cores for efficient doxorubicin loading and triggered release. J. Mater. Chem. 2012, 22, 14168–14179. [Google Scholar] [CrossRef]
- Du, J.-Z.; Sun, T.-M.; Song, W.-J.; Wu, J.; Wang, J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew. Chem. Int. Ed. 2010, 49, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X.; Yao, X.; Zhang, Y.; Wu, W.; Jiang, X. Hyaluronic acid nanogels with enzyme-sensitive cross-linking group for drug delivery. J. Controll. Release 2015, 205, 206–217. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cattran, A.W.; Nguyen, T.; Nieminen, A.-L.; Xu, P. Triple-responsive expansile nanogel for tumor and mitochondria targeted photosensitizer delivery. Biomaterials 2014, 35, 9546–9553. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y.-K. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef] [PubMed]
- Salehi, R.; Rasouli, S.; Hamishehkar, H. Smart thermo/ph responsive magnetic nanogels for the simultaneous delivery of doxorubicin and methotrexate. Int. J. Pharm. 2015, 487, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Mitra, N.; Yan, E.C.Y.; Zhou, S. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano 2010, 4, 4831–4839. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choe, K.; Jeong, Y.; Yoo, J.; Lee, S.M.; Park, J.-H.; Kim, P.; Kim, Y.-C. Establishment of a controlled insulin delivery system using a glucose-responsive double-layered nanogel. RSC Adv. 2015, 5, 14482–14491. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Guo, H.; Li, C.; Yu, D. An injectable and glucose-sensitive nanogel for controlled insulin release. J. Mater. Chem. 2012, 22, 22788–22796. [Google Scholar] [CrossRef]
- Yao, R.-S.; Zhang, W.-B.; Yang, X.-Z.; Liu, J.; Liu, H.-T. HPMC/PAA hybrid nanogels via aqueous-phase synthesis for controlled delivery of insulin. Biomater. Sci. 2014, 2, 1761–1767. [Google Scholar] [CrossRef]
- Park, C.W.; Yang, H.-M.; Lee, H.J.; Kim, J.-D. Core-shell nanogel of PEG-poly(aspartic acid) and its pH-responsive release of rH-insulin. Soft Matter 2013, 9, 1781–1788. [Google Scholar] [CrossRef]
- Liu, J.; Detrembleur, C.; Debuigne, A.; De Pauw-Gillet, M.-C.; Mornet, S.; Vander Elst, L.; Laurent, S.; Duguet, E.; Jerome, C. Glucose-, pH- and thermo-responsive nanogels crosslinked by functional superparamagnetic maghemite nanoparticles as innovative drug delivery systems. J. Mater. Chem. B 2014, 2, 1009–1023. [Google Scholar] [CrossRef]
- De Backer, L.; Braeckmans, K.; Demeester, J.; De Smedt, S.C.; Raemdonck, K. The influence of natural pulmonary surfactant on the efficacy of siRNA-loaded dextran nanogels. Nanomedicine 2013, 8, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Coll Ferrer, M.C.; Shuvaev, V.V.; Zern, B.J.; Composto, R.J.; Muzykantov, V.R.; Eckmann, D.M. ICAM-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. PLoS ONE 2014, 9, e102329. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Hu, S.-H.; Chian, C.-S.; Chen, S.-Y.; Lai, H.-Y.; Chen, Y.-Y. Self-assembling PVA-f127 thermosensitive nanocarriers with highly sensitive magnetically-triggered drug release for epilepsy therapy in vivo. J. Mater. Chem. 2012, 22, 8566–8573. [Google Scholar] [CrossRef]
