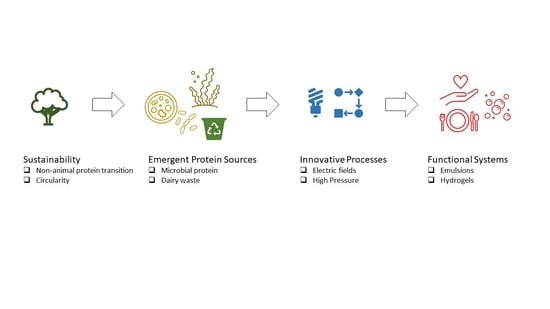
Emergent Proteins-Based Structures—Prospects towards Sustainable Nutrition and Functionality
Abstract
:1. Introduction
2. Promising Protein Sources and their Challenges
2.1. Food Waste
2.2. Microbial Protein—Yeast, Fungi, and Bacteria
2.3. Insect Protein
2.4. Algae Protein
3. Downstream Processing
3.1. US
3.2. MW
3.3. HHP
3.4. Electric Field Processing—A Promising Approach
4. Structured Systems for Functional Food and Health
4.1. Milk-Derived Proteins
4.2. Microalgae Proteins
4.3. Emergent Sources
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, S.; Roy, P.K.; Hossain, M.I.; Byun, K.-H.; Choi, C.; Ha, S.-D. COVID-19 pandemic crisis and food safety: Implications and inactivation strategies. Trends Food Sci. Technol. 2021, 109, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Béné, C.; Bakker, D.; Chavarro, M.J.; Even, B.; Melo, J.; Sonneveld, A. Global assessment of the impacts of COVID-19 on food security. Glob. Food Sec. 2021, 31, 100575. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Boostani, S.; Van der Meeren, P. Phytoparticles for the stabilization of Pickering emulsions in the formulation of novel food colloidal dispersions. Trends Food Sci. Technol. 2020, 98, 117–128. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Pereira, R.N.; Vicente, A.A.; Cavaco-Paulo, A.; Ribeiro, A. Ohmic heating as a new tool for protein scaffold engineering. Mater. Sci. Eng. C 2021, 120, 111784. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Herneke, A.; Lendel, C.; Johansson, D.; Newson, W.; Hedenqvist, M.; Karkehabadi, S.; Jonsson, D.; Langton, M. Protein Nanofibrils for Sustainable Food–Characterization and Comparison of Fibrils from a Broad Range of Plant Protein Isolates. ACS Food Sci. Technol. 2021, 1, 854–864. [Google Scholar] [CrossRef]
- Tarrega, A.; Rizo, A.; Murciano, A.; Laguna, L.; Fiszman, S. Are mixed meat and vegetable protein products good alternatives for reducing meat consumption? A case study with burgers. Curr. Res. Food Sci. 2020, 3, 30–40. [Google Scholar] [CrossRef]
- Malek, L.; Umberger, W.J.; Goddard, E. Committed vs. uncommitted meat eaters: Understanding willingness to change protein consumption. Appetite 2019, 138, 115–126. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.M.R.; Genisheva, Z.; Ferreira-Santos, P.; Rodrigues, R.; Vicente, A.A.; Teixeira, J.A.; Pereira, R.N. Electric field-based technologies for valorization of bioresources. Bioresour. Technol. 2018, 254, 325–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loveday, S.M. Food Proteins: Technological, Nutritional, and Sustainability Attributes of Traditional and Emerging Proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food proteins from plants and fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Prandi, B.; Faccini, A.; Lambertini, F.; Bencivenni, M.; Jorba, M.; Van Droogenbroek, B.; Bruggeman, G.; Schöber, J.; Petrusan, J.; Elst, K.; et al. Food wastes from agrifood industry as possible sources of proteins: A detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem. 2019, 286, 567–575. [Google Scholar] [CrossRef]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent Advances in the Recovery Techniques of Plant-Based Proteins from Agro-Industrial By-Products. Food Rev. Int. 2021, 37, 447–468. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, F.; de Oliveira Santana, W.; Gregolon, J.G.N.; Kist, T.B.L.; Fontana, R.C.; Camassola, M. Production of edible mycoprotein using agroindustrial wastes: Influence on nutritional, chemical and biological properties. Innov. Food Sci. Emerg. Technol. 2019, 58, 102227. [Google Scholar] [CrossRef]
