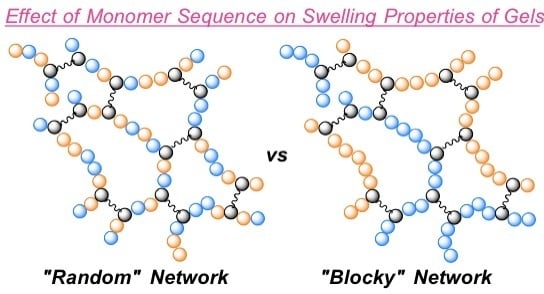
Effect of Monomer Sequence along Network Chains on Thermoresponsive Properties of Polymer Gels
Abstract
:1. Introduction
2. Results and Discussion
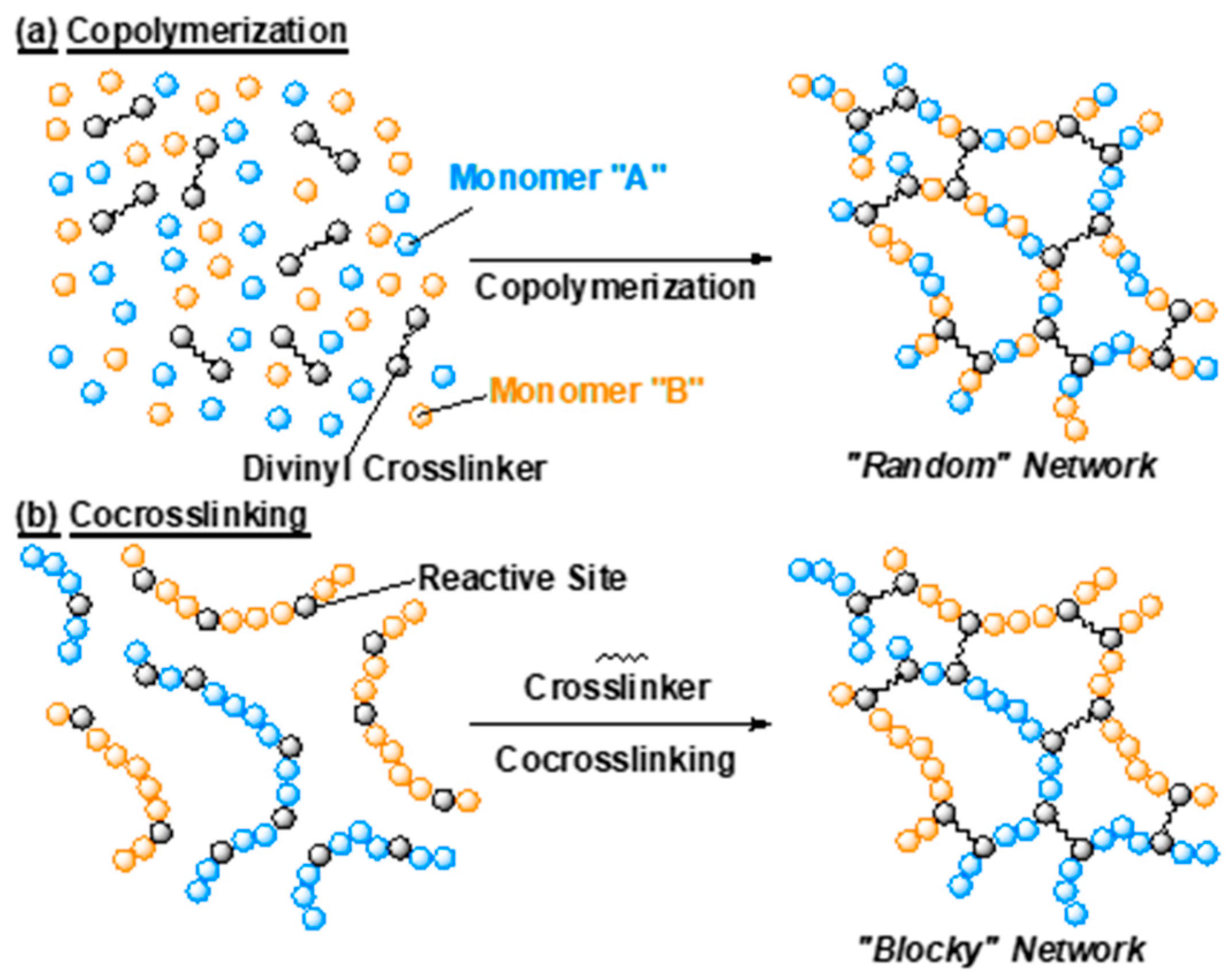
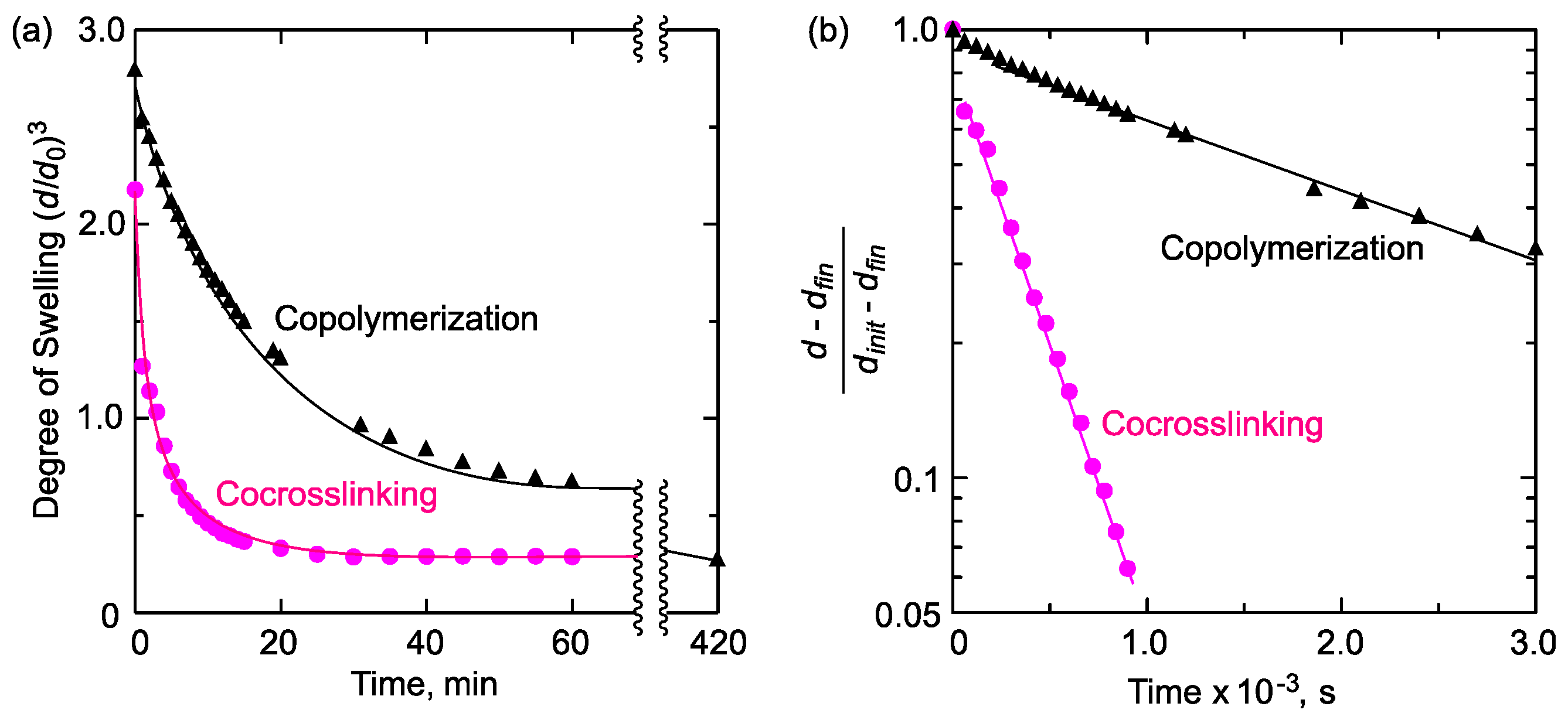
2.1. Gel Synthesis by Copolymerization and Co-crosslinking
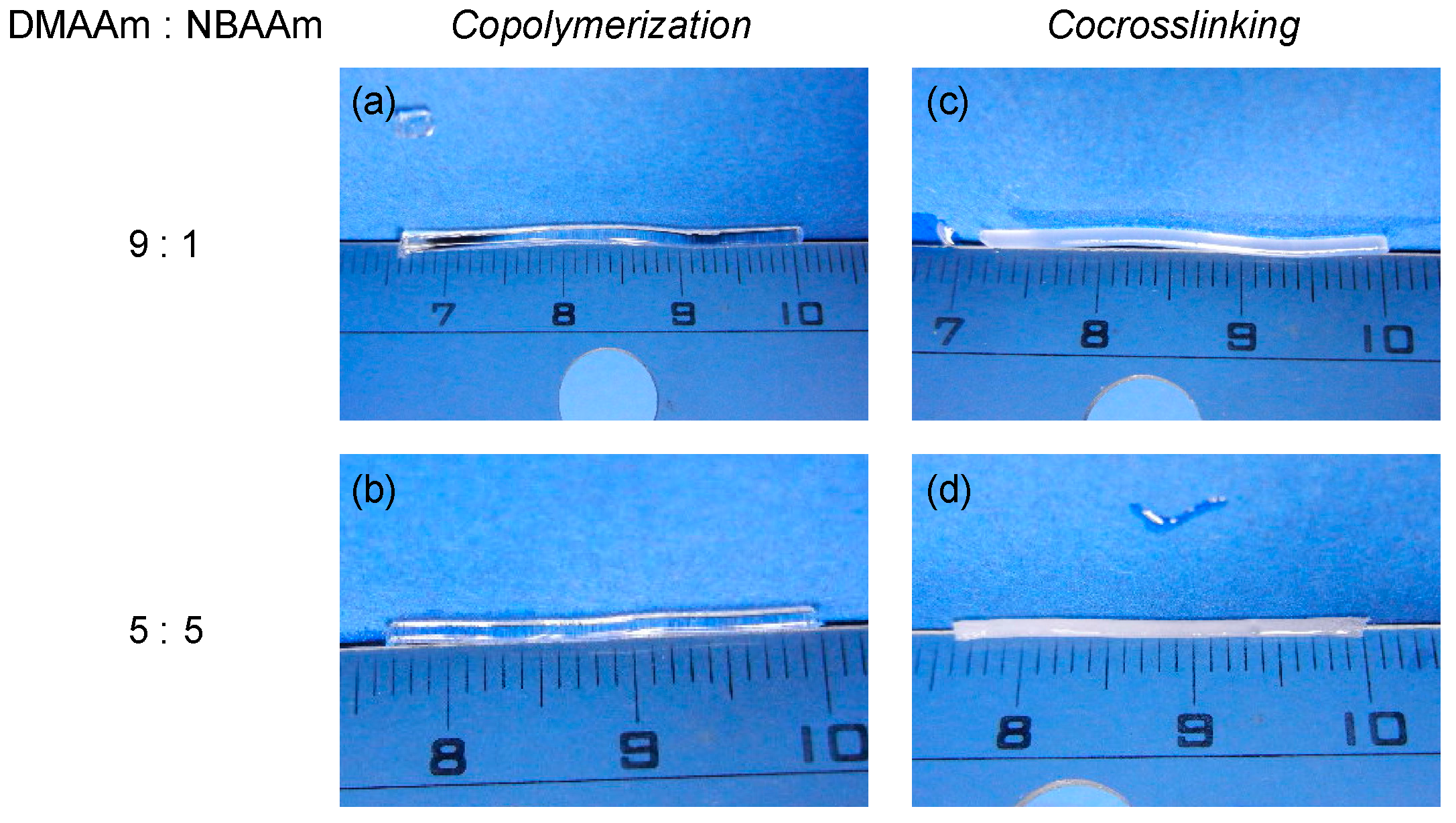
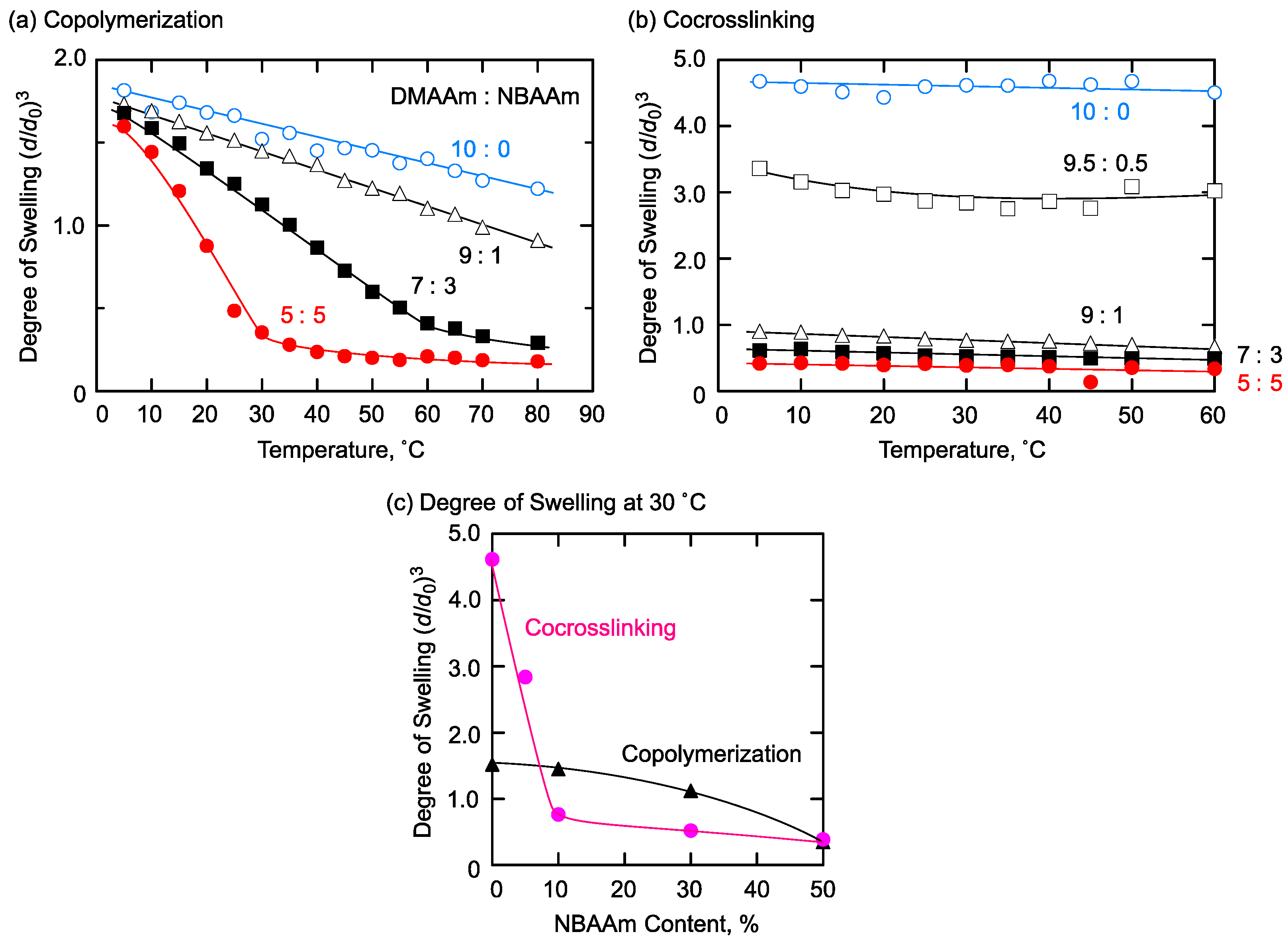
2.2. Hydrophilic/Hydrophobic Combination
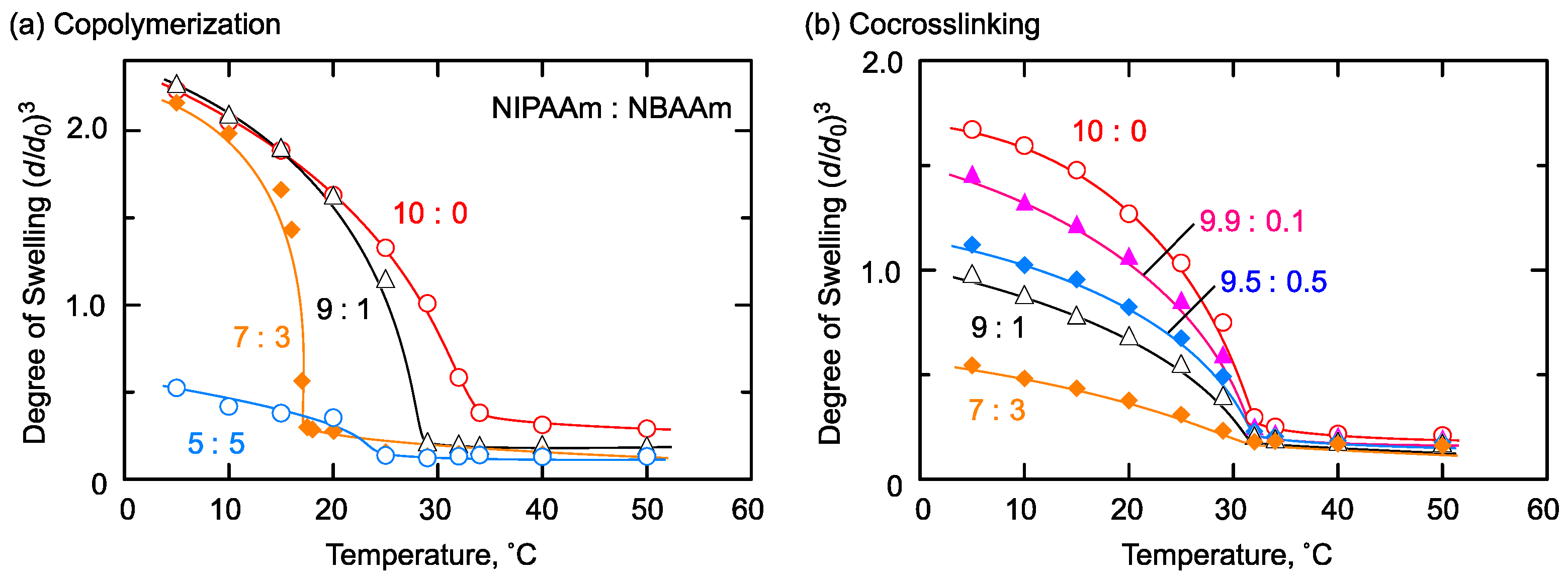
2.3. Thermoresponsive/Hydrophobic Combination
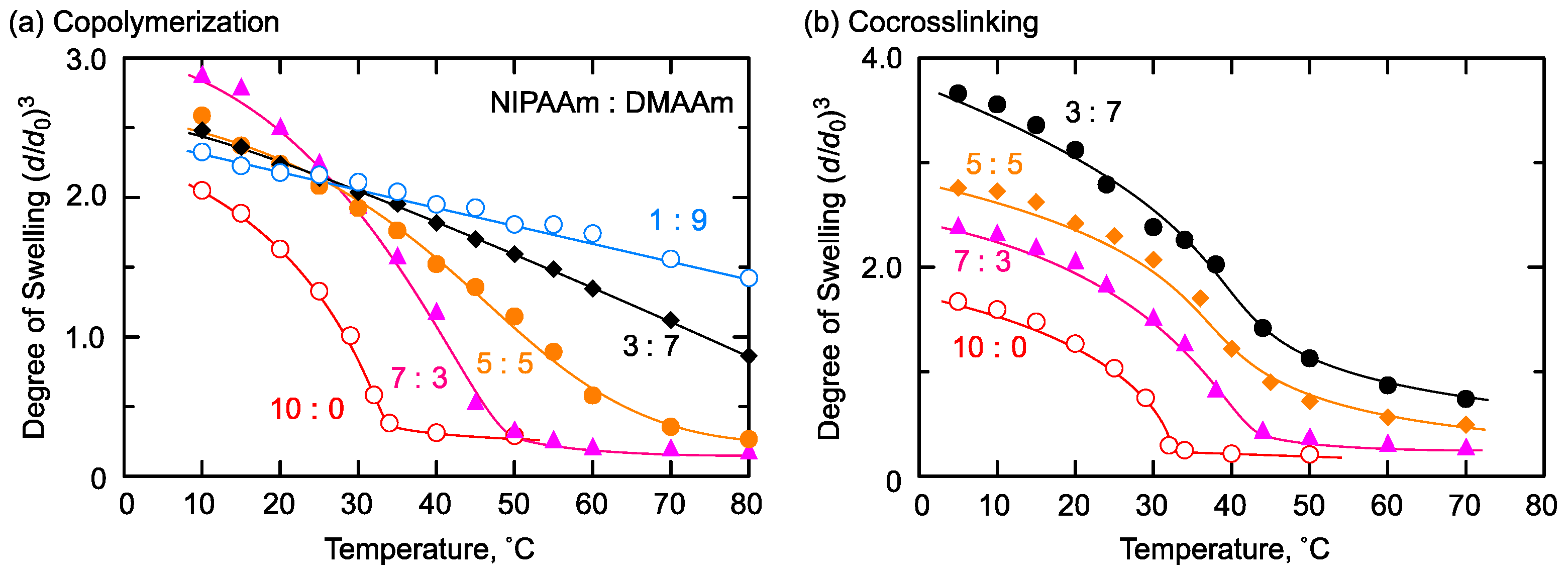
2.4. Thermoresponsive/Hydrophiliic Combination
3. Conclusions
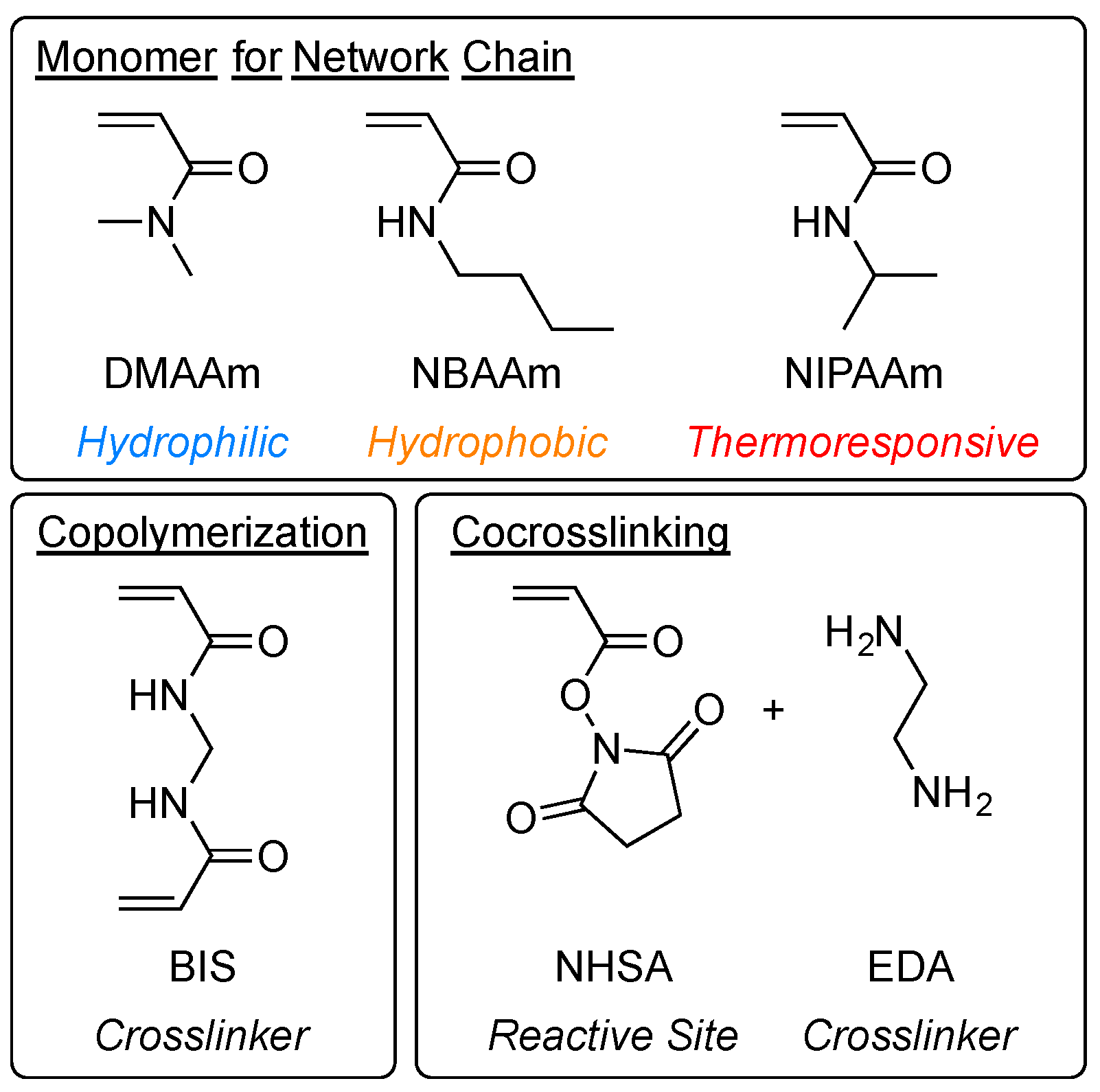
4. Materials and Methods
4.1. Materials
4.2. Copolymerization Gel Synthesis
4.3. Co-crosslinking Gel Synthesis
4.4. Characterization
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanaka, T. Collapse of Gels and the Critical Endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.; Sun, S.-T.; Nishio, I.; Swislow, G.; Shah, A. Phase Transitions in Ionic Gels. Phys. Rev. Lett. 1980, 45, 1636–1639. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Osada, Y.; Gong, J. Stimuli-responsive polymer gels and their application to chemomechanical systems. Prog. Polym. Sci. 1993, 18, 187–226. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 53–77. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [PubMed]
- Diaz Diaz, D.; Kuhbeck, D.; Koopmans, R.J. Stimuli-Responsive Gels as Reaction Vessels and Reusable Catalysts. Chem. Soc. Rev. 2011, 40, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Temperature-responsive bioconjugates. 2. Molecular design for temperature-modulated bioseparations. Bioconjug. Chem. 1993, 4, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Katakai, R.; Saito, K.; Sorimachi, M.; Hiroki, A.; Kinuno, T.; Nakajima, T.; Shimizu, M.; Kubota, H.; Yoshida, M. Hydrophobic Site-Specific Control of the Volume Phase Transition of Hydrogels. Macromolecules 1998, 31, 3383–3384. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Park, J.-S.; Kataoka, K. Precise Control of Lower Critical Solution Temperature of Thermosensitive Poly(2-isopropyl-2-oxazoline) via Gradient Copolymerization with 2-Ethyl-2-oxazoline as a Hydrophilic Comonomer. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Confortini, O.; Du Prez, F.E. Functionalized Thermo-Responsive Poly(vinyl ether) by Living Cationic Random Copolymerization of Methyl Vinyl Ether and 2-Chloroethyl Vinyl Ether. Macromol. Chem. Phys. 2007, 208, 1871–1882. [Google Scholar] [CrossRef]
- Ida, S.; Kawahara, T.; Fujita, Y.; Tanimoto, S.; Hirokawa, Y. Thermoresponsive Properties of Copolymer Gels Induced by Appropriate Hydrophilic/Hydrophobic Balance of Monomer Combination. Macromol. Symp. 2015, 350, 14–21. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Georgiou, T.K. Covalent amphiphilic polymer networks. Curr. Opin. Colloid Interface Sci. 2003, 8, 76–85. [Google Scholar] [CrossRef]
- Erdodi, G.; Kennedy, J.P. Amphiphilic conetworks: Definition, synthesis, applications. Prog. Polym. Sci. 2006, 31, 1–18. [Google Scholar] [CrossRef]
- Weber, M.; Stadler, R. Hydrophilic-hydrophobic two-component polymer networks: 1. Synthesis of reactive poly(ethylene oxide) telechelics. Polymer 1988, 29, 1064–1070. [Google Scholar] [CrossRef]
- Weber, M.; Stadler, R. Hydrophilic-hydrophobic two-component polymer networks: 2. Synthesis and characterization of poly(ethylene oxide)-linked-polybutadiene. Polymer 1988, 29, 1071–1078. [Google Scholar] [CrossRef]
- Simmons, M.R.; Yamasaki, E.N.; Patrickios, C.S. Cationic Amphiphilic Model Networks: Synthesis by Group Transfer Polymerization and Characterization of the Degree of Swelling. Macromolecules 2000, 33, 3176–3179. [Google Scholar] [CrossRef]
- Triftaridou, A.I.; Hadjiyannakou, S.C.; Vamvakaki, M.; Patrickios, C.S. Synthesis, Characterization, and Modeling of Cationic Amphiphilic Model Hydrogels: Effects of Polymer Composition and Architecture. Macromolecules 2002, 35, 2506–2513. [Google Scholar] [CrossRef]
- Erdodi, G.; Kennedy, J.P. Ideal tetrafunctional amphiphilic PEG/PDMS conetworks by a dual-purpose extender/crosslinker. I. Synthesis. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4953–4964. [Google Scholar] [CrossRef]
- Erdodi, G.; Kennedy, J.P. Ideal tetrafunctional amphiphilic PEG/PDMS conetworks by a dual-purpose extender/crosslinker. II. Characterization and properties of water-swollen membranes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4965–4971. [Google Scholar] [CrossRef]
- Guan, Y.; Ding, X.; Zhang, W.; Wan, G.; Peng, Y. Polytetrahydrofuran Amphiphilic Networks, 5. Synthesis and Swelling Behavior of Thermosensitive Poly(N-isopropylacrylamide)-l-polytetrahydrofuran Networks. Macromol. Chem. Phys. 2002, 203, 900–908. [Google Scholar] [CrossRef]
- Lequieu, W.; Du Prez, F.E. Segmented polymer networks based on poly(N-isopropyl acrylamide) and poly(tetrahydrofuran) as polymer membranes with thermo-responsive permeability. Polymer 2004, 45, 749–757. [Google Scholar] [CrossRef]
- Hirotsu, S. Anomalous Kinetics of the Volume Phase Transition in Poly-N-Isopropylacrylamide Gels. Jpn. J. Appl. Phys. 1998, 37, L284. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Aoyagi, T.; Kikuchi, A.; Sakurai, Y.; Okano, T. Rapid Deswelling Response of Poly(N-isopropylacrylamide) Hydrogels by the Formation of Water Release Channels Using Poly(ethylene oxide) Graft Chains. Macromolecules 1998, 31, 6099–6105. [Google Scholar] [CrossRef]
- Zheng, Q.; Zheng, S. From poly(N-isopropylacrylamide)-block-poly(ethylene oxide)-block-poly(N-isopropylacrylamide) triblock copolymer to poly(N-isopropylacrylamide)-block-poly(ethylene oxide) hydrogels: Synthesis and rapid deswelling and reswelling behavior of hydrogels. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1717–1727. [Google Scholar] [CrossRef]
- Kamata, H.; Chung, U.; Shibayama, M.; Sakai, T. Anomalous volume phase transition in a polymer gel with alternative hydrophilic-amphiphilic sequence. Soft Matter 2012, 8, 6876–6879. [Google Scholar] [CrossRef]
- Kamata, H.; Akagi, Y.; Kayasuga-Kariya, Y.; Chung, U.-I.; Sakai, T. “Nonswellable” Hydrogel Without Mechanical Hysteresis. Science 2014, 343, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sanson, N.; Hourdet, D.; Marcellan, A. Thermoresponsive Toughening with Crack Bifurcation in Phase-Separated Hydrogels under Isochoric Conditions. Adv. Mater. 2016, 28, 5857–5864. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Mussault, C.; Brûlet, A.; Marcellan, A.; Hourdet, D.; Sanson, N. Thermoresponsive Toughening in LCST-Type Hydrogels with Opposite Topology: From Structure to Fracture Properties. Macromolecules 2016, 49, 4295–4306. [Google Scholar] [CrossRef]
- Ida, S.; Kitanaka, H.; Ishikawa, T.; Kanaoka, S.; Hirokawa, Y. Swelling properties of thermoresponsive/hydrophilic co-networks with functional crosslinked domain structures. Polym. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Ida, S.; Katsurada, A.; Yoshida, R.; Hirokawa, Y. Effect of reaction conditions on poly(N-isopropylacrylamide) gels synthesized by post-polymerization crosslinking system. React. Funct. Polym. 2017, 115, 73–80. [Google Scholar] [CrossRef]
- Theato, P. Synthesis of well-defined polymeric activated esters. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6677–6687. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Li, Y.; Tanaka, T. Kinetics of swelling and shrinking of gels. J. Chem. Phys. 1990, 92, 1365–1371. [Google Scholar] [CrossRef]
- Hirose, H.; Shibayama, M. Kinetics of volume phase transition in poly(N-isopropylacrylamide-co-acrylic acid) gels. Macromolecules 1998, 31, 5336–5342. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Xu, X.-D.; Cheng, S.-X.; Zhuo, R.-X. Strategies to improve the response rate of thermosensitive PNIPAAm hydrogels. Soft Matter 2008, 4, 385–391. [Google Scholar] [CrossRef]
- Yan, H.; Fujiwara, H.; Sasaki, K.; Tsujii, K. Rapid Swelling/Collapsing Behavior of Thermoresponsive Poly(N-isopropylacrylamide) Gel Containing Poly(2-(methacryloyloxy)decyl phosphate) Surfactant. Angew. Chem. Int. Ed. 2005, 44, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tsujii, K. A Novel Hydrogel Showing Super-Rapid Shrinking but Slow Swelling Behavior. Macromolecules 2006, 39, 8550–8552. [Google Scholar] [CrossRef]
- Pollak, A.; Blumenfeld, H.; Wax, M.; Baughn, R.L.; Whitesides, G.M. Enzyme immobilization by condensation copolymerization into crosslinked polyacrylamide gels. J. Am. Chem. Soc. 1980, 102, 6324–6336. [Google Scholar] [CrossRef]
| Prepolymer | NHSA Content (%) 2 | Mn 3 | Mw/Mn 3 |
|---|---|---|---|
| PDMAAm | 7.7 | 43,900 | 1.78 |
| PNBAAm | 6.7 | 53,900 | 1.71 |
| PNIPAAm | 6.4 | 76,700 | 1.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ida, S.; Kawahara, T.; Kawabata, H.; Ishikawa, T.; Hirokawa, Y. Effect of Monomer Sequence along Network Chains on Thermoresponsive Properties of Polymer Gels. Gels 2018, 4, 22. https://doi.org/10.3390/gels4010022
Ida S, Kawahara T, Kawabata H, Ishikawa T, Hirokawa Y. Effect of Monomer Sequence along Network Chains on Thermoresponsive Properties of Polymer Gels. Gels. 2018; 4(1):22. https://doi.org/10.3390/gels4010022
Chicago/Turabian StyleIda, Shohei, Toru Kawahara, Hidekazu Kawabata, Tatsuya Ishikawa, and Yoshitsugu Hirokawa. 2018. "Effect of Monomer Sequence along Network Chains on Thermoresponsive Properties of Polymer Gels" Gels 4, no. 1: 22. https://doi.org/10.3390/gels4010022
APA StyleIda, S., Kawahara, T., Kawabata, H., Ishikawa, T., & Hirokawa, Y. (2018). Effect of Monomer Sequence along Network Chains on Thermoresponsive Properties of Polymer Gels. Gels, 4(1), 22. https://doi.org/10.3390/gels4010022