Polyampholyte Hydrogels in Biomedical Applications
Abstract
:1. Introduction
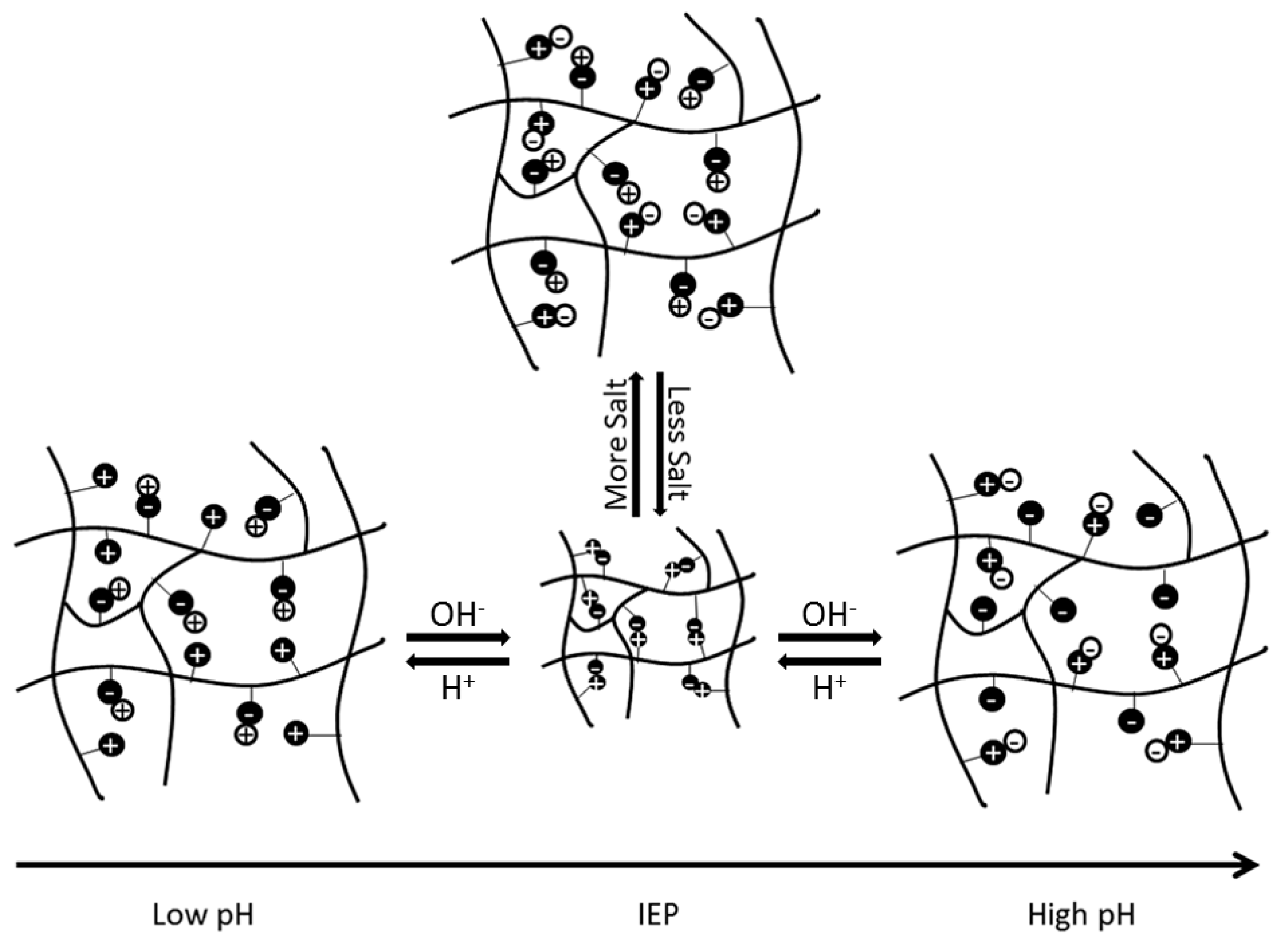
2. General Polyampholyte Characteristics
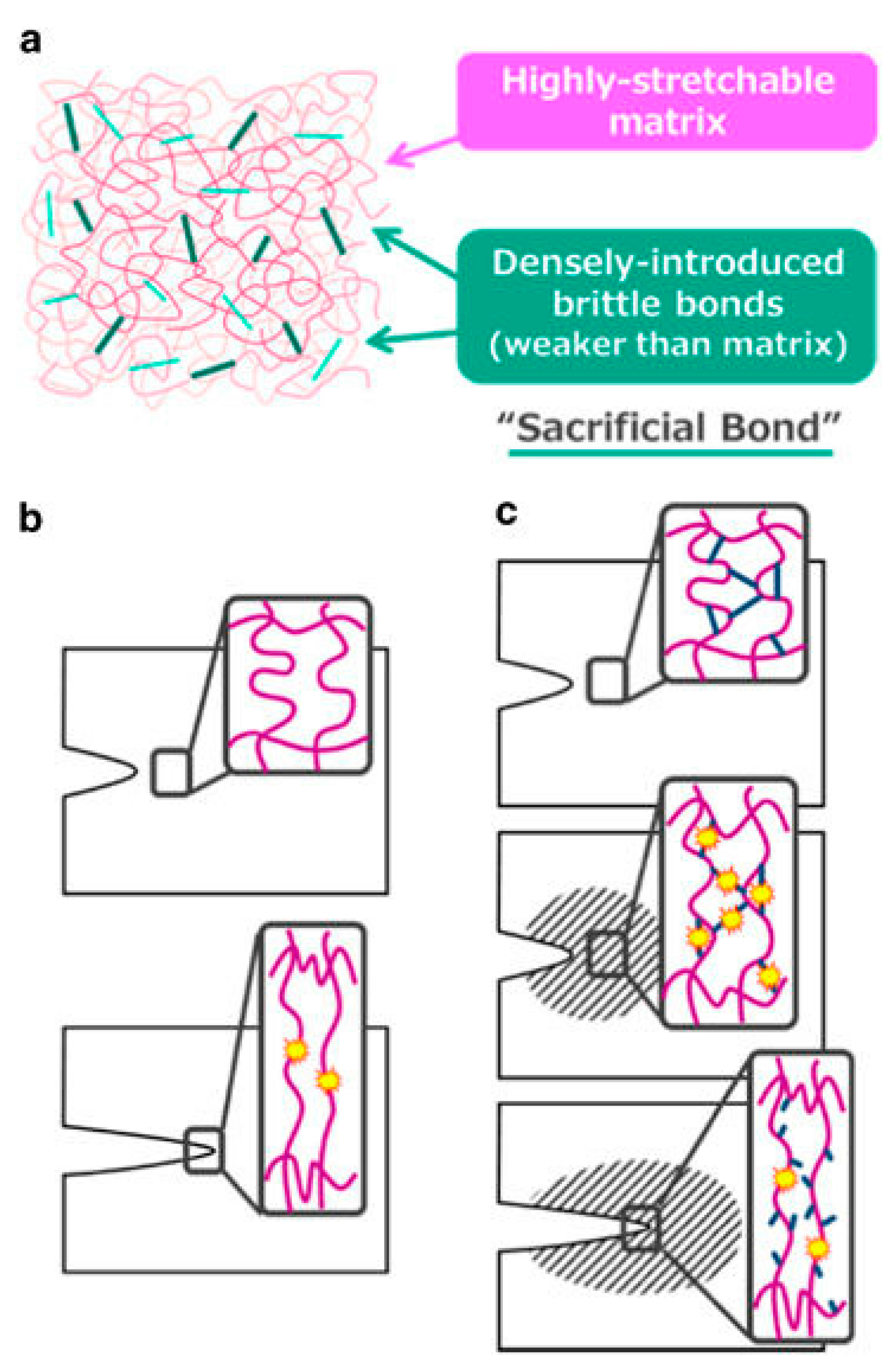
3. Mechanical Properties
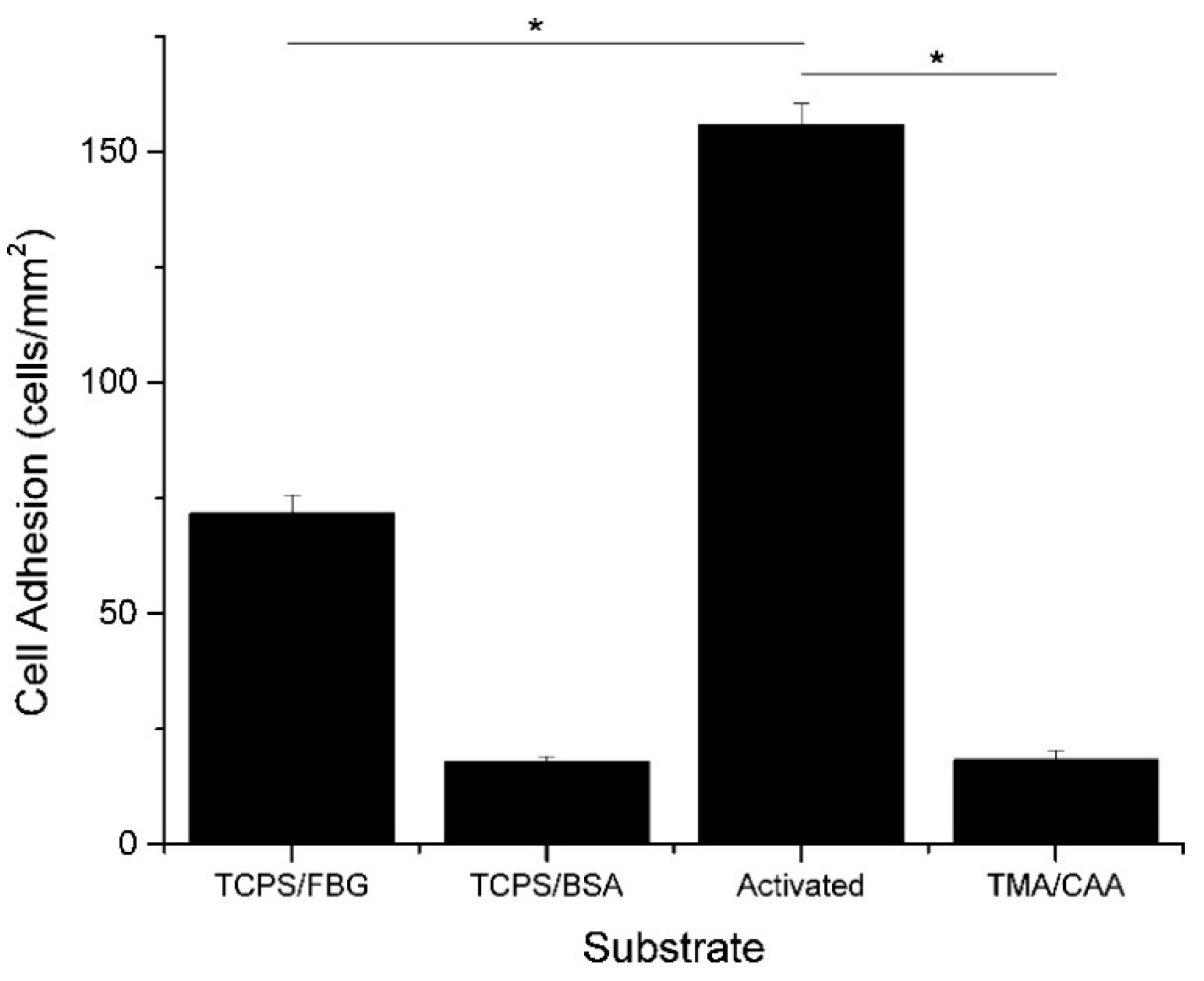
4. Tissue Engineering Applications
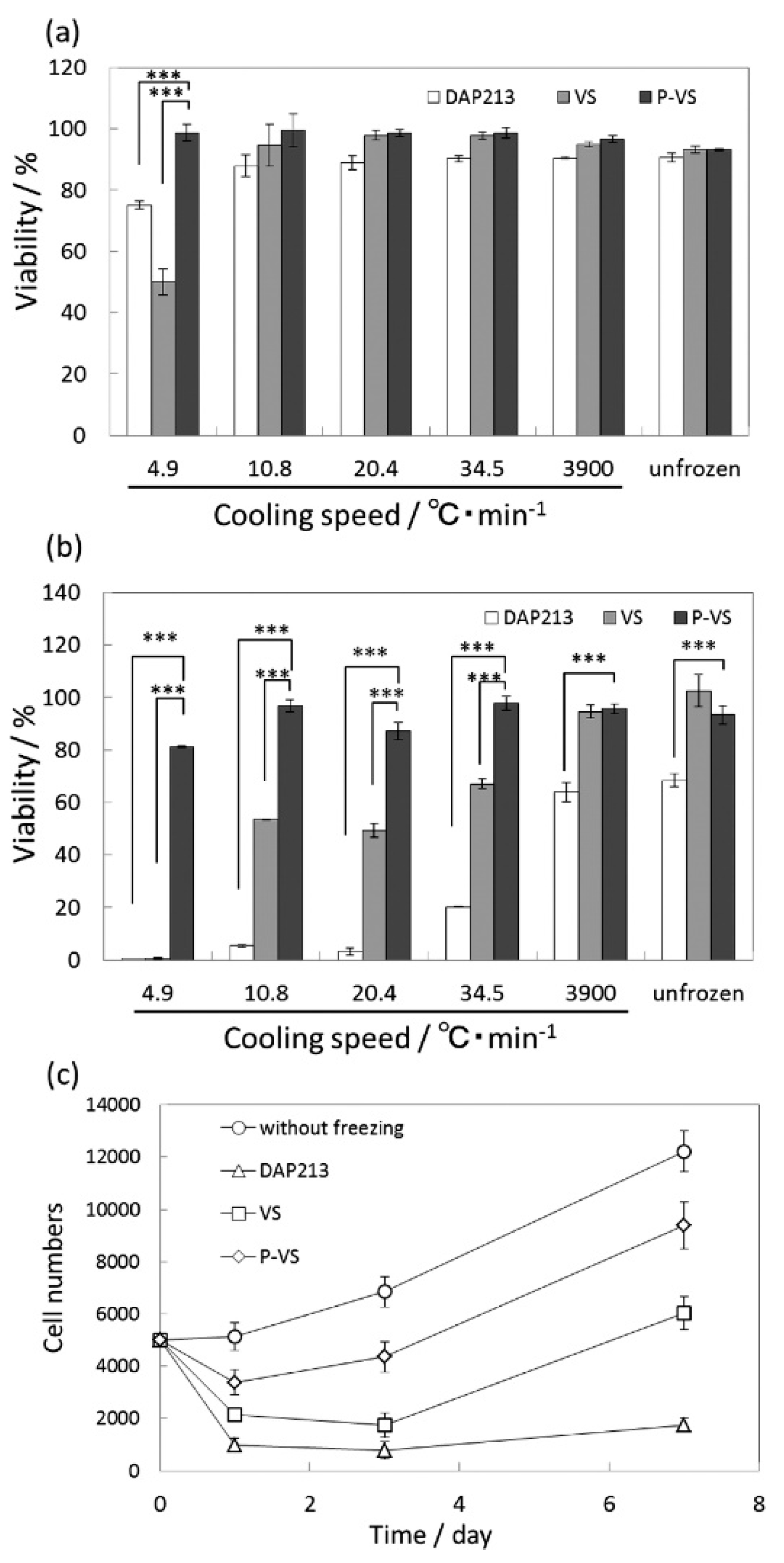
5. Cryopreservation Applications
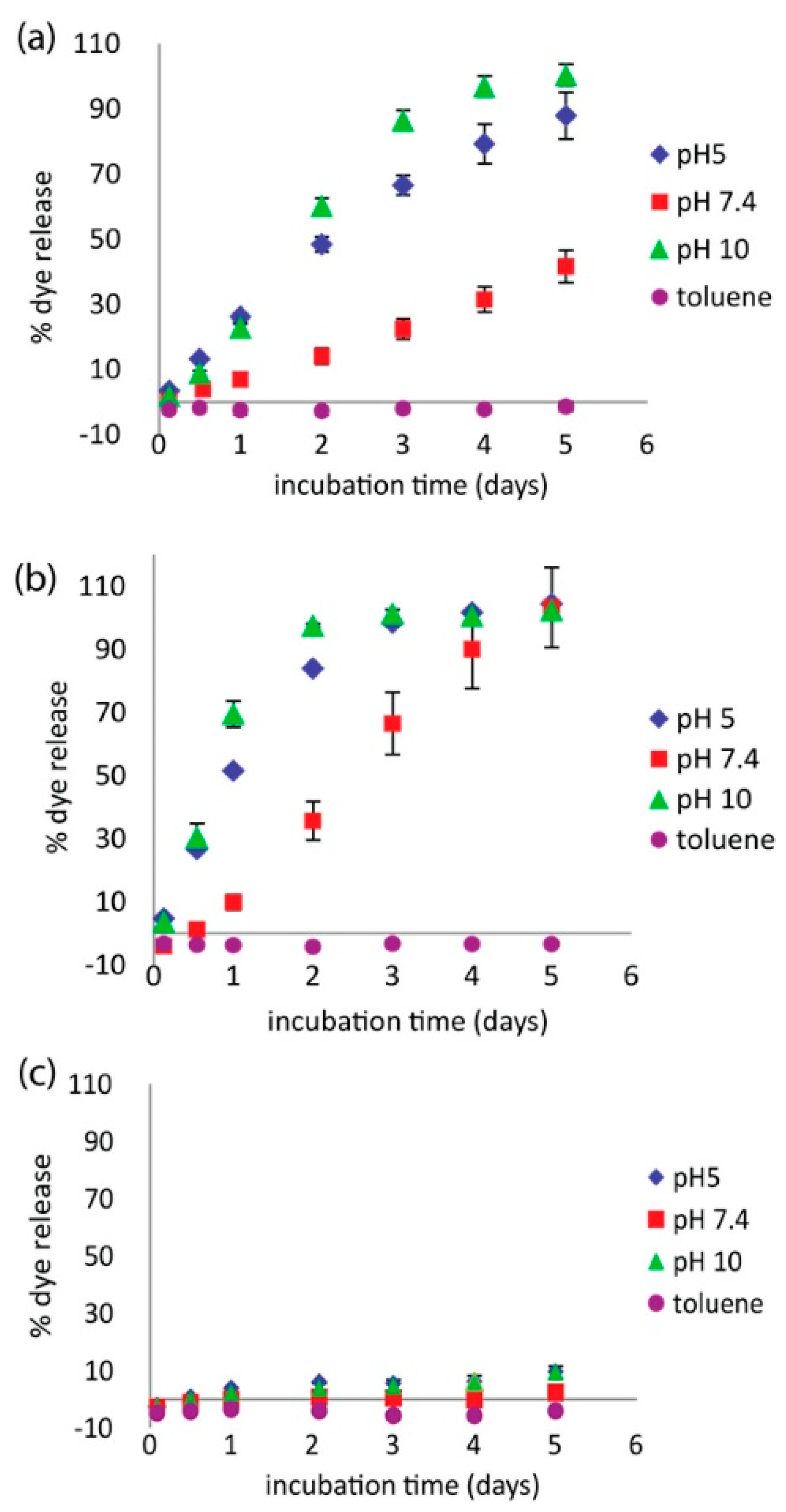
6. Drug Delivery Applications
7. Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zurick, K.M.; Bernards, M. Recent Biomedical Advances with Polyampholyte Polymers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Bernards, M.; He, Y. Polyampholyte polymers as a versatile zwitterionic biomaterial platform. J. Biomater. Sci.-Polym. Ed. 2014, 25, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Laschewsky, A. Structures and Synthesis of Zwitterionic Polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Gao, M.; Gawel, K.; Stokke, B.T. Polyelectrolyte and antipolyelectrolyte effects in swelling of polyampholyte and polyzwitterionic charge balanced and charge offset hydrogels. Eur. Polym. J. 2014, 53, 65–74. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Nuraje, N.; Khutoryanskiy, V.V. Amphoteric nano-, micro-, and macrogels, membranes, and thin films. Soft Matter 2012, 8, 9302–9321. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177–4189. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Oeda, I.; Nakamura, T. The preparation, swelling characteristics, and albumin adsorption and release behaviors of a novel chitosan-based polyampholyte hydrogel. React. Funct. Polym. 2013, 73, 97–107. [Google Scholar] [CrossRef]
- Yilmaz, E.; Yalinca, Z.; Yahya, K.; Sirotina, U. pH responsive graft copolymers of chitosan. Int. J. Biol. Macromol. 2016, 90, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yin, X.; Zhao, Y.; Chen, L. Antifouling enhancement of PVDF membrane tethered with polyampholyte hydrogel layers. Polym. Eng. Sci. 2015, 55, 1367–1373. [Google Scholar] [CrossRef]