- Soni, S.; Babbar, A.K.; Sharma, R.k.; Maitra, A. Delivery of hydrophobised 5-Fluorouracil derivative to brain tissue through intravenous route using surface modified nanogels. J. Drug Target. 2006, 14, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Azadi, A.; Hamidi, M.; Rouini, M.-R. Methotrexate-loaded chitosan nanogels as ‘trojan horses’ for drug delivery to brain: Preparation and in vitro/in vivo characterization. Int. J. Biol. Macromol. 2013, 62, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Azadi, A.; Rouini, M.-R.; Hamidi, M. Neuropharmacokinetic evaluation of methotrexate-loaded chitosan nanogels. Int. J. Biol. Macromol. 2015, 79, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Rashed, E.R.; Abd El-Rehim, H.A.; El-Ghazaly, M.A. Potential efficacy of dopamine loaded-PVP/PAA nanogel in experimental models of parkinsonism: Possible disease modifying activity. J. Biomed. Mater. Res. Part A 2015, 103, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V.; Batrakova, E.V.; Kabanov, A.V. Nanogels for oligonucleotide delivery to the brain. Bioconj. Chem. 2004, 15, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.; Makarov, E.; Lu, Y.; Senanayake, T.; Rivera, K.; Gorantla, S.; Poluektova, L.Y.; Vinogradov, S.V. Amphiphilic cationic nanogels as brain-targeted carriers for activated nucleoside reverse transcriptase inhibitors. J. Neuroimmune Pharmacol. 2015, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, T.; Nyan, M.; Shimoda, A.; Yamamoto, Y.; Kuroda, S.; Shiota, M.; Akiyoshi, K.; Kasugai, S. Exploitation of a novel polysaccharide nanogel cross-linking membrane for guided bone regeneration (GBR). J. Tissue Eng. Regenerative. Med. 2012, 6, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Katakura, O.; Morimoto, N.; Akiyoshi, K.; Kasugai, S. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Alles, N.; Soysa, N.S.; Hussain, M.D.A.; Tomomatsu, N.; Saito, H.; Baron, R.; Morimoto, N.; Aoki, K.; Akiyoshi, K.; Ohya, K. Polysaccharide nanogel delivery of a TNF-α and RANKL antagonist peptide allows systemic prevention of bone loss. Eur. J. Pharm. Sci. 2009, 37, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Ota, M.S.; Shimoda, A.; Nakahama, K.-I.; Akiyoshi, K.; Miyamoto, Y.; Iseki, S. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 2012, 33, 7613–7620. [Google Scholar] [CrossRef] [PubMed]
- Caldorera-Moore, M.E.; Liechty, W.B.; Peppas, N.A. Responsive theranostic systems: Integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc. Chem. Res. 2011, 44, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Martin, B.; Fernandez-Barbero, A. Multifunctional hybrid nanogels for theranostic applications. Soft Matter 2015, 11, 8205–8216. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Aiello, M.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials 2010, 31, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Gai, Z.; Hong, K.; Banerjee, P.; Zhou, S. Multi-functional core-shell hybrid nanogels for pH-dependent magnetic manipulation, fluorescent pH-sensing, and drug delivery. Biomaterials 2011, 32, 9876–9887. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.; Qiu, R.; Shi, L.; Wu, W.; Zhou, S. Responsive fluorescent Bi2O3@PVA hybrid nanogels for temperature-sensing, dual-modal imaging, and drug delivery. Biomaterials 2012, 33, 3058–3069. [Google Scholar] [CrossRef] [PubMed]