- Farag, M.A.; Jomaa, S.A.; El-Wahed, A.A.; El-Seedi, A.H.R. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; de Souza Vandenberghe, L.P.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation—A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, T.J.A.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The Future of Nutritious Nonmeat Protein, a Symposium Review. Curr. Dev. Nutr. 2019, 3, nzz021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnigan, T.J.A. Mycoprotein: Origins, production and properties. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2011; pp. 335–352. ISBN 978-1-84569-758-7. [Google Scholar]
- Derbyshire, E.; Ayoob, K.-T. Mycoprotein: Nutritional and Health Properties. Nutr. Today 2019, 54, 7–15. [Google Scholar] [CrossRef]
- Jacobson, M.F.; DePorter, J. Self-reported adverse reactions associated with mycoprotein (Quorn-brand) containing foods. Ann. Allergy Asthma Immunol. 2018, 120, 626–630. [Google Scholar] [CrossRef]
- Sillman, J.; Nygren, L.; Kahiluoto, H.; Ruuskanen, V.; Tamminen, A.; Bajamundi, C.; Nappa, M.; Wuokko, M.; Lindh, T.; Vainikka, P.; et al. Bacterial protein for food and feed generated via renewable energy and direct air capture of CO2: Can it reduce land and water use? Glob. Food Secur. 2019, 22, 25–32. [Google Scholar] [CrossRef]
- Katona, S.J.; Kaminski, E.R. Sensitivity to Quorn mycoprotein (Fusarium venenatum) in a mould allergic patient. J. Clin. Pathol. 2002, 55, 876–877. [Google Scholar] [CrossRef] [Green Version]
- Dickie, F.; Miyamoto, M.; Collins, C.M. The Potential of Insect Farming to Increase Food Security. In Edible Insects; Heimo, M., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-636-1. [Google Scholar]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.P.; Ramos, O.L.; Vicente, A.A. Emergent food proteins—Towards sustainability, health and innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef] [Green Version]
- Van Huis, A.; Dunkel, F.V. Edible Insects: A Neglected and Promising Food Source. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Elsevier: Amsterdam, The Netherlands, Laboratorium voor Entomologie; 2016; pp. 341–355. ISBN 9780128027783. [Google Scholar]
- Geada, P.; Rodrigues, R.; Loureiro, L.; Pereira, R.; Fernandes, B.; Teixeira, J.A.; Vasconcelos, V.; Vicente, A.A. Electrotechnologies applied to microalgal biotechnology—Applications, techniques and future trends. Renew. Sustain. Energy Rev. 2018, 94, 656–668. [Google Scholar] [CrossRef] [Green Version]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Suarez Garcia, E.; van Leeuwen, J.; Safi, C.; Sijtsma, L.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Selective and energy efficient extraction of functional proteins from microalgae for food applications. Bioresour. Technol. 2018, 268, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleurence, J. The enzymatic degradation of algal cell walls: A useful approach for improving protein accessibility? J. Appl. Phycol. 1999, 11, 313–314. [Google Scholar] [CrossRef]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodrigues, M.M.; Estrada-Beristain, C.; Metri-Ojeda, J.; Pérez-Alva, A.; Baigts-Allende, D.K. Spirulina platensis Protein as Sustainable Ingredient for Nutritional Food Products Development. Sustainability 2021, 13, 6849. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Cian, R.E.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [Green Version]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.R.; Chew, K.W.; Zaid, H.F.M.; Chu, D.-T.; Tao, Y.; Show, P.L. Microalgal Protein Extraction From Chlorella vulgaris FSP-E Using Triphasic Partitioning Technique With Sonication. Front. Bioeng. Biotechnol. 2019, 7, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingwascharapong, P.; Chaijan, M.; Karnjanapratum, S. Ultrasound-assisted extraction of protein from Bombay locusts and its impact on functional and antioxidative properties. Sci. Rep. 2021, 11, 17320. [Google Scholar] [CrossRef]
