Physical Properties of the Extracellular Matrix of Decellularized Porcine Liver
Abstract
:1. Introduction
2. Results and Discussion
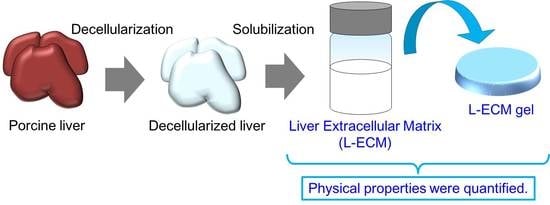
2.1. Decellularization of Porcine Liver Tissue and Preparation of L-ECM
2.2. Characterization of L-ECM
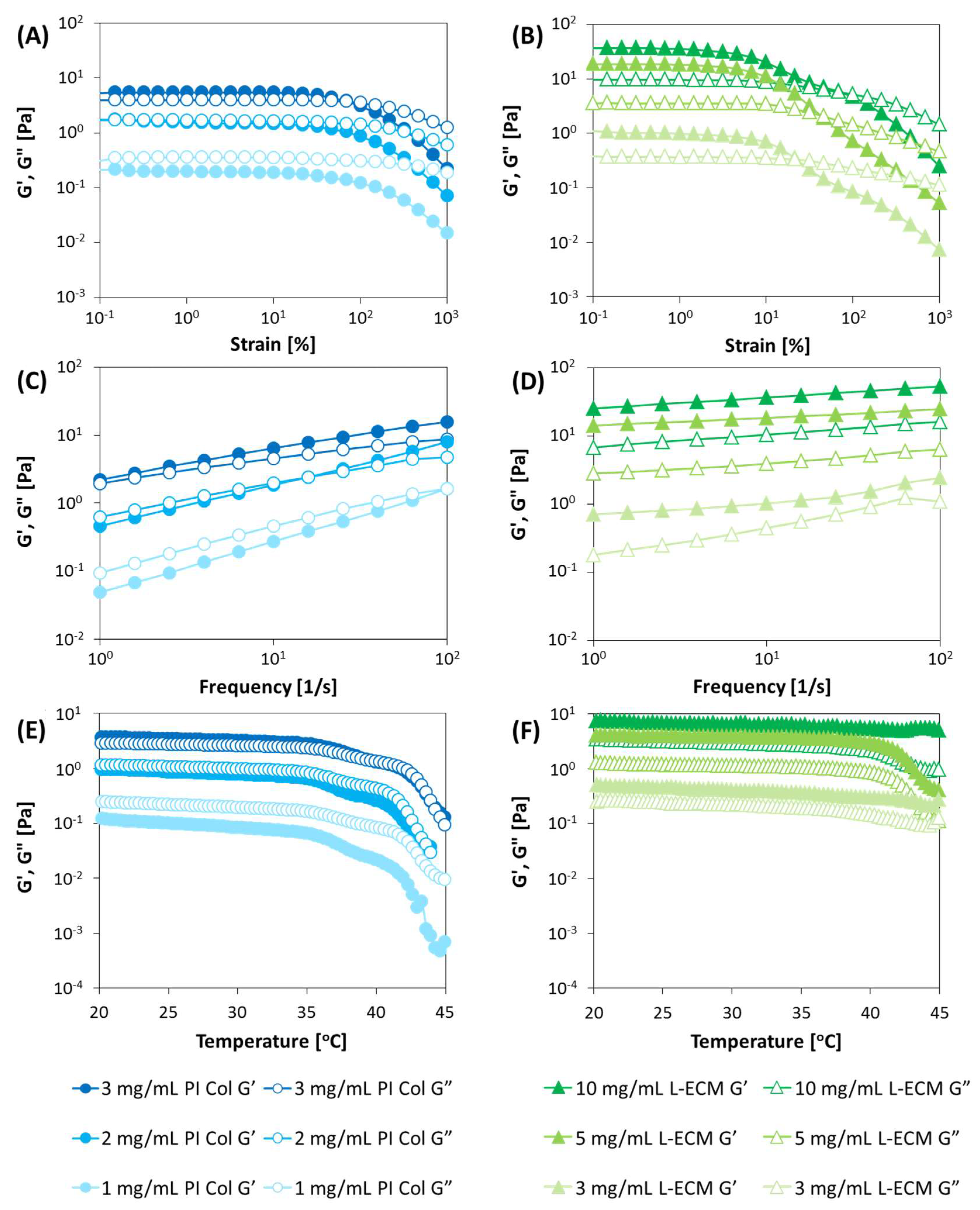
2.3. Rheological Properties of L-ECM Based on Dynamic Viscoelastic Evaluation
2.3.1. Strain Dispersion Test
2.3.2. Frequency Dispersion Test
2.3.3. Temperature Dependence
2.4. SEM Gel Observations
2.5. Immunostaining of L-ECM Gel
2.6. Rheological Properties of Gelation Behavior of L-ECM
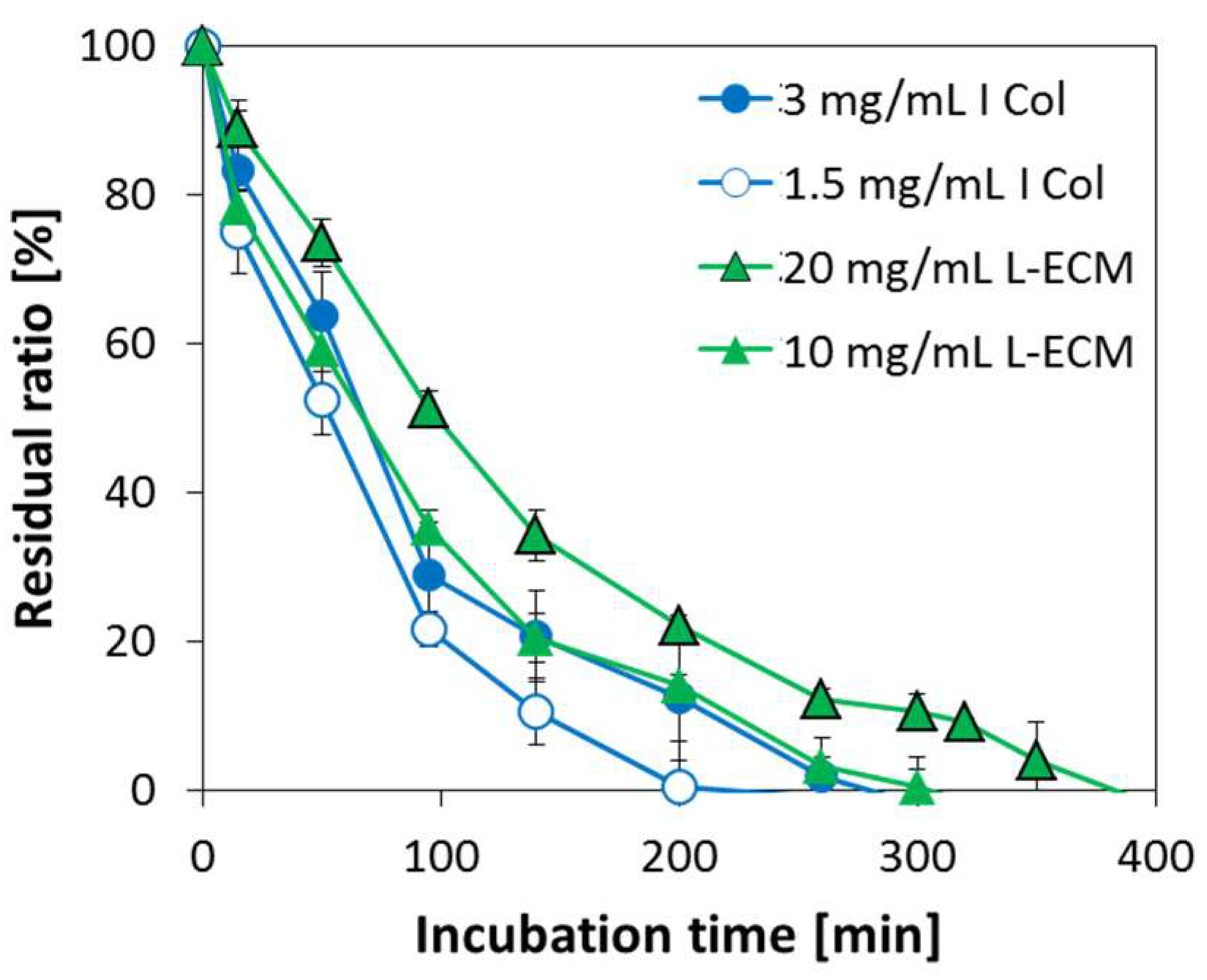
2.7. Degradation Behavior of L-ECM Gel
2.8. Stress on Compression of L-ECM Gel
3. Conclusions
4. Materials and Methods
4.1. Decellularization of Porcine Liver
4.2. Preparation of Liver-Specific ECM-Solubilized Substrate and Preparation of Hydrogel
4.3. Immunostaining
4.4. Molecular Weight Distribution of L-ECM
4.5. SEM Gel Observations
4.6. Rheology of L-ECM
4.7. Rheology of Gelation Behavior of L-ECM
4.8. Degradation Behavior of L-ECM Gel
4.9. Stress on the Compression of L-ECM Gel
Author Contributions
Conflicts of Interest
References
- Sasaki, S.; Funamoto, S.; Hashimoto, Y.; Kimura, T.; Honda, T.; Hattori, S.; Kobayashi, H.; Kishida, A.; Mochizuki, M. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol. Vis. 2009, 15, 2022–2028. [Google Scholar] [PubMed]
- Poh, M.; Boyer, M.; Solan, A.; Dahl, S.L.; Pedrotty, D.; Banik, S.S.; McKee, J.A.; Klinger, R.Y.; Counter, C.M.; Niklason, L.E. Blood vessels engineered from human cells. Lancet 2005, 365, 2122–2124. [Google Scholar] [CrossRef]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.M.; Siddiqui, M.M.; Lozier, G.; Rodriguez, S.R.; Atala, A.; Soker, S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011, 53, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Butter, A.; Aliyev, K.; Hillebrandt, K.H.; Raschzok, N.; Kluge, M.; Seiffert, N.; Tang, P.; Napierala, H.; Muhamma, A.I.; Reutzel-Selke, A.; et al. Evolution of graft morphology and function after recellularization of decellularized rat livers. J. Tissue Eng. Regen. Med. 2018, 12, e807–e816. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Yasuchika, K.; Fukumitsu, K.; Ishii, T.; Ogiso, S.; Miyauchi, Y.; Yamaoka, R.; Kawai, T.; Katayama, H.; Yoshitoshi-Uebayashi, E.Y.; et al. Establishment of practical recellularized liver graft for blood perfusion using primary rat hepatocytes and liver sinusoidal endothelial cells. Am. J. Transplant. 2018. [Google Scholar] [CrossRef] [PubMed]
- Shirakigawa, N.; Takei, T.; Ijima, H. Base structure consisting of an endothelialized vascular-tree network and hepatocytes for whole liver engineering. J. Biosci. Bioeng. 2013, 116, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yan, S.; Gao, J.J.; Wang, Y.Y.; Lu, Z.J.; Cui, C.W.; Zhang, Y.H.; Wang, Y.; Meng, X.Q.; Zhou, L.; et al. In-vivo organ engineering: Perfusion of hepatocytes in a single liver lobe scaffold of living rats. Int. J. Biochem. Cell Biol. 2016, 80, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ijima, H. Practical and functional culture technologies for primary hepatocytes. Biochem. Eng. J. 2010, 48, 332–336. [Google Scholar] [CrossRef]
- Shirakigawa, N.; Ijima, H. Nucleus number in clusters of transplanted fetal liver cells increases by partial hepatectomy of recipient rats. J. Biosci. Bioeng. 2013, 115, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ijima, H. Solubilized matrix derived from decellularized liver as a growth factor-immobilizable scaffold for hepatocyte culture. J. Biosci. Bioeng. 2013, 116, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Loneker, A.E.; Faulk, D.M.; Hussey, G.S.; D’Amore, A.; Badylak, S.F. Solubilized liver extracellular matrix maintains primary rat hepatocyte phenotype in-vitro. J. Biomed. Mater. Res. A 2016, 104, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Saheli, M.; Sepantafar, M.; Pournasr, B.; Farzaneh, Z.; Vosough, M.; Piryaei, A.; Baharvand, H. Three-Dimensional Liver-derived Extracellular Matrix Hydrogel Promotes Liver Organoids Function. J. Cell Biochem. 2018, 119, 4320–4333. [Google Scholar] [CrossRef] [PubMed]
- Damania, A.; Kumar, A.; Teotia, A.K.; Kimura, H.; Kamihira, M.; Ijima, H.; Sarin, S.K.; Kumar, A. Decellularized liver matrix-modified cryogel scaffolds as potential hepatocyte carriers in bioartificial liver support systems and implantable liver constructs. ACS Appl. Mater. Interfaces 2018, 10, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, J.; Park, H.M.; Kim, Y.G.; Kim, B.G.; Oh, J.W.; Cho, S.W. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules 2014, 15, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, Y.; Bharadwaj, S.; Hammam, N.; Carnagey, K.; Myers, R.; Atala, A.; Dyke, M.V. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009, 30, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- DeQuach, J.A.; Lin, J.E.; Cam, C.; Hu, D.; Salvatore, M.A.; Sheikh, F.; Christman, K.L. Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model. Eur. Cells Mater. 2012, 23, 400–412. [Google Scholar] [CrossRef]
- You, J.; Park, S.A.; Shin, D.S.; Patel, D.; Raghunathan, V.K.; Kim, M.; Murphy, C.J.; Tae, G.; Revzin, A. Characterizing the effects of heparin gel stiffness on function of primary hepatocytes. Tissue Eng. Part A 2013, 19, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Bairati, A.; Garrone, R. Biology of Invertebrate and Lower Vertebrate Collagen; Plenum Press: New York, NY, USA, 1985; ISBN 978-1-4684-7638-5. [Google Scholar] [CrossRef]
- Yoshimura, K.; Terashima, M.; Hozan, D.; Shirai, K. Preparation and Dynamic Viscoelasticity Characterization of Alkali-Solubilized Collagen from Shark Skin. J. Agric. Food Chem. 2000, 48, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.R.; Whittaker, R.E. Low strain dynamic properties of filled rubbers. Rubber Chem. Technol. 1971, 44, 440–478. [Google Scholar] [CrossRef]
- Freakly, P.K.; Payne, A.R. Theory and Practice of Engineering with Rubber; Applied Science Publishers LTD: London, UK, 1978; ISBN 978-0853347729. [Google Scholar]
- Snowdend, J.M.; Swann, D.A. The formation and thermal stability of in vitro assembled fibrils from acid-soluble and pepsin-treated collagens. Biochem. Biophys. Acta 1979, 580, 372–381. [Google Scholar]
- Lang, R.; Stern, M.M.; Smith, L.; Liu, Y.; Bharadwaj, S.; Liu, G.; Baptista, P.M.; Bergman, C.R.; Soker, S.; Yoo, J.J.; et al. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials 2011, 32, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Ijima, H.; Matsushita, T.; Nakazawa, K.; Fujii, Y.; Funatsu, K. Hepatocyte spheroids in polyurethane foams: Functional analysis and application for a hybrid artificial liver. Tissue Eng. 1998, 4, 213–226. [Google Scholar] [CrossRef]
- Ijima, H.; Nakazawa, K.; Mizumoto, H.; Matsushita, T.; Funatsu, K. Formation of a spherical multicellular aggregate (spheroid) of animal cells in the pores of polyurethane foam as a cell culture substratum and its application to a hybrid artificial liver. J. Biomater. Sci. Polym. Ed. 1998, 9, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Chonan, Y.; Shirai, K. Reactivity of Shark, Pig, and Bovine Skin Collagens with Formaldehyde and Basic Chromium Sulfate. Anim. Sci. Technol. (Jpn.) 1997, 68, 285–292. [Google Scholar] [CrossRef]
- Liao, J.; Joyce, E.M.; Sacks, M.S. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials 2008, 29, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Piez, K.A. Extracellular Matrix Biochemistry; Piaz, K.A., Reddi, A.H., Eds.; Elsevier: New York, NY, USA, 1988; pp. 9–11. ISBN 978-0444007995. [Google Scholar]
- Bond, M.D.; Van Wart, H.E. Characterization of the individual collagenases from Clostridium histolyticum. Biochemistry 1984, 23, 3085–3091. [Google Scholar] [CrossRef] [PubMed]








| Sample | Concentration (mg/mL) | Upper Yield Point (N/mm2) | Elastic Modulus (N/mm2) |
|---|---|---|---|
| I Col | 3 | 0.0112 | 0.0825 |
| 2 | 0.0069 | 0.0428 | |
| 1 | 0.0025 | 0.0175 | |
| L-ECM | 10 | 0.0026 | 0.0318 |
| 5 | 0.0014 | 0.0153 | |
| 3 | 0.0008 | 0.0057 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijima, H.; Nakamura, S.; Bual, R.; Shirakigawa, N.; Tanoue, S. Physical Properties of the Extracellular Matrix of Decellularized Porcine Liver. Gels 2018, 4, 39. https://doi.org/10.3390/gels4020039
Ijima H, Nakamura S, Bual R, Shirakigawa N, Tanoue S. Physical Properties of the Extracellular Matrix of Decellularized Porcine Liver. Gels. 2018; 4(2):39. https://doi.org/10.3390/gels4020039
Chicago/Turabian StyleIjima, Hiroyuki, Shintaro Nakamura, Ronald Bual, Nana Shirakigawa, and Shuichi Tanoue. 2018. "Physical Properties of the Extracellular Matrix of Decellularized Porcine Liver" Gels 4, no. 2: 39. https://doi.org/10.3390/gels4020039