- DiSanto, R.M.; Subramanian, V.; Gu, Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Cameron, F.J.; Wherrett, D.K. Care of diabetes in children and adolescents: Controversies, changes, and consensus. Lancet 2015, 385, 2096–2106. [Google Scholar] [CrossRef]
- Lapeyre, V.; Ancla, C.; Catargi, B.; Ravaine, V. Glucose-responsive microgels with a core–shell structure. J. Coll. Interface Sci. 2008, 327, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiao, C.; Ding, J.; Zhuang, X.; Gai, G.; Wang, L.; Chen, X. Competitive binding-accelerated insulin release from a polypeptide nanogel for potential therapy of diabetes. Polym. Chem. 2015, 6, 3807–3815. [Google Scholar] [CrossRef]
- Rehor, A.; Botterhuis, N.E.; Hubbell, J.A.; Sommerdijk, N.A.J.M.; Tirelli, N. Glucose sensitivity through oxidation responsiveness. An example of cascade-responsive nano-sensors. J. Mater. Chem. 2005, 15, 4006–4009. [Google Scholar] [CrossRef]
- Ye, T.; Yan, S.; Hu, Y.; Ding, L.; Wu, W. Synthesis and volume phase transition of Concanavalin A-based glucose-responsive nanogels. Polym. Chem. 2014, 5, 186–194. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, L.; Yu, H.; Wang, J.; Chen, Z. Organization of glucose-responsive systems and their properties. Chem. Rev. 2011, 111, 7855–7875. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. Breaking down barriers. Science 2002, 297, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9, S5. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, E.; Andrieux, K.; Gil, S.; Couvreur, P. Colloidal carriers and blood–brain barrier (BBB) translocation: A way to deliver drugs to the brain? Int. J. Pharm. 2005, 298, 274–292. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V. New technologies for drug delivery across the blood brain barrier. Curr. Pharm. Des. 2004, 10, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Cosco, D.; Molinaro, R.; Celia, C.; Fresta, M. Supramolecular devices to improve the treatment of brain diseases. Drug Discov. Today 2011, 16, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, E.; Ansorena, E.; Blanco-Prieto, M.J. Brain drug delivery systems for neurodegenerative disorders. Curr. Pharm. Biotechnol. 2012, 13, 2388–2402. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Hoekstra, D.; Zuhorn, I.S. Smuggling drugs into the brain: An overview of ligands targeting transcytosis for drug delivery across the blood–brain barrier. Pharmaceutics 2014, 6, 557–583. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Boridy, S.; Takahashi, H.; Akiyoshi, K.; Maysinger, D. The binding of pullulan modified cholesteryl nanogels to Aβ oligomers and their suppression of cytotoxicity. Biomaterials 2009, 30, 5583–5591. [Google Scholar] [CrossRef] [PubMed]