- Hargreaves, G.; Buttress, A.; Dimitrakis, G.; Dodds, C.; Martin-Tanchereau, P.; Unthank, M.G.; Irvine, D.J. The importance of ionic conduction in microwave heated polyesterifications. React. Chem. Eng. 2020, 5, 495–505. [Google Scholar] [CrossRef]
- Bohr, H.; Bohr, J. Microwave-enhanced folding and denaturation of globular proteins. Phys. Rev. E 2000, 61, 4310–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedin, S.; Netto, F.M.; Bragagnolo, N.; Taranto, O.P. Reduction of the process time in the achieve of rice bran protein through ultrasound-assisted extraction and microwave-assisted extraction. Sep. Sci. Technol. 2020, 55, 300–312. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Lee, S.Y.; Zhu, L.; Show, P.L. Enhanced microalgal protein extraction and purification using sustainable microwave-assisted multiphase partitioning technique. Chem. Eng. J. 2019, 367, 1–8. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Gordon, T.A.; Villalobos-Carvajal, R.; Moreno-Osorio, L.; Salazar, F.N.; Pérez-Won, M.; Acuña, S. Effects of high hydrostatic pressure (HHP) on the protein structure and thermal stability of Sauvignon blanc wine. Food Chem. 2014, 155, 214–220. [Google Scholar] [CrossRef]
- Katzav, H.; Chirug, L.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. Comparison of Thermal and High-Pressure Gelation of Potato Protein Isolates. Foods 2020, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Avelar, Z.; Vicente, A.A.; Petersen, S.B.; Pereira, R.N. Influence of moderate electric fields in β-lactoglobulin thermal unfolding and interactions. Food Chem. 2020, 304, 125442. [Google Scholar] [CrossRef] [Green Version]
- Linsberger-Martin, G.; Weiglhofer, K.; Thi Phuong, T.P.; Berghofer, E. High hydrostatic pressure influences antinutritional factors and in vitro protein digestibility of split peas and whole white beans. LWT Food Sci. Technol. 2013, 51, 331–336. [Google Scholar] [CrossRef]
- Al-Ruwaih, N.; Ahmed, J.; Mulla, M.F.; Arfat, Y.A. High-pressure assisted enzymatic proteolysis of kidney beans protein isolates and characterization of hydrolysates by functional, structural, rheological and antioxidant properties. LWT 2019, 100, 231–236. [Google Scholar] [CrossRef]
- Franck, M.; Perreault, V.; Suwal, S.; Marciniak, A.; Bazinet, L.; Doyen, A. High hydrostatic pressure-assisted enzymatic hydrolysis improved protein digestion of flaxseed protein isolate and generation of peptides with antioxidant activity. Food Res. Int. 2019, 115, 467–473. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.; Mikolajczak, M.; Salmon, J.-M.; Abecassis, J.; Chaunier, L.; Guessasma, S.; Lourdin, D.; Belhabib, S.; Leroy, E.; Trystram, G. Small-scale food process engineering—Challenges and perspectives. Innov. Food Sci. Emerg. Technol. 2018, 46, 122–130. [Google Scholar] [CrossRef]
- Alkanan, Z.T.; Altemimi, A.B.; Al-Hilphy, A.R.S.; Watson, D.G.; Pratap-Singh, A. Ohmic Heating in the Food Industry: Developments in Concepts and Applications during 2013–2020. Appl. Sci. 2021, 11, 2507. [Google Scholar] [CrossRef]