- Shen, X.; Yin, X.B.; Zhao, Y.P.; Chen, L. Improved protein fouling resistance of PVDF membrane grafted with the polyampholyte layers. Colloid Polym. Sci. 2015, 293, 1205–1213. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, K.M.; Gu, H.C. Investigations on the Interactions of Proteins with Polyampholyte-Coated Magnetite Nanoparticles. J. Phys. Chem. B 2013, 117, 14129–14135. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.L.; Zhao, L.; Du, G.F.; Wei, X.; Guo, J.X.; Wang, X.Y.; Guo, G.S.; Pu, Q.S. Charge Tunable Zwitterionic Polyampholyte Layers Formed in Cyclic Olefin Copolymer Microchannels through Photochemical Graft Polymerization. ACS Appl. Mater. Interfaces 2013, 5, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Tah, T.; Bernards, M.T. Nonfouling polyampholyte polymer brushes with protein conjugation capacity. Colloids Surf. B Biointerfaces 2012, 93, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Bin Ihsan, A.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; King, D.R.; Sun, T.L.; Nonoyama, T.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Energy-Dissipative Matrices Enable Synergistic Toughening in Fiber Reinforced Soft Composites. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Cao, S.; Barcellona, M.N.; Pfeiffer, F.; Bernards, M.T. Tunable multifunctional tissue engineering scaffolds composed of three-component polyampholyte polymers. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Jain, M.; Matsumura, K. Polyampholyte- and nanosilicate-based soft bionanocomposites with tailorable mechanical and cell adhesion properties. J. Biomed. Mater. Res. Part A 2016, 104, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Bin Ihsan, A.; Sun, T.L.; Kuroda, S.; Haque, M.A.; Kurokawa, T.; Nakajima, T.; Gong, J.P. A phase diagram of neutral polyampholyte—From solution to tough hydrogel. J. Mater. Chem. B 2013, 1, 4555–4562. [Google Scholar] [CrossRef]
