Microfluidic Spun Alginate Hydrogel Microfibers and Their Application in Tissue Engineering
Abstract
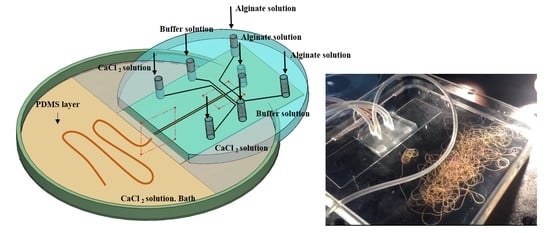
:1. Introduction
2. Microfluidic Spinning Method
2.1. Parallel Laminar Flows
2.2. Coaxial Laminar Flows
2.3. Valve-Involved Spinning Method
3. Alginate Hydrogel Microfibers as Scaffolds for Cell Culture
3.1. Three-Dimensional Cell Culture
3.2. Biomimetic Microorganoids
3.3. Cell Guidance
4. Alginate Hydrogel Microfibers as Scaffolding Elements for Higher-Order Tissue
4.1. Microfluidic Printing
4.2. Guided-Assembly Method
5. Conclusions and Future Perspective
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 20, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Khademhosseini, A. Modular tissue engineering: Engineering biological tissues from the bottom up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, C.A.M.; Rengarajan, V.; Finley, T.D.; Keles, H.O.; Sung, Y.R.; Li, B.Q.; Gurkan, U.A.; Demirci, U. Three-dimensional magnetic assembly of microscale hydrogels. Adv. Mater. 2011, 23, 4254–4260. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Mun, C.H.; Kang, E.; No, D.Y.; Ju, J.; Lee, S.H. One-stop microfiber spinning and fabrication of a fibrous cell-encapsulated scaffold on a single microfluidic platform. Biofabrication 2014, 6, 024108. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.T.; Morimoto, Y.; Takeuchi, S. Molding cell beads for rapid construction of macroscopic 3D tissue architecture. Adv. Mater. 2011, 12, H90–H94. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.S.; Philippi, J.A.; Sitti, M.; MacKrell, J.; Campbell, P.G.; Amon, C. Control of cell behavior by aligned micro/nanofibrous biomaterial scaffolds fabricated by spinneret-based tunable engineered parameters (STEP) technique. Small 2008, 4, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.M.; Warren, S.P.; Hilgart, D.A.; Schworer, A.T.; Pabba, S.; Gobin, A.S.; Cohn, R.W.; Keynton, R.S. Endothelial cell scaffolds generated by 3D direct writing of biodegradable polymer microfibers. Biomaterials 2011, 32, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jun, Y.; Qin, J.H.; Lee, S.H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.C.; Sharifi, F.; Wrede, A.H.; Kimlinger, D.F.; Thomas, D.G.; Vander Wiel, J.B.; Vander Wiel, J.B.; Chen, Y.F.; Montazami, R.; Hashemi, N.N. Microfibers as physiologically relevant platforms for creation of 3D cell cultures. Macromol. Biosci. 2017, 17, 1700279. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Atencia, J.; Beebe, D.J. Controlled microfluidic interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.B.; Vishwanath, A.; Brody, J.P.; Austin, R.H. Hydrodynamic focusing on a silicon chip: Mixing nanoliters in microseconds. Phys. Rev. Lett. 1998, 80, 3863–3866. [Google Scholar] [CrossRef]
- Ng, J.M.K.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for integrated poly (dimethylsiloxane) microfluidic systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef]
- Yamada, M.; Sugaya, S.; Naganuma, Y.; Seki, M. Microfluidic synthesis of chemically and physically anisotropic hydrogel microfibers for guided cell growth and networking. Soft Matter 2012, 8, 3122–3130. [Google Scholar] [CrossRef]
- Lin, Y.S.; Huang, K.S.; Yang, C.H.; Wang, C.Y.; Yang, Y.S.; Hsu, H.C.; Liao, Y.J.; Tsai, C.W. Microfluidic synthesis of microfibers for magnetic-responsive controlled drug release and cell culture. PLoS ONE 2012, 7, e33184. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Huang, Q.; Shi, Q.; Wang, H.P.; Liu, X.M.; Seki, M.; Nakajima, M.; Fukuda, T. Magnetic assembly of microfluidic spun alginate microfibers for fabricating three-dimensional cell-laden hydrogel constructs. Microfluid. Nanofluid. 2015, 19, 1169–1180. [Google Scholar] [CrossRef]
