Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors
Abstract
:1. Introduction
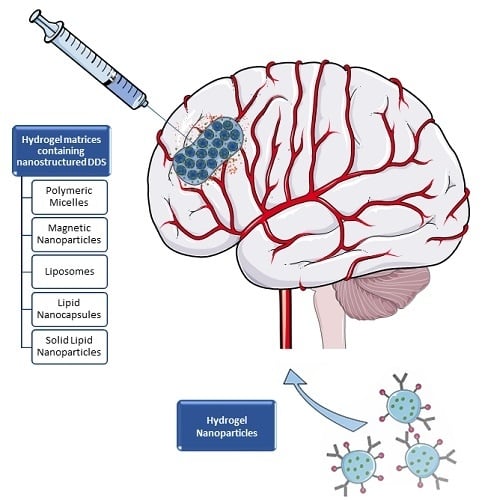
2. Hydrogel Matrices Containing Nanostructured DDS
2.1. Polymeric Micelles
2.2. Polymeric Nanoparticles
2.3. Magnetic Nanoparticles
2.4. Lipid-Based Drug Delivery Systems
3. Hydrogel Nanoparticles
4. Some In Silico Insights
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ohgaki, H.; Kleihues, P. Population-Based Studies on Incidence, Survival Rates, and Genetic Alterations in Astrocytic and Oligodendroglial Gliomas. J. Neuropathol. Exp. Neurol. 2005, 64, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobusawa, S.; Watanabe, T.; Kleihues, P.; Ohgaki, H. IDH1 Mutations as Molecular Signature and Predictive Factor of Secondary Glioblastomas. Clin. Cancer Res. 2009, 15, 6002–6007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Juratli, T.A.; Schackert, G.; Krex, D. Current Status of Local Therapy in Malignant Gliomas—A Clinical Review of Three Selected Approaches. Pharmacol. Ther. 2013, 139, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.; Chambers, A.; Spithoff, K.; Laperriere, N. Gliadel® Wafers in the Treatment of Malignant Glioma: A Systematic Review. Curr. Oncol. 2007, 14, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Merrill, E.W. Poly(vinyl alcohol) Hydrogels: Reinforcement of Radiation-Crosslinked Networks by Crystallization. J. Polym. Sci. Part A 1976, 14, 441–457. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design Properties of Hydrogel Tissue-Engineering Scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Larraneta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, e13. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent Advances in Hydrogel-Based Drug Delivery for Melanoma Cancer Therapy: A Mini Review. J. Drug Deliv. 2017, 2017, 7275985. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable Hydrogel-based Drug Delivery Systems for Local Cancer Therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Danhier, P.; Préat, V.; Danhier, F. Anticancer Drug-Loaded Hydrogels as Drug Delivery Systems for the Local Treatment of Glioblastoma. J. Control. Release 2016, 243, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Pourgholi, F.; hajivalili, M.; Farhad, J.-N.; Kafil, H.S.; Yousefi, M. Nanoparticles: Novel Vehicles in Treatment of Glioblastoma. Biomed. Pharmacother. 2016, 77, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the Treatment of Glioblastoma Multiforme: Current State-of-the-Art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Pedron, S.; Becka, E.; Harley, B.A.C. Regulation of Glioma Cell Phenotype in 3D Matrices by Hyaluronic Acid. Biomaterials 2013, 34, 7408–7417. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tong, X.; Yang, F. Bioengineered 3D Brain Tumor Model To Elucidate the Effects of Matrix Stiffness on Glioblastoma Cell Behavior Using PEG-Based Hydrogels. Mol. Pharm. 2014, 11, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tong, L.; Yi, L.; Zhang, C.; Hai, L.; Li, T.; Yu, S.; Wang, W.; Tao, Z.; Ma, H. Three-Dimensional Hydrogel is Suitable for Targeted Investigation of Amoeboid Migration of Glioma Cells. Mol. Med. Rep. 2018, 17, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jiguet Jiglaire, C.; Baeza-Kallee, N.; Denicolaï, E.; Barets, D.; Metellus, P.; Padovani, L.; Chinot, O.; Figarella-Branger, D.; Fernandez, C. Ex Vivo Cultures of Glioblastoma in Three-Dimensional Hydrogel Maintain the Original Tumor Growth Behavior and are Suitable for Preclinical Drug and Radiation Sensitivity Screening. Exp. Cell Res. 2014, 321, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Kaneko, D.; Hashizawa, K.; Imai, Y.; Tagami, T.; Okada, H. Combination Therapy of Surgical Tumor Resection with Implantation of a Hydrogel Containing Camptothecin-Loaded Poly(lactic-co-glycolic acid) Microspheres in a C6 Rat Glioma Model. Biol. Pharm. Bull. 2012, 35, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Hashizawa, K.; Kaneko, D.; Imai, Y.; Okada, H. Treatment of Rat Brain Tumors Using Sustained-Release of Camptothecin from Poly(lactic-co-glycolic acid) Microspheres in a Thermoreversible Hydrogel. Chem. Pharm. Bull. 2010, 58, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Kee, I.; Krantz, W.B.; Chow, P.K.-H.; Wang, C.-H. Hydrogel Matrix Entrapping PLGA-Paclitaxel Microspheres: Drug Delivery with Near Zero-Order Release and Implantability Advantages for Malignant Brain Tumour Chemotherapy. Pharm. Res. 2009, 26, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Benny, O.; Joki, T.; Menon, L.G.; Machluf, M.; Abe, T.; Carroll, R.S.; Black, P.M. Novel Local Drug Delivery System Using Thermoreversible Gel in Combination with Polymeric Microspheres or Liposomes. Anticancer Res. 2010, 30, 1057–1064. [Google Scholar] [PubMed]