- Luo, F.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Li, X.F.; Guo, H.L.; Huang, Y.W.; Zhang, H.J.; Gong, J.P. Tough polyion-complex hydrogels from soft to stiff controlled by monomer structure. Polymer 2017, 116, 487–497. [Google Scholar] [CrossRef]
- Wang, H.W.; Li, P.C.; Xu, K.; Tan, Y.; Lu, C.G.; Li, Y.L.; Liang, X.C.; Wang, P.X. Synthesis and characterization of multi-sensitive microgel-based polyampholyte hydrogels with high mechanical strength. Colloid Polym. Sci. 2016, 294, 367–380. [Google Scholar] [CrossRef]
- Li, G.; Zhang, G.P.; Sun, R.; Wong, C.P. Dually pH-responsive polyelectrolyte complex hydrogel composed of polyacrylic acid and poly (2-(dimthylamino) ethyl methacrylate). Polymer 2016, 107, 332–340. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.H.; Yu, H.C.; Luo, F.; Li, J.H.; Tan, H. Structure and properties of tough polyampholyte hydrogels: Effects of a methyl group in the cationic monomer. RSC Adv. 2016, 6, 114532–114540. [Google Scholar] [CrossRef]
- Long, R.; Hui, C.Y. Fracture toughness of hydrogels: Measurement and interpretation. Soft Matter 2016, 12, 8069–8086. [Google Scholar] [CrossRef] [PubMed]
- Karobi, S.N.; Sun, T.L.; Kurokawa, T.; Luo, F.; Nakajima, T.; Nonoyama, T.; Gong, J.P. Creep Behavior and Delayed Fracture of Tough Polyampholyte Hydrogels by Tensile Test. Macromolecules 2016, 49, 5630–5636. [Google Scholar] [CrossRef]
- Sun, T.L.; Luo, F.; Hong, W.; Cui, K.P.; Huang, Y.W.; Zhang, H.J.; King, D.R.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Bulk Energy Dissipation Mechanism for the Fracture of Tough and Self-Healing Hydrogels. Macromolecules 2017, 50, 2923–2931. [Google Scholar] [CrossRef]
- Luo, F.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Zhao, Y.; Bin Ihsan, A.; Guo, H.L.; Li, X.F.; Gong, J.P. Crack Blunting and Advancing Behaviors of Tough and Self-healing Polyampholyte Hydrogel. Macromolecules 2014, 47, 6037–6046. [Google Scholar] [CrossRef]