- Pereira, R.N.; Vicente, A.A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Pagels, F.; Pereira, R.N.; Amaro, H.M.; Vasconcelos, V.; Guedes, A.C.; Vicente, A.A. Continuous pressurized extraction versus electric fields-assisted extraction of cyanobacterial pigments. J. Biotechnol. 2021, 334, 35–42. [Google Scholar] [CrossRef]
- Su, L.-C.; Hsu, Y.-H.; Wang, H.-Y. Enhanced labeling of microalgae cellular lipids by application of an electric field generated by alternating current. Bioresour. Technol. 2012, 111, 323–327. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Menegol, T.; Rech, R.; Mercali, G.D.; Marczak, L.D.F. Carotenoid and lipid extraction from Heterochlorella luteoviridis using moderate electric field and ethanol. Process Biochem. 2016, 51, 1636–1643. [Google Scholar] [CrossRef]
- Coustets, M.; Joubert-Durigneux, V.; Hérault, J.; Schoefs, B.; Blanckaert, V.; Garnier, J.-P.; Teissié, J. Optimization of protein electroextraction from microalgae by a flow process. Bioelectrochemistry 2015, 103, 74–81. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Furtado, G.; Pereira, R.N.; Vicente, A.A.; Cunha, R.L. Cold gel-like emulsions of lactoferrin subjected to ohmic heating. Food Res. Int. 2018, 103, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.N.; Rodrigues, R.M.; Altinok, E.; Ramos, Ó.L.; Xavier Malcata, F.; Maresca, P.; Ferrari, G.; Teixeira, J.A.; Vicente, A.A. Development of iron-rich whey protein hydrogels following application of ohmic heating—Effects of moderate electric fields. Food Res. Int. 2017, 99, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.N.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Effects of electric fields on protein unfolding and aggregation: Influence on edible films formation. Biomacromolecules 2010, 11, 2912–2918. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.M.; Martins, A.J.A.J.; Ramos, O.L.O.L.; Malcata, F.X.X.; Teixeira, J.A.J.A.; Vicente, A.A.A.; Pereira, R.N. Influence of moderate electric fields on gelation of whey protein isolate. Food Hydrocoll. 2015, 43, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.M.; Fasolin, L.H.; Avelar, Z.; Petersen, S.B.; Vicente, A.A.; Pereira, R.N. Effects of moderate electric fields on cold-set gelation of whey proteins—From molecular interactions to functional properties. Food Hydrocoll. 2020, 101, 105505. [Google Scholar] [CrossRef] [Green Version]
- Caruggi, N.; Lucisano, M.; Feyissa, A.H.; Rahimi Yazdi, S.; Mohammadifar, M.A. Effect of Ohmic Heating on the Formation and Texture of Acid Milk Gels. Food Biophys. 2019, 14, 249–259. [Google Scholar] [CrossRef]
- Moreira, T.C.P.; Pereira, R.N.; Vicente, A.A.; da Cunha, R.L. Effect of Ohmic heating on functionality of sodium caseinate—A relationship with protein gelation. Food Res. Int. 2019, 116, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ye, C.; Tian, Y.; Pan, S.; Wang, L. Effect of ohmic heating on fundamental properties of protein in soybean milk. J. Food Process Eng. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Jaeger, H.; Roth, A.; Toepfl, S.; Holzhauser, T.; Engel, K.H.; Knorr, D.; Vogel, R.F.; Bandick, N.; Kulling, S.; Heinz, V.; et al. Opinion on the use of ohmic heating for the treatment of foods. Trends Food Sci. Technol. 2016, 55, 84–97. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Ramos, Ó.L.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Vicente, A.A. Electric Field Processing: Novel Perspectives on Allergenicity of Milk Proteins. J. Agric. Food Chem. 2018, 66, 11227–11233. [Google Scholar] [CrossRef]