- Fourniols, T.; Randolph, L.D.; Staub, A.; Vanvarenberg, K.; Leprince, J.G.; Préat, V.; des Rieux, A.; Danhier, F. Temozolomide-loaded photopolymerizable PEG-DMA-based hydrogel for the treatment of glioblastoma. J. Control. Release 2015, 210, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, J.; Hu, Y.; Yang, Y.; Gou, Z.; Du, T.; Mao, J.; Gou, M. A Conformal Hydrogel Nanocomposite for Local Delivery of Paclitaxel. J. Biomater. Sci. Polym. Ed. 2017, 28, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Shea, L.D. Lentivirus Immobilization to Nanoparticles for Enhanced and Localized Delivery from Hydrogels. Mol. Ther. 2010, 18, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.W.; Chen, P.Y.; Wei, K.C.; Huang, C.Y.; Wang, C.K.; Yang, H.W. Rapid In Situ MRI Traceable Gel-forming Dual-drug Delivery for Synergistic Therapy of Brain Tumor. Theranostics 2017, 7, 2524–2536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.; Sasmal, P.K.; Lee, K.-B. Photo-Triggerable Hydrogel-Nanoparticle Hybrid Scaffolds for Remotely Controlled Drug Delivery. J. Mater. Chem. B 2014, 2, 7685–7693. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, M.; Sun, Y.; Gao, P.; Duan, Y. Polymer Nanocomposites Based Thermo-Sensitive Gel for Paclitaxel and Temozolomide Co-Delivery to Glioblastoma Cells. J. Nanosci. Nanotechnol. 2015, 15, 9777–9787. [Google Scholar] [CrossRef] [PubMed]
- Meenach, S.A.; Hilt, J.Z.; Anderson, K.W. Poly(ethylene glycol)-Based Magnetic Hydrogel Nanocomposites for Hyperthermia Cancer Therapy. Acta Biomater. 2010, 6, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Meenach, S.A.; Shapiro, J.M.; Hilt, J.Z.; Anderson, K.W. Characterization of PEG-Iron Oxide Hydrogel Nanocomposites for Dual Hyperthermia and Paclitaxel Delivery. J. Biomater. Sci. Polym. Ed. 2013, 24, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Lee, B.S.; Chun, C.; Cho, J.K.; Kim, S.Y.; Song, S.C. Long-Term Theranostic Hydrogel System for Solid Tumors. Biomaterials 2012, 33, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, B.; Chun, C.; Lee, S.H.; Song, S.C. MRI-Monitored Long-Term Therapeutic Hydrogel System for Brain Tumors without Surgical Resection. Biomaterials 2012, 33, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Vanvarenberg, K.; Ucakar, B.; Pitorre, M.; Bastiat, G.; Lagarce, F.; Preat, V.; Danhier, F. Lauroyl-Gemcitabine-Loaded Lipid Nanocapsule Hydrogel for the Treatment of Glioblastoma. J. Control. Release 2016, 225, 283–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastiancich, C.; Bianco, J.; Vanvarenberg, K.; Ucakar, B.; Joudiou, N.; Gallez, B.; Bastiat, G.; Lagarce, F.; Préat, V.; Danhier, F. Injectable Nanomedicine Hydrogel for Local Chemotherapy of Glioblastoma after Surgical Resection. J. Control. Release 2017, 264, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Formulation Design and Development of Anti-EGFR-BSA-CYP-SLNs In Situ Gel for Nasal Administration. Asian J. Pharm. 2016, 10, S750–S769. [Google Scholar]
- Shatsberg, Z.; Zhang, X.; Ofek, P.; Malhotra, S.; Krivitsky, A.; Scomparin, A.; Tiram, G.; Calderón, M.; Haag, R.; Satchi-Fainaro, R. Functionalized Nanogels Carrying an Anticancer microRNA for Glioblastoma Therapy. J. Control. Release 2016, 239, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z. Targeted Delivery of CRISPR/Cas9-Mediated Cancer Gene Therapy via Liposome-templated Hydrogel Nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036. [Google Scholar] [CrossRef] [PubMed]