- Nakajima, T. Generalization of the sacrificial bond principle for gel and elastomer toughening. Polym. J. 2017, 49, 477–485. [Google Scholar] [CrossRef]
- Su, E.; Okay, O. Polyampholyte hydrogels formed via electrostatic and hydrophobic interactions. Eur. Polym. J. 2017, 88, 191–204. [Google Scholar] [CrossRef]
- Dyakonova, M.A.; Stavrouli, N.; Popescu, M.T.; Kyriakos, K.; Grillo, I.; Philipp, M.; Jaksch, S.; Tsitsilianis, C.; Papadakis, C.M. Physical Hydrogels via Charge Driven Self-Organization of a Triblock Polyampholyte—Rheological and Structural Investigations. Macromolecules 2014, 47, 7561–7572. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Shull, K.R. High-Toughness Polycation Cross-Linked Triblock Copolymer Hydrogels. Macromolecules 2017, 50, 3637–3646. [Google Scholar] [CrossRef]
- Luo, F.; Sun, T.L.; Nakajima, T.; King, D.R.; Kurokawa, T.; Zhao, Y.; Bin Ihsan, A.; Li, X.F.; Guo, H.L.; Gong, J.P. Strong and Tough Polyion-Complex Hydrogels from Oppositely Charged Polyelectrolytes: A Comparative Study with Polyampholyte Hydrogels. Macromolecules 2016, 49, 2750–2760. [Google Scholar] [CrossRef]
- Bin Ihsan, A.; Sun, T.L.; Kurokawa, T.; Karobi, S.N.; Nakajima, T.; Nonoyama, T.; Roy, C.K.; Luo, F.; Gong, J.P. Self-Healing Behaviors of Tough Polyampholyte Hydrogels. Macromolecules 2016, 49, 4245–4252. [Google Scholar] [CrossRef]
- Na, Y.H. Double network hydrogels with extremely high toughness and their applications. Korea-Aust. Rheol. J. 2013, 25, 185–196. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sun, T.L.; Luo, F.; Kurokawa, T.; Karobi, S.N.; Nakajima, T.; Gong, J.P. Molecular structure of self-healing polyampholyte hydrogels analyzed from tensile behaviors. Soft Matter 2015, 11, 9355–9366. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.P.; Sun, T.L.; Kurokawa, T.; Nakajima, T.; Nonoyama, T.; Chen, L.; Gong, J.P. Stretching-induced ion complexation in physical polyampholyte hydrogels. Soft Matter 2016, 12, 8833–8840. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Mehlich, A.; Koga, N.; Huang, J.Q.; Koga, R.; Gao, X.Y.; Hu, C.G.; Jin, C.; Rief, M.; Kast, J.; et al. Forced protein unfolding leads to highly elastic and tough protein hydrogels. Nat. Commun. 2013, 4, 2974. [Google Scholar] [CrossRef] [PubMed]