- Yamada, M.; Utoh, R.; Ohashi, K.; Tatsumi, K.; Yamato, M.; Okano, T.; Seki, M. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials 2012, 33, 304–8315. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Yamakoshi, K.; Yajima, Y.; Utoh, R.; Yamada, M.; Seki, M. Preparation of stripe-patterned heterogeneous hydrogel sheets using microfluidic devices for high-density coculture of hepatocytes and fibroblasts. J. Biosci. Bioeng. 2013, 116, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, J.Y.; Lee, J.Y.; Park, H.; Park, Y.D.; Lee, K.B.; Whang, C.M.; Lee, S.H. “On the fly” continuous generation of alginate fibers using a microfluidic device. Langmuir 2007, 23, 9104–9108. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wei, W.B.; Wang, Y.Q.; Xu, C.; Guo, Y.Q.; Qin, J.H. Simple Spinning of Heterogeneous Hollow Microfibers on Chip. Adv. Mater. 2016, 23, 6649–6655. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Shin, S.J.; Park, Y.; Lee, S.H. Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications. Small 2009, 5, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zheng, F.Y.; Lu, J.; Shang, L.R.; Xie, Z.Y.; Zhao, Y.J.; Chen, Y.P.; Gu, Z.Z. Bioinspired multicompartmental Microfibers from Microfluidics. Adv. Mater. 2014, 26, 5184–5190. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, A.S.; Sajjadi, S. Flexible asymmetric encapsulation for dehydration-responsive hybrid microfibers. Small 2016, 12, 4146–4155. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.J.; Wang, W.; Xie, R.; Ju, X.J.; Liu, Z.; Chu, L.Y. Microfluidic generation of hollow ca-alginate microfibers. Lab Chip 2016, 16, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Wang, L.; Yin, F.C.; Yu, Y.; Wang, Y.Q.; Liu, H.; Wang, H.; Sun, N.; Liu, H.T.; Qin, J.H. A hollow fiber system for simple generation of human brain organoids. Intergr. Biol. (Camb.) 2017, 9, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Park, D.; Jun, Y.; Lee, J.; Hyun, J.; Lee, S.H. Biomimetic spinning of silk fibers and in situ cell encapsulation. Lab Chip 2016, 16, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- He, X.H.; Wang, W.; Liu, Y.M.; Jiang, M.Y.; Wu, F.; Deng, K.; Liu, Z.; Ju, X.J.; Xie, R.; Chu, L.Y. Microfluidic Fabrication of Bio-Inspired Microfibers with Controllable Magnetic Spindle-Knots for 3D Assembly and Water Collection. ACS Appl. Mater. Interfaces 2015, 7, 17471–17481. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Hu, C.Z.; Nakajima, M.; Takeuchi, M.; Seki, M.; Yue, T.; Shi, Q.; Fukuda, T. On-chip fabrication and magnetic force estimation of peapod-like hybrid microfibers using a microfluidic device. Microfluid. Nanofluid. 2015, 18, 1177–1187. [Google Scholar] [CrossRef]
- Yu, Y.R.; Fu, F.F.; Shang, L.R.; Cheng, Y.; Gu, Z.Z.; Zhao, Y.J. Bioinspired Helical Microfibers from Microfluidics. Adv. Mater. 2017, 29, 1605765. [Google Scholar] [CrossRef] [PubMed]
- Totton, S.; Takeuchi, S. Formation of liquid rope coils in a coaxial microfluidic device. RSC Adv. 2015, 5, 33691–33695. [Google Scholar]
- Kang, E.; Shin, S.J.; Lee, K.H.; Lee, S.H. Novel PDMS cylindrical channels that generate coaxial flow, and application to fabrication of microfibers and particles. Lab Chip 2010, 10, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Choi, Y.Y.; Chae, S.K.; Moon, J.H.; Chang, J.Y.; Lee, S.H. Microfluidic Spinning of Flat Alginate Fibers with Grooves for Cell-Aligning Scaffolds. Adv. Mater. 2012, 24, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Kobayashi, K.; Tanaka, D.; Sekiguchi, T.; Shoji, S. Simple microfluidic formation of highly heterogeneous microfibers using a combination of sheath units. Lab Chip 2017, 17, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wen, H.; Ma, J.Y.; Lykkemark, S.; Xu, H.; Qin, J.H. Flexible Fabrication of Biomimetic Bamboo-Like Hybrid Microfibers. Adv. Mater. 2014, 26, 2494–2499. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jeong, G.S.; Choi, Y.Y.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater. 2011, 10, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Kim, M.J.; Hwang, Y.H.; Jeon, E.A.; Kang, A.R.; Lee, S.H.; Lee, D.Y. Microfluidics-generated pancreatic islet microfibers for enhanced immunoprotection. Biomaterials 2013, 34, 8122–8130. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.Y.; Okitsu, T.; Onoe, H.; Kiyosawa, M.; Teramae, H.; Iwanaga, S.; Kazama, T.; Matsumoto, T.; Takeuchi, S. Smooth Muscle-Like Tissue Constructs with Circumferentially Oriented Cells Formed by the Cell Fiber Technology. PLoS ONE 2015, 10, e0119010. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Piao, Y.; Seo, T.S. Circumferential alignment of vascular smooth muscle cells in a circular microfluidic channel. Biomaterials 2014, 35, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Lee, K.H.; Kang, E.; Kim, D.S.; Lee, S.H. Microfluidic wet spinning of chitosan-alginate microfibers and encapsulation of HepG2 cells in fibers. Biomicrofluidics 2011, 5, 022208. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, J.; Bolderson, J.; Zhong, M.L.; Dalby, M.J.; Cusack, M.; Yin, H.B.; Fan, H.S.; Zhang, X.D. Continuous Fabrication and Assembly of Spatial Cell-Laden Fibers for a Tissue-Like Construct via a Photolithographic-Based Microfluidic Chip. ACS Appl. Mater. Interfaces 2017, 9, 14606–14617. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.C.; He, X.H.; Yang, Y.; Wei, D.; Sun, J.; Zhong, M.L.; Xie, R.; Fan, H.S.; Zhang, X.D. Microfluidic-based generation of functional microfibers for biomimetic complex tissue construction. Acta Biomater. 2016, 38, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.C.; Tompkins, R.G.; Yarmush, M.L. Hepatocytes in collagen sandwich: Evidence for transcriptional and translational regulation. J. Cell Biol. 1992, 116, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; He, Y.; Fu, J.Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.T.; Gungor-Qzkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.J.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.D.; Xie, R.X.; Liu, Y.P.; Luo, G.A.; Ding, M.Y.; Liang, Q.L. Bioinspired microfibers with embedded perfusable helical channels. Adv. Mater. 2017, 29, 1701664. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Naganuma, Y.; Yajima, Y.; Yamada, M.; Seki, M. Patterned hydrogel microfibers prepared using multilayered microfluidic devices for guiding network formation of neural cells. Biofabrication 2014, 6, 035011. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Shi, Q.; Huang, Q.; Wang, H.P.; Xiong, X.L.; Hu, C.Z.; Fukuda, T. Magnetic alginate microfibers as scaffolding elements for the fabrication of microvascular-like structures. Acta Biomater. 2018, 66, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, L.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanian, S.; Qasaimeh, M.A.; Akbari, M.; Tamayol, A.; Juncker, D. Microfluidic direct writer with integrated declogging mechanism for fabricating cell-laden hydrogel constructs. Biomed. Microdevices 2014, 16, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Shi, Q.; Wang, H.P.; Sun, T.; Huang, Q.; Fukuda, T. Magnetically-guided assembly of microfluidic fibers for ordered construction of diverse netlike modules. J. Micromech. Microeng. 2017, 27, 125014. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Li, X.; Shi, Q.; Wang, H.; Huang, Q.; Fukuda, T. Microfluidic Spun Alginate Hydrogel Microfibers and Their Application in Tissue Engineering. Gels 2018, 4, 38. https://doi.org/10.3390/gels4020038
Sun T, Li X, Shi Q, Wang H, Huang Q, Fukuda T. Microfluidic Spun Alginate Hydrogel Microfibers and Their Application in Tissue Engineering. Gels. 2018; 4(2):38. https://doi.org/10.3390/gels4020038
Chicago/Turabian StyleSun, Tao, Xingfu Li, Qing Shi, Huaping Wang, Qiang Huang, and Toshio Fukuda. 2018. "Microfluidic Spun Alginate Hydrogel Microfibers and Their Application in Tissue Engineering" Gels 4, no. 2: 38. https://doi.org/10.3390/gels4020038