- Shirakura, T.; Ray, A.; Kopelman, R. Polyethylenimine Incorporation into Hydrogel Nanomatrices for Enhancing Nanoparticle-Assisted Chemotherapy. RSC Adv. 2016, 6, 48016–48024. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Q.; Mu, K.; Xie, H.; Zhu, Y.; Zhu, W.; Zhao, Y.; Xu, H.; Yang, X. pH/Temperature Sensitive Magnetic Nanogels Conjugated with Cy5.5-Labled Lactoferrin for MR and Fluorescence Imaging of Glioma in Rats. Biomaterials 2013, 34, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Hah, H.J.; Kim, G.; Lee, Y.E.; Qin, M.; Ratani, T.S.; Fotiadis, P.; Miller, A.; Kochi, A.; Gao, D.; et al. Hydrogel Nanoparticles with Covalently Linked Coomassie Blue for Brain Tumor Delineation Visible to the Surgeon. Small 2012, 8, 884–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, M.; Zong, H.; Kopelman, R. Click Conjugation of Peptide to Hydrogel Nanoparticles for Tumor-Targeted Drug Delivery. Biomacromolecules 2014, 15, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Baklaushev, V.P.; Nukolova, N.N.; Khalansky, A.S.; Gurina, O.I.; Yusubalieva, G.M.; Grinenko, N.P.; Gubskiy, I.L.; Melnikov, P.A.; Kardashova, K.S.; Kabanov, A.V. Treatment of Glioma by Cisplatin-Loaded Nanogels Conjugated with Monoclonal Antibodies against Cx43 and BSAT1. Drug Deliv. 2015, 22, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Achazi, K.; Schade, B.; Haag, R. Charge-Conversional and Reduction-Sensitive Poly (vinyl alcohol) Nanogels for Enhanced Cell Uptake and Efficient Intracellular Doxorubicin Release. J. Control. Release 2015, 205, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Neshastehriz, A.; Khateri, M.; Ghaznavi, H.; Shakeri-Zadeh, A. Investigating the Therapeutic Effects of Alginate Nanogel Co-Loaded with Gold Nanoparticles and Cisplatin on U87-MG Human Glioblastoma Cells. Anticancer Agents Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Dey, G.; Bharti, R.; Mandal, P.; Mandal, M.; Chattopadhyay, S. Metal Ion Ornamented Ultrafast Light-Sensitive Nanogel for Potential in Vivo Cancer Therapy. Chem. Mater. 2016, 28, 8598–8610. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent Adaptable Networks: Smart, Reconfigurable and Responsive Network Systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed]
- Kean Zachary, S.; Hawk Jennifer, L.; Lin, S.; Zhao, X.; Sijbesma Rint, P.; Craig Stephen, L. Increasing the Maximum Achievable Strain of a Covalent Polymer Gel Through the Addition of Mechanically Invisible Cross-Links. Adv. Mater. 2014, 26, 6013–6018. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Deng, G.; Tang, C.; Li, F.; Jiang, H.; Chen, Y. Covalent Cross-Linked Polymer Gels with Reversible Sol−Gel Transition and Self-Healing Properties. Macromolecules 2010, 43, 1191–1194. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-Responsive Hydrogels in Drug Delivery and Tissue Engineering. Drug Deliv. 2016, 23, 758–780. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Shan, G.; Bao, Y.; Wu, Z.L.; Pan, P. Thermoresponsive Physical Hydrogels of Poly(lactic acid)/Poly(ethylene glycol) Stereoblock Copolymers Tuned by Stereostructure and Hydrophobic Block Sequence. Soft Matter 2016, 12, 4628–4637. [Google Scholar] [CrossRef] [PubMed]
- Vellimana, A.K.; Recinos, V.R.; Hwang, L.; Fowers, K.D.; Li, K.W.; Zhang, Y.; Okonma, S.; Eberhart, C.G.; Brem, H.; Tyler, B.M. Combination of Paclitaxel Thermal Gel Depot with Temozolomide and Radiotherapy Significantly Prolongs Survival in an Experimental Rodent Glioma Model. J. Neuro-Oncol. 2013, 111, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gothwal, A.; Khan, I.; Gupta, U. Polymeric Micelles: Recent Advancements in the Delivery of Anticancer Drugs. Pharm. Res. 2016, 33, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, K. Polymeric Micelles and Alternative Nanonized Delivery Vehicles for Poorly Soluble Drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S.K. Polymeric Nanoparticles for Cancer Therapy. J. Drug Targets 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Polymeric Nanoparticles for Drug Delivery. In Cancer Nanotechnology: Methods and Protocols; Grobmyer, S.R., Moudgil, B.M., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 163–175. [Google Scholar]
- Pardo, Y.; Ma, M. Smart Cell Culture for Tissue Engineering. In Smart Materials for Tissue Engineering: Applications; The Royal Society of Chemistry: Cambridge, UK, 2017; pp. 409–438. [Google Scholar]
- Akash, M.S.H.; Rehman, K.; Chen, S. Pluronic F127-Based Thermosensitive Gels for Delivery of Therapeutic Proteins and Peptides. Polym. Rev. 2014, 54, 573–597. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Huang, S. Magnetic Nanoparticles in Cancer Diagnosis, Drug Delivery and Treatment. Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.M. The Application of Magnetic Nanoparticles in the Treatment and Monitoring of Cancer and Infectious Diseases. Biosci. Horiz. 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite Nanoparticles for Cancer Diagnosis, Treatment, and Treatment Monitoring: Recent Advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Labhasetwar, V. Nanotech Approaches to Drug Delivery and Imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Yoshioka, H.; Mikami, M.; Mori, Y.; Tsuchida, E. A Synthetic Hydrogel with Thermoreversible Gelation. I. Preparation and Rheological properties. J. Macromol. Sci. 1994, 31, 113–120. [Google Scholar] [CrossRef]
- Taylor, L.D.; Cerankowski, L.D. Preparation of Films Exhibiting a Balanced Temperature Dependence to Permeation by Aqueous Solutions—A Study of Lower Consolute Behavior. J. Polym. Sci. Part A 1975, 13, 2551–2570. [Google Scholar] [CrossRef]
- Tang, S.; Floy, M.; Bhandari, R.; Dziubla, T.; Hilt, J.Z. Development of Novel N-Isopropylacrylamide (NIPAAm) Based Hydrogels with Varying Content of Chrysin Multiacrylate. Gels 2017, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Floy, M.; Bhandari, R.; Sunkara, M.; Morris, A.J.; Dziubla, T.D.; Hilt, J.Z. Synthesis and Characterization of Thermoresponsive Hydrogels Based on N-Isopropylacrylamide Crosslinked with 4,4′-Dihydroxybiphenyl Diacrylate. ACS Omega 2017, 2, 8723–8729. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Bhandari, R.; Delaney, S.P.; Munson, E.J.; Dziubla, T.D.; Hilt, J.Z. Synthesis and Characterization of Thermally Responsive N-Isopropylacrylamide Hydrogels Copolymerized with Novel Hydrophobic Polyphenolic Crosslinkers. Mater. Today Commun. 2017, 10, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Maire, C.L.; Lamszus, K. EGFR as a Target for Glioblastoma Treatment: An Unfulfilled Promise. CNS Drugs 2017, 31, 723–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, T.E.; Furnari, F.B.; Cavenee, W.K. Targeting EGFR for Treatment of Glioblastoma: Molecular Basis to Overcome Resistance. Curr. Cancer Drug Targets 2012, 12, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H. Intranasal Delivery Bypasses the Blood-Brain Barrier to Target Therapeutic Agents to the Central Nervous System and Treat Neurodegenerative Disease. BMC Neurosci. 2008, 9, S5. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel Carrier Design for Targeted Drug Delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The Development of Microgels/Nanogels for Drug Delivery Applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer Nanogels: A Versatile Nanoscopic Drug Delivery Platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Bobone, S.; Miele, E.; Cerroni, B.; Roversi, D.; Bocedi, A.; Nicolai, E.; Di Venere, A.; Placidi, E.; Ricci, G.; Rosato, N. Liposome-Templated Hydrogel Nanoparticles as Vehicles for Enzyme-Based Therapies. Langmuir 2015, 31, 7572–7580. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic Concepts and Recent Advances in Nanogels as Carriers for Medical Applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Achazi, K.; Steinhilber, D.; Kratz, F.; Dernedde, J.; Haag, R. A Facile Approach for Dual-Responsive Prodrug Nanogels Based on Dendritic Polyglycerols with Minimal Leaching. J. Control. Release 2014, 174, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Purow, B.W.; Haque, R.M.; Noel, M.W.; Su, Q.; Burdick, M.J.; Lee, J.; Sundaresan, T.; Pastorino, S.; Park, J.K.; Mikolaenko, I. Expression of Notch1 and its Ligands, Delta-like-1 and Jagged-1, is Critical for Glioma Cell Survival and Proliferation. Cancer Res. 2005, 65, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-B.; Ma, M.-W.; Dong, L.-J.; Wang, F.; Chen, L.-X.; Li, X.-R. MicroRNA-34a Targets Notch1 and Inhibits Cell Proliferation in Glioblastoma Multiforme. Cancer Biol. Ther. 2011, 12, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Intra, J.; Salem, A.K. Characterization of the Transgene Expression Generated by Branched and Linear Polyethylenimine-Plasmid DNA Nanoparticles in vitro and after Intraperitoneal Injection in vivo. J. Control. Release 2008, 130, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Urban-Klein, B.; Werth, S.; Abuharbeid, S.; Czubayko, F.; Aigner, A. RNAi-Mediated Gene-Targeting through Systemic Application of Polyethylenimine (PEI)-Complexed siRNA in vivo. Gene Ther. 2005, 12, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhu, J.-L.; Zeng, X.; Jing, Y.; Zhang, X.-Z.; Zhuo, R.-X. Targeted Gene Delivery Mediated by Folate-Polyethylenimine-Block-Poly (ethylene glycol) with Receptor Selectivity. Bioconjug. Chem. 2009, 20, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gu, G.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; Yao, L. F3 Peptide-Functionalized PEG-PLA Nanoparticles Co-administrated with tLyp-1 Peptide for Anti-Glioma Drug Velivery. Biomaterials 2013, 34, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Orringer, D.A.; Koo, Y.-E.L.; Chen, T.; Kim, G.; Hah, H.J.; Xu, H.; Wang, S.; Keep, R.; Philbert, M.A.; Kopelman, R. In vitro Characterization of a Targeted, Dye-Loaded Nanodevice for Intraoperative Tumor Delineation. Neurosurgery 2009, 64, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zou, Y.; Zhong, Z.; Haag, R. Cyclo (RGD)-Decorated Reduction-Responsive Nanogels Mediate Targeted Chemotherapy of Integrin Overexpressing Human Glioblastoma In Vivo. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Backos, D.S.; Franklin, C.C.; Reigan, P. The Role of Glutathione in Brain Tumor Drug Resistance. Biochem. Pharmacol. 2012, 83, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Hjelmeland, A.B.; Wu, Q.; Heddleston, J.; Choudhary, G.; MacSwords, J.; Lathia, J.; McLendon, R.; Lindner, D.; Sloan, A.; Rich, J.N. Acidic Stress Promotes a Glioma Stem Cell Phenotype. Cell Death Differ. 2011, 18, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Pandey, P.; Rao, P.; Mahmoud, A.; Goldman, A.; Sabbisetti, V.; Parcha, S.; Natarajan, S.K.; Chandrasekar, V.; Dinulescu, D.; et al. Algorithm for Designing Nanoscale Supramolecular Therapeutics with Increased Anticancer Efficacy. ACS Nano 2016, 10, 8154–8168. [Google Scholar] [CrossRef] [PubMed]
- Ramezanpour, M.; Leung, S.S.W.; Delgado-Magnero, K.H.; Bashe, B.Y.M.; Thewalt, J.; Tieleman, D.P. Computational and Experimental Approaches for Investigating Nanoparticle-Based Drug Delivery Systems. Biochim. Biophys. Acta 2016, 1858, 1688–1709. [Google Scholar] [CrossRef] [PubMed]
- Sambasivam, A.; Sangwai, A.V.; Sureshkumar, R. Self-Assembly of Nanoparticle-Surfactant Complexes with Rodlike Micelles: A Molecular Dynamics Study. Langmuir 2016, 32, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Weeber, R.; Hermes, M.; Schmidt, A.M.; Holm, C. Polymer Architecture of Magnetic Gels: A Review. J. Phys. Condens. Matter 2018, 30, 063002. [Google Scholar] [CrossRef] [PubMed]
- Kosovan, P.; Richter, T.; Holm, C. Molecular Simulations of Hydrogels. In Intelligent Hydrogels, Progess in Colloid and Polymer Science; Sadowski, G., Richtering, W., Eds.; Springer International Publishing: Cham, Switzerland, 2013; Volume 140, pp. 247–263. [Google Scholar]
- Dhasaiyan, P.; Prasad, B.L. Self-Assembly of Bolaamphiphilic Molecules. Chem. Rec. 2017, 17, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Carnal, F.; Clavier, A.; Stoll, S. Polypeptide-Nanoparticle Interactions and Corona Formation Investigated by Monte Carlo Simulations. Polymers 2016, 8, 203. [Google Scholar] [CrossRef]
- Shirakura, T.; Smith, C.; Hopkins, T.J.J.; Koo Lee, Y.-E.; Lazaridis, F.; Argyrakis, P.; Kopelman, R. Matrix Density Engineering of Hydrogel Nanoparticles with Simulation-Guided Synthesis for Tuning Drug Release and Cellular Uptake. ACS Omega 2017, 2, 3380–3389. [Google Scholar] [CrossRef] [PubMed]
- Stornes, M.; Linse, P.; Dias, R.S. Monte Carlo Simulations of Complexation between Weak Polyelectrolytes and a Charged Nanoparticle. Influence of Polyelectrolyte Chain Length and Concentration. Macromolecules 2017, 50, 5978–5988. [Google Scholar] [CrossRef]
- Adroher-Benitez, I.; Martin-Molina, A.; Ahualli, S.; Quesada-Perez, M.; Odriozola, G.; Moncho-Jorda, A. Competition between Excluded-Volume and Electrostatic Interactions for Nanogel Swelling: Effects of the Counterion Valence and Nanogel Charge. Phys. Chem. Chem. Phys. 2017, 19, 6838–6848. [Google Scholar] [CrossRef] [PubMed]
- Ahualli, S.; Martín-Molina, A.; Maroto-Centeno, J.A.; Quesada-Pérez, M. Interaction between Ideal Neutral Nanogels: A Monte Carlo Simulation Study. Macromolecules 2017, 50, 2229–2238. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Maroto-Centeno, J.A.; Martín-Molina, A.; Moncho-Jordá, A. Direct Determination of Forces between Charged Nanogels through Coarse-Grained Simulations. Phys. Rev. E 2018, 97, 042608. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mas, L.; Martin-Molina, A.; Quesada-Perez, M.; Moncho-Jorda, A. Maximizing the Absorption of Small Cosolutes inside Neutral Hydrogels: Steric Exclusion versus Hydrophobic Adhesion. Phys. Chem. Chem. Phys. 2018, 20, 2814–2825. [Google Scholar] [CrossRef] [PubMed]