- Byette, F.; Laventure, A.; Marcotte, I.; Pellerin, C. Metal-Ligand Interactions and Salt Bridges as Sacrificial Bonds in Mussel Byssus-Derived Materials. Biomacromolecules 2016, 17, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Duan, H.D.; Zhu, L.P.; Li, G.Q.; Ban, Q.; Lucia, L.A. A semi-interpenetrating network polyampholyte hydrogel simultaneously demonstrating remarkable toughness and antibacterial properties. New J. Chem. 2016, 40, 10520–10525. [Google Scholar] [CrossRef]
- Dobbins, S.C.; McGrath, D.E.; Bernards, M.T. Nonfouling Hydrogels Formed from Charged Monomer Subunits. J. Phys. Chem. B 2012, 116, 14346–14352. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.E.; Zurick, K.M.; McGrath, D.E.; Bernards, M.T. Multifunctional Polyampholyte Hydrogels with Fouling Resistance and Protein Conjugation Capacity. Biomacromolecules 2013, 14, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Hayashi, F.; Nagashima, T.; Hyon, S.H. Long-term cryopreservation of human mesenchymal stem cells using carboxylated poly-l-lysine without the addition of proteins or dimethyl sulfoxide. J. Biomater. Sci.-Polym. Ed. 2013, 24, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Kawamoto, K.; Takeuchi, M.; Yoshimura, S.; Tanaka, D.; Hyon, S.H. Cryopreservation of a Two-Dimensional Monolayer Using a Slow Vitrification Method with Polyampholyte to Inhibit Ice Crystal Formation. ACS Biomater. Sci. Eng. 2016, 2, 1023–1029. [Google Scholar] [CrossRef]
- Rajan, R.; Jain, M.; Matsumura, K. Cryoprotective properties of completely synthetic polyampholytes via reversible addition-fragmentation chain transfer (RAFT) polymerization and the effects of hydrophobicity. J. Biomater. Sci.-Polym. Ed. 2013, 24, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Hayashi, F.; Nagashima, T.; Matsumura, K. Toward a Molecular Understanding of the Mechanism of Cryopreservation by Polyampholytes: Cell Membrane Interactions and Hydrophobicity. Biomacromolecules 2016, 17, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Rajan, R.; Hyon, S.H.; Matsumura, K. Hydrogelation of dextran-based polyampholytes with cryoprotective properties via click chemistry. Biomater. Sci. 2014, 2, 308–317. [Google Scholar] [CrossRef]
- Jain, M.; Matsumura, K. Thixotropic injectable hydrogel using a polyampholyte and nanosilicate prepared directly after cryopreservation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 69, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Das, E.; Matsumura, K. Tunable Phase-Separation Behavior of Thermoresponsive Polyampholytes Through Molecular Design. J. Polym. Sci. Part A-Polym. Chem. 2017, 55, 876–884. [Google Scholar] [CrossRef]
- Ahmed, S.; Hayashi, F.; Nagashima, T.; Matsumura, K. Protein cytoplasmic delivery using polyampholyte nanoparticles and freeze concentration. Biomaterials 2014, 35, 6508–6518. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Fujitab, S.; Matsumura, K. Enhanced protein internalization and efficient endosomal escape using polyampholyte-modified liposomes and freeze concentration. Nanoscale 2016, 8, 15888–15901. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel carrier design for targeted drug delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef] [PubMed]
- Barcellona, M.N.; Johnson, N.; Bernards, M.T. Characterizing Drug Release from Nonfouling Polyampholyte Hydrogels. Langmuir 2015, 31, 13402–13409. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergenov, S.E.; Tatykhanova, G.S.; Klivenko, A.N. Complexation of macroporous amphoteric cryogels based on N,N-dimethylaminoethyl methacrylate and methacrylic acid with dyes, surfactant, and protein. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Rudov, A.A.; Gelissen, A.P.H.; Lotze, G.; Schmid, A.; Eckert, T.; Pich, A.; Richtering, W.; Potemkin, I.I. Intramicrogel Complexation of Oppositely Charged Compartments As a Route to Quasi-Hollow Structures. Macromolecules 2017, 50, 4435–4445. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ramasamy, K.; Ban, N.N.; Majeed, A.B.A. Synthesis of poly 3-(methacryloylamino)propyl trimethylammonium chloride-co-methacrylic acid copolymer hydrogels for controlled indomethacin delivery. J. Appl. Polym. Sci. 2013, 128, 3365–3374. [Google Scholar] [CrossRef]
- Cao, Z.F.; Jin, Y.; Miao, Q.; Ma, C.Y.; Zhang, B. Preparation and properties of a dually responsive hydrogels based on polyampholyte for oral delivery of drugs. Polym. Bull. 2013, 70, 2675–2689. [Google Scholar] [CrossRef]
- Sankar, R.M.; Meera, K.M.S.; Samanta, D.; Jithendra, P.; Mandal, A.B.; Jaisankar, S.N. The pH-sensitive polyampholyte nanogels: Inclusion of carbon nanotubes for improved drug loading. Colloids Surf. B-Biointerfaces 2013, 112, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Grolman, J.M.; Rizvi, A.; Hisao, G.S.; Rienstra, C.M.; Zimmerman, S.C. pH-Triggered Release from Polyamide Microcapsules Prepared by Interfacial Polymerization of a Simple Diester Monomer. ACS Macro Lett. 2017, 6, 321–325. [Google Scholar] [CrossRef]
- Schulze, N.; Tiersch, B.; Zenke, I.; Koetz, J. Polyampholyte-tuned lyotrop lamellar liquid crystalline systems. Colloid Polym. Sci. 2013, 291, 2551–2559. [Google Scholar] [CrossRef]
- Ekici, S.; Tetik, A. Development of polyampholyte hydrogels based on laponite for electrically stimulated drug release. Polym. Int. 2015, 64, 335–343. [Google Scholar] [CrossRef]
- Ali, S.A.; Al-Muallem, H.A.; Al-Hamouz, O.; Estaitie, M.K. Synthesis of a novel zwitterionic bisphosphonate cyclopolymer containing residues of alendronic acid. React. Funct. Polym. 2015, 86, 80–86. [Google Scholar] [CrossRef]
- Asayama, S.; Seno, K.; Kawakami, H. Synthesis of Carboxymethyl Poly(1-vinylimidazole) as a Polyampholyte for Biocompatibility. Chem. Lett. 2013, 42, 358–360. [Google Scholar] [CrossRef]