- Rekha, M.R.; Sharma, C.P. Blood compatibility and in vitro transfection studies on cationically modified pullulan for liver cell targeted gene delivery. Biomaterials 2009, 30, 6655–6664. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, M.A.; Khan, M.A.A.M.; Alles, N.; Matsui, M.; Tabata, Y.; Ohya, K.; Aoki, K. Gelatin hydrogel carrier with the W9-peptide elicits synergistic effects on BMP-2-induced bone regeneration. J. Oral Biosci. 2013, 55, 217–223. [Google Scholar] [CrossRef]

 ); pH 6.5 (
); pH 6.5 (  ), pH 6.9 (
), pH 6.9 (  ), pH 7.5 (
), pH 7.5 (  ) and pH 7.9 (
) and pH 7.9 (  ). (Reprinted with permission from reference [25]. Copyright 2014 Wiley).
). (Reprinted with permission from reference [25]. Copyright 2014 Wiley).
 ); pH 6.5 (
); pH 6.5 (  ), pH 6.9 (
), pH 6.9 (  ), pH 7.5 (
), pH 7.5 (  ) and pH 7.9 (
) and pH 7.9 (  ). (Reprinted with permission from reference [25]. Copyright 2014 Wiley).
). (Reprinted with permission from reference [25]. Copyright 2014 Wiley).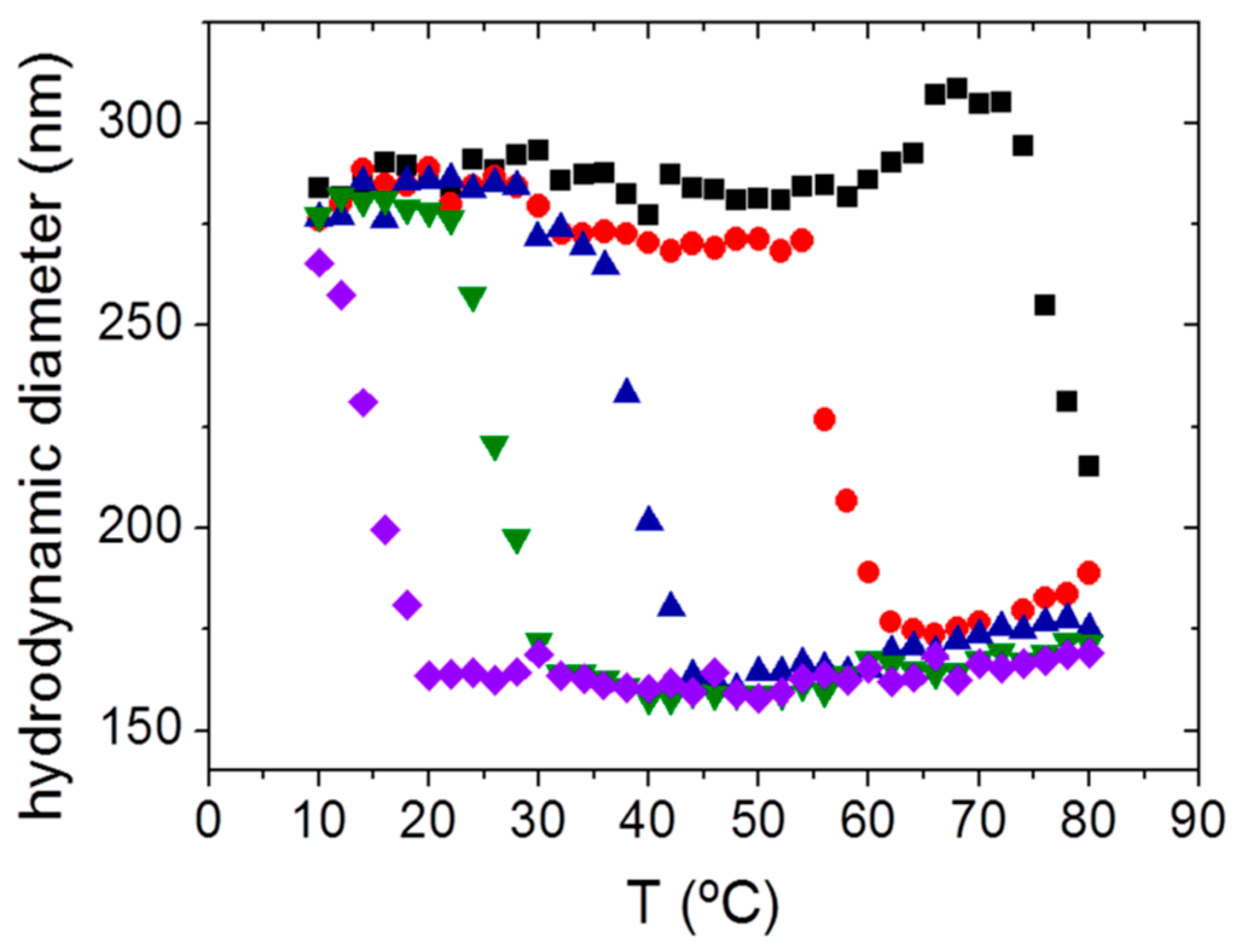

| Nanogels Based on | Synthesis Process | Drug | Stimuli-Responsiveness | Therapeutic Field | Reference |
|---|---|---|---|---|---|
| Methacrylate hyaluronic acid and di(ethylene glycol) diacrylate (MAHA-DEGDA) | Radical copolymerization | Doxorubicin | Physiological enzymes and pH | Chemotherapy | [142] |
| 4-Methoxybenzoic acid-poly[(2-(pyridin-2-yldisulfanyl)-co-[poly(ethylene glycol)]-poly(N-isopropyl methacrylamide (MBA-PDA-PEG-PNIPAM) | Free radical polymerization | Silicon phthalocyanine photosensitizer, Pc4 | pH, temperature and redox potential | Chemotherapy | [148] |
| Hyaluronic acid (HA) | Emulsion | Graphene and Doxorubicin conjugates | Light, temperature, and redox potential | Optical imaging and thermo-chemotherapy | [149] |
| Poly(ethylene glycol)-Hyaluronic acid with Ag-Au Nanoparticles (Ag-Au@PEG-HA) | Precipitation polymerization | Temozolomide | Temperature and light | Optical imaging and chemo-photo-thermal-therapy | [76] |
| Poly(N-isopropyl methacrylamide-methacrylic acid-quaternary ammonium alkyl halide with Fe3O4 nanoparticles P(NIPAM-MAA-DMAEMAQ) and poly(N-isopropyl methacrylamide)-methacrylic acid-hydroxyl ethyl methacrylate-quaternary ammonium alkyl halide with Fe3O4 nanoparticles P(NIPAAm-MAA-HEMA-DMAEMAQ) | Free radical polymerization | Doxorubicin and methotrexate | pH, temperature and magnetic field | Chemotherapy | [150] |
| 4-Vinylphenylboronic acid-2-(dimethylamino)ethyl acrylate with Ag Nanoparticles (Ag@P(VPBA-DMAEA) | Emulsion polymerization | Insulin | Glucose and light | Diabetes treatment | [151] |
| Glycol chitosan-sodium alginate-poly(l-glutmate-co-N-3-l-glutamylphenylboronic acid) (GC/SA-PGGA) | Isotropic gelation method and electrostatic interactions | Insulin | Glucose | Diabetes treatment | [152] |
| Poly(N-isopropyl methacrylamide)-dextran-maleic acid-phenylboronic acid P(NIPAM–Dex–PBA) | Polymerization | Insulin | pH, temperature and glucose | Diabetes treatment | [153] |
| Hydroxypropylmethylcellulose/poly-(acrylic acid) (HPMC/PAA) | Surfactant free polymerization | Insulin | pH | Diabetes treatment | [154] |
| Poly(ethylene glycol)-poly(aspartic acid) (PEG-PAsp) | Self assembling and cross-linking | Insulin | pH | Diabetes treatment | [155] |
| Boronic acid-Fe2O3 Nanoparticles-poly(vinyl alcohol)-b-poly(N-vinylcaprolactam) PVOH-b-PNVCL | Micelle thermo-formation | Tamoxifen | pH, temperature, glucose and magnetic field | Thermo-chemotherapy and optical imaging | [156] |
| Dextran-(2-[methacryloyloxy]-ethyl) trimethylammonium chloride DEX-NGs | Inverse miniemulsion photopolymerization | siRNA | Physiological stimuli (natural pulmonary surfactant) | Pulmonary diseases | [157] |
| Dextran-Lysozyme (Ab-NG-DEX) | Enzymatic reaction | Antibody ICAM-1 and dexamethasone | Physiological stimuli (intercellular adhesion molecule-1) | Acute pulmonary inflammation | [158] |
| Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide)-Polyvinyl alcohol with Fe3O4 nanoparticles (F-127-PVA) | Self-assembly | Ethosiximide | Magnetic field and temperature | Epilepsy | [159] |
| Poly(N-isopropyl methacrylamide and N-vinylpirrolidone (PNIPAM-VP) | Free radical polymerization | N-hexylcarbamoyl-5-Fluorouracil | Temperature | Brain tumors | [160] |
| Polysorbate 80-coated chitosan | Ionic gelation | Methotrexate | Surface modification | Brain tumors | [161,162] |
| Polyvinylpyrrolidone-poly(acrylic acid) (PVP/PAAc) | g radiation-induced polymerization | Dopamine | pH | Parkinson disease | [163] |
| Poly(ethylene glycol) and polyethylenimine PEG-PEI | Emulsification-solvent evaporation | Oligonucleotides | Surface functionalization | Brain diseases | [164] |
| Cholesterol-Polylysine | Emulsification-solvent evaporation | Nucleoside reverse transcriptase inhibitors | Surface functionalization | Human Immunodeficiency Virus (HIV)-associated encephalitis and neurodegeneration | [165] |
| Cholesterol-bearing pullulan (CHP) | Self-assembly | CHP nanogel membrane | Natural-based nanogels | Bone regeneration | [166] |
| Cholesterol-bearing pullulan (CHP) | Self-assembly | Prostaglandin E1 | Natural-based nanogels | Wound healing | [167] |
| Cholesterol-bearing pullulan (CHP) | Self-assembly | W9-peptide | Natural-based nanogels | Bone regeneration | [168] |
| Cholesteryl group- and acryloyl group-bearing pullulan (CHPOA) | Self-assembly | Human bone morphogenetic protein 2 and recombinant human fibroblast growth factor 18 | Natural-based nanogels | Bone regeneration | [169] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicario-de-la-Torre, M.; Forcada, J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels 2017, 3, 16. https://doi.org/10.3390/gels3020016
Vicario-de-la-Torre M, Forcada J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels. 2017; 3(2):16. https://doi.org/10.3390/gels3020016
Chicago/Turabian StyleVicario-de-la-Torre, Marta, and Jacqueline Forcada. 2017. "The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy" Gels 3, no. 2: 16. https://doi.org/10.3390/gels3020016
APA StyleVicario-de-la-Torre, M., & Forcada, J. (2017). The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels, 3(2), 16. https://doi.org/10.3390/gels3020016