- Hofzumahaus, C.; Hebbeker, P.; Schneider, S. Monte Carlo Simulations of Weak Polyelectrolyte Microgels: PH-Dependence of Conformation and Ionization. Soft Matter 2018, 14, 4087–4100. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Miranda, A.; Cova, T.; Gonçalves, L.; Almeida, A.J.; Sousa, J.J.; do Vale, M.L.C.; Marques, E.F.; Pais, A.; Vitorino, C. Modeling of Ultra-Small Lipid Nanoparticle Surface Charge for Targeting Glioblastoma. Eur. J. Pharm. Sci. 2018, 117, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Nieh, M.-P.; Li, Y. Decorating Nanoparticle Surface for Targeted Drug Delivery: Opportunities and Challenges. Polymers 2016, 8, 83. [Google Scholar] [CrossRef]
- Van Lehn, R.C.; Atukorale, P.U.; Carney, R.P.; Yang, Y.-S.; Stellacci, F.; Irvine, D.J.; Alexander-Katz, A. Effect of Particle Diameter and Surface Composition on the Spontaneous Fusion of Monolayer-Protected Gold Nanoparticles with Lipid Bilayers. Nano Lett. 2013, 13, 4060–4067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Lehn, R.C.; Alexander-Katz, A. Free Energy Change for Insertion of Charged, Monolayer-Protected Nanoparticles into Lipid Bilayers. Soft Matter 2014, 10, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Van Lehn, R.C.; Alexander-Katz, A. Fusion of Ligand-Coated Nanoparticles with Lipid Bilayers: Effect of Ligand Flexibility. J. Phys. Chem. A 2014, 118, 5848–5856. [Google Scholar] [CrossRef] [PubMed]
- Haume, K.; Mason, N.J.; Solov’yov, A.V. Modeling of Nanoparticle Coatings for Medical Applications. Eur. Phys. J. D 2016, 70, 181. [Google Scholar] [CrossRef]
- Van Lehn, R.C.; Ricci, M.; Silva, P.H.J.; Andreozzi, P.; Reguera, J.; Voïtchovsky, K.; Stellacci, F.; Alexander-Katz, A. Lipid Tail Protrusions Mediate the Insertion of Nanoparticles into Model Cell Membranes. Nat. Commun. 2014, 5, 4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deyev, S.; Proshkina, G.; Ryabova, A.; Tavanti, F.; Menziani, M.C.; Eidelshtein, G.; Avishai, G.; Kotlyar, A. Synthesis, Characterization, and Selective Delivery of DARPin–Gold Nanoparticle Conjugates to Cancer Cells. Bioconjug. Chem. 2017, 28, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Bandyopadhyay, A.; Chakraborty, A.; Sarkar, K. Enhancement of Anticancer Activity and Drug Delivery of Chitosan-Curcumin Nanoparticle via Molecular Docking and Simulation Analysis. Carbohydr. Polym. 2018, 182, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xue, Z.; Liu, Y.; Xiao, J.; Chen, J.; Zhang, L.; Guo, J.; Lin, W. Delivery of Anticancer Drug using pH-sensitive Micelles from Triblock Copolymer MPEG-b-PBAE-b-PLA. Mater. Sci. Eng. C 2018, 84, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, S.-G.; Wang, P.; Wang, Y.; Lv, X.; Liu, Y.; Wang, F.; Gu, Z.; Yang, Z.; Weber, J.K.; et al. Molecular Mechanism of Gd@C82(OH)22 increasing Collagen Expression: Implication for Encaging Tumor. Biomaterials 2018, 152, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, L.; Zhao, L.; He, J.; Yuan, Q.; Zhang, P.; Zhao, Y.; Gao, X. Peptide-Au Clusters Induced Tumor Cells Apoptosis via Targeting Glutathione Peroxidase-1: The Molecular Dynamics Assisted Experimental Studies. Sci. Rep. 2017, 7, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.; Sehrt, J.; Vrabec, J.; Hasse, H. Molecular Dynamics and Experimental Study of Conformation Change of Poly(N-isopropylacrylamide) Hydrogels in Mixtures of Water and Methanol. J. Phys. Chem. B 2012, 116, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Cova, T.F.G.G.; Milne, B.F.; Nunes, S.C.C.; Pais, A.A.C.C. Drastic Stabilization of Junction Nodes in Supramolecular Structures Based on Host-Guest Complexes. Macromolecules 2018, 51, 2732–2741. [Google Scholar] [CrossRef]
- Cova, T.F.G.G.; Nunes, S.C.C.; Pais, A.A.C.C. Free-Energy Patterns in Inclusion Complexes: The Relevance of Non-Included Moieties in the Stability Constants. Phys. Chem. Chem. Phys. 2017, 19, 5209–5221. [Google Scholar] [CrossRef] [PubMed]
- Zidek, J.; Milchev, A.; Jancar, J.; Vilgis, T.A. Deformation-Induced Damage and Recovery in Model Hydrogels—A Molecular Dynamics Simulation. J. Mech. Phys. Solids 2016, 94, 372–387. [Google Scholar] [CrossRef]
- Weeber, R.; Holm, C. Interplay between Particle Microstructure, Network Topology and Sample Shape in Magnetic Gels—A Molecular Dynamics Simulation Study. arXiv, 2017; arXiv:1704.06578. [Google Scholar]
- Minina, E.S.; Sánchez, P.A.; Likos, C.N.; Kantorovich, S.S. The Influence of the Magnetic Filler Concentration on the Properties of a Microgel Particle: Zero-field Case. J. Magn. Magn. Mater. 2018, 459, 226–230. [Google Scholar] [CrossRef]








| Delivery System | Hybrid System | Carried Agent | Route of Admin. | Trigger | Main Achievements | Ref. |
|---|---|---|---|---|---|---|
| Hydrogel matrices containing nanostructured DDS | ||||||
| Polymeric Micelles | PEG750-p(CL-co-TMC) micelles + PEG-DMA-based in situ hydrogel | TMZ | Local Delivery | UV light | Fast in situ photopolymerization; sustained release of TMZ over 1 week; potent in vivo antitumor efficacy. | [28] |
| mPEG-PDLLA micelles + macroporous gelatin hydrogel | PTX | Local Delivery | Enzymatic | Controlled release of PTX and high inhibition of tumor cell proliferation in glioma C6 cells. | [29] | |
| HA NPs + collagen hydrogel | Lentivirus | Local Delivery | - | HA within the hydrogel increased the lentivirus activity for 72 h and delayed its release; Transduction activity in invasive C6 glioma cells with the hydrogel was ~80% of the control. | [30] | |
| BSA NPs + CMC-g-PNIPAAmMA and DTPAGd/bPEI hydrogel | PTX + EPI | Local Delivery | Temp. | MBR 614 cell line: sustained drug release and inhibition of tumor cells growth; human glioma U87 MG tumor-bearing mouse: effective tumor reduction and average survival increase. | [31] | |
| Silica NPs + PEG-based hydrogel | CPT | Local Delivery | UV light | U87 MG cells: marked decreased in cell viability. | [32] | |
| mPEG-PLGA NPs + PF127 hydrogel | PTX + TMZ | Local Delivery | Temp. | Drug release was controlled by gel composition; High growth inhibition and apoptosis-inducing effects in both U87 MG and C6 cell lines. | [33] | |
| Magnetic NPs | Fe3O4 NPs + PEG-based hydrogel | PTX | Local Delivery | AMF | Effective delivery of PTX simultaneously with hyperthermia in M059K glioma cells. | [34,35] |
| P-CoFe2O4 NPs + PPZ hydrogel | SN-38 | Local Delivery | - | Drug sustained release; long-term inhibition of tumor growth in U87 MG tumor-bearing mice; proved MR imaging abilities. | [36,37] | |
| Liposomes | Liposomes + P(NIPAAm-co-BMA) and PEG-based hydrogel | DOX | Local Delivery | Temp. | Increased sustained release over 52 days; Significant reduction of tumor growth (38 days vs. 12 days with free DOX in hydrogel). | [27] |
| LNC | Lipid nanocapsule-based hydrogel | GemC12 | Local Delivery | - | Sustained drug release over one month with a significant increase of survival/reduction of recurrences in tumor xenograft models. | [38,39] |
| SLNs | Anti-EGFR-SLNs + in situ gel | CP | Intranasal | - | The optimized formulation presented good viscosity, gelling strength, drug content as well as favorable adhering properties to the nasal mucosa, stability and dissolution profile, among others. | [40] |
| Hydrogel Nanoparticles | ||||||
| Hydrogel Nanoparticles | Polyglycerol-scaffold nano-polyplexes in polymeric nanogels | miR-34a | Local Delivery | pH/redox | Significant reduction in tumor size growth of abdominal GBM xenograft models. | [41] |
| Liposome-templated hydrogel NPs | CRISPR/Cas9 | IV | - | Controlled release of DNA and protein; Marked cytotoxicity over U87 MG cells; reduction of tumor growth and improvement of overall survival of mice models. | [42] | |
| PEI-modified PAA-based hydrogel NPs | CIS | - | - | Uptake of nanogels promoted by PEI-9L glioma cells interaction; higher toxicity of NPs over glioma cells than free CIS. | [43] | |
| Fe3O4 NPs loaded MPNA nanogel | Cy5.5 | IV | pH/Temp. | Proved MR/fluorescence imaging abilities; Good and specific uptake by C6 glioma cells in rat model; cellular uptake favored at pH 6.8 (tumor environment) using lactoferrin. | [44] | |
| PAA-based hydrogel NPs | CB | IV | - | Covalently linked CB nanoparticles, functionalized with F3 peptide and PEG, effectively target and clearly identify GBM cells. | [45] | |
| F3 peptide-conjugated co(CEA-AAm) nanogel | DOX | - | - | Surface modification with F3 peptide increased NPs uptake by 9 L glioma cells; NPs show controlled release of DOX (42% in the first 24 h). | [46] | |
| Anti-Cx43 and anti-BSAT1-conjugated nanogels | CIS | IV | - | Sustained release of CIS (50% after one week); Despite the lower cytotoxicity over C6 cells than free CIS, NPs improved the overall survival of 101/8 cells implanted in rats. | [47] | |
| cRGD-decorated PVA nanogels | DOX | IV | pH/redox | Triggered release of DOX caused by low pH and redox environment; cRGD modified nanogels effectively reduce tumor growth in vivo. | [48] | |
| Alginate nanogel co-loaded with gold nanoparticles | CIS | - | X-Ray | Higher toxicity on U87 MG cells, when compared to free CIS; marked apoptotic effect after X-ray irradiation. | [49] | |
| Fe3+-crosslinked pentaerythritol poly-(caprolactone)-b-poly(acrylic acid) nanogels | DOX | IV | Light | Nanoparticles effectively release DOX after the exposure of the tumor to light, with 91% of tumor growth inhibition and no adverse effects. | [50] | |
| Hydrogel matrices containing microstructured DDS | ||||||
| Microspheres | PLGA microspheres + TGP hydrogel | CPT | Local Delivery | Temp. | Sustained drug release of CPT at the tumor site; hydrogel administration and tumor resection significantly increased overall survival of the models (over 60 days). | [24,25] |
| PLGA microspheres + alginate hydrogel | PTX | Local Delivery | - | After a low initial burst, microspheres exhibited a controlled drug release over more than 60 days; higher cytotoxicity over C6 cells when compared to reference; reduction of tumor volume in tumor-bearing models. | [26] | |
| PLGA microspheres + P(NIPAAm-co-BMA) and PEG-based hydrogel | DOX | Local Delivery | Temp. | Increased sustained release over 30 days; significant reduction of tumor growth (32 days vs. 12 days with free DOX in hydrogel). | [27] | |
| Critical Quality Attributes | Justification |
|---|---|
| Easy to synthetize | They can be easily scaled up for large-scale production, in addition to being an eco-friendly chemistry approach. |
| Nanosize | Their small size eases the passage through biological barriers and avoids clearance by phagocytic cells, thus increasing their blood circulation time. |
| Viscoelasticity | Given their ability to deform, i.e., to switch between solid-like and liquid-like states, hydrogel nanoparticles pass more easily through biological barriers and cell membranes. |
| Swelling capacity | Occurring in aqueous fluids, swelling is controlled by chemical structure of the gel matrix and its crosslinking degree, as well as environmental variables (temperature, pH and ionic strength). |
| Response to stimuli | They can respond to biological stimuli, ensuring site-specific and controlled release of drugs. Such response involves changes in physicochemical properties of the hydrogel nanoparticles (swelling, permeability, viscoelasticity). |
| Encapsulation stability | Their crosslinked structure allows an extend drug circulation time in bloodstream, protecting drugs from enzymatic/chemical degradation. |
| Passive and active targeting | A wide range of bioactives (drugs, peptides, proteins, antibodies and vaccines) can be coupled to the surface of hydrogel nanoparticles in order to target specific tissues. In addition, stimuli-responsive hydrogels (as referred above) are another strategy of active targeting. Extravasation in the pathological sites and retention in the microvasculature could represent passive targeting approaches of hydrogel nanoparticles. All of them increase therapeutic efficacy and reduce undesired effects. |
| Controlled and sustained drug release | Internal crosslinking modulation of hydrogel nanoparticle networks can control both drug loading capacity and drug release. |
| Minimal toxicity | They are biocompatible, non-immunogenic and biodegradable. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors. Gels 2018, 4, 62. https://doi.org/10.3390/gels4030062
Basso J, Miranda A, Nunes S, Cova T, Sousa J, Vitorino C, Pais A. Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors. Gels. 2018; 4(3):62. https://doi.org/10.3390/gels4030062
Chicago/Turabian StyleBasso, João, Ana Miranda, Sandra Nunes, Tânia Cova, João Sousa, Carla Vitorino, and Alberto Pais. 2018. "Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors" Gels 4, no. 3: 62. https://doi.org/10.3390/gels4030062