- Ladika, M.; Kalantar, T.H.; Shao, H.; Dean, S.L.; Harris, J.K.; Sheskey, P.J.; Coppens, K.; Balwinski, K.M.; Holbrook, D.L. Polyampholyte Acrylic Latexes for Tablet Coating Applications. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]

| Chemical Name | Acronym | Monomer Formula | Strength of Functional Group |
|---|---|---|---|
| Acrylamide | AM | CH2=CHCONH2 | Weak cation |
| N-[3-(Dimethylamino)propyl] acrylamide | DMAPAA | CH2=CHCONH(CH2)3N(CH3)2 | Weak cation |
| 2-(Dimethylamino)ethyl methacrylate | DMAEM | CH2=C(CH3)COOCH2CH2N(CH3)2 | Weak cation |
| 2-(Diethylamino)ethyl methacrylate | DEAEM | CH2=C(CH3)CO2CH2CH2N(C2H5)2 | Weak cation |
| [2-(Methacryloyloxy)ethyl] trimethylammonium chloride | TM | CH2=C(CH3)CO2CH2CH2N(CH3)3Cl | Strong cation |
| 2-(Acryloyloxy ethyl)trimethyl ammonium chloride | TMA | CH2=CHCO2CH2CH2N(CH3)3Cl | Strong cation |
| [3-(Methacryloylamino)propyl] trimethylammonium chloride | MAPTAC | CH2=C(CH3)CONH(CH2)3N(CH3)3Cl | Strong cation |
| 2-Carboxyethyl acrylate | CAA | CH2=CHCO2(CH2)2CO2H | Weak anion |
| Methacrylic acid | MAA | CH2=C(CH3)COOH | Weak anion |
| Acrylic acid | AA | CH2=CHCOOH | Weak anion |
| Carboxylated poly-l-lysine | COOH-PLL | NH2(CH2)4CHNH2COOH | Weak anion |
| 3-Sulfopropyl methacrylate potassium salt | SA | H2C=C(CH3)CO2(CH2)3SO3K | Strong anion |
| 2-Sulfoethyl methacrylate | SE | H2C=C(CH3)CO2(CH2)2SO3H | Strong anion |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haag, S.L.; Bernards, M.T. Polyampholyte Hydrogels in Biomedical Applications. Gels 2017, 3, 41. https://doi.org/10.3390/gels3040041
Haag SL, Bernards MT. Polyampholyte Hydrogels in Biomedical Applications. Gels. 2017; 3(4):41. https://doi.org/10.3390/gels3040041
Chicago/Turabian StyleHaag, Stephanie L., and Matthew T. Bernards. 2017. "Polyampholyte Hydrogels in Biomedical Applications" Gels 3, no. 4: 41. https://doi.org/10.3390/gels3040041