- Pereira, R.N.; Costa, J.; Rodrigues, R.M.; Villa, C.; Machado, L.; Mafra, I.; Vicente, A. Effects of ohmic heating on the immunoreactivity of β-lactoglobulin—A relationship towards structural aspects. Food Funct. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.N.; Rodrigues, R.M.; Machado, L.; Ferreira, S.; Costa, J.; Villa, C.; Barreiros, M.P.; Mafra, I.; Teixeira, J.A.; Vicente, A.A. Influence of ohmic heating on the structural and immunoreactive properties of soybean proteins. LWT 2021, 148, 111710. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Claro, B.; Bastos, M.; Pereira, R.N.; Vicente, A.A.; Petersen, S.B. Multi-step thermally induced transitions of β-lactoglobulin—An in situ spectroscopy approach. Int. Dairy J. 2020, 100, 104562. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Avelar, Z.; Machado, L.; Pereira, R.N.; Vicente, A.A. Electric field effects on proteins – Novel perspectives on food and potential health implications. Food Res. Int. 2020, 137, 109709. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Cultivation and downstream processing of microalgae and cyanobacteria to generate protein-based technofunctional food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 2961–2989. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Wei, G.; Ma, Y.; Bhandari, B.; Zhou, P. 3D printed milk protein food simulant: Improving the printing performance of milk protein concentration by incorporating whey protein isolate. Innov. Food Sci. Emerg. Technol. 2018, 49, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Picchio, M.L.; Cuggino, J.C.; Nagel, G.; Wedepohl, S.; Minari, R.J.; Alvarez Igarzabal, C.I.; Gugliotta, L.M.; Calderón, M. Crosslinked casein-based micelles as a dually responsive drug delivery system. Polym. Chem. 2018, 9, 3499–3510. [Google Scholar] [CrossRef] [Green Version]
- Ding, G.-J.; Zhu, Y.-J.; Cheng, G.-F.; Ruan, Y.-J.; Qi, C.; Lu, B.-Q.; Chen, F.; Wu, J. Porous Microspheres of Casein/Amorphous Calcium Phosphate Nanocomposite: Room Temperature Synthesis and Application in Drug Delivery. Curr. Nanosci. 2016, 12, 70–78. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Owonubi, S.J.; Aderibigbe, B.A.; Mukwevho, E.; Sadiku, E.R.; Ray, S.S. Characterization and in vitro release kinetics of antimalarials from whey protein-based hydrogel biocomposites. Int. J. Ind. Chem. 2018, 9, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Henriques, M.; Gomes, D.; Borges, A.; Pereira, C. Liquid whey protein concentrates as primary raw material for acid dairy gels. Food Sci. Technol. 2020, 40(2), 361–369. [Google Scholar] [CrossRef] [Green Version]
- Pires, A.F.; Marnotes, N.G.; Bella, A.; Viegas, J.; Gomes, D.M.; Henriques, M.H.F.; Pereira, C.J.D. Use of ultrafiltrated cow’s whey for the production of whey cheese with Kefir or probiotics. J. Sci. Food Agric. 2021, 101, 555–563. [Google Scholar] [CrossRef]
- Dinika, I.; Verma, D.K.; Balia, R.; Utama, G.L.; Patel, A.R. Potential of cheese whey bioactive proteins and peptides in the development of antimicrobial edible film composite: A review of recent trends. Trends Food Sci. Technol. 2020, 103, 57–67. [Google Scholar] [CrossRef]
- Nascimento, L.G.L.; Casanova, F.; Silva, N.F.N.; de Carvalho Teixeira, A.V.N.; de Carvalho, A.F. Casein-based hydrogels: A mini-review. Food Chem. 2020, 314, 126063. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Aquib, M.; Ghayas, S.; Bushra, R.; Haleem Khan, D.; Parveen, A.; Wang, B. Whey protein: A functional and promising material for drug delivery systems recent developments and future prospects. Polym. Adv. Technol. 2019, 30, 2183–2191. [Google Scholar] [CrossRef]
- Agnieray, H.; Glasson, J.L.; Chen, Q.; Kaur, M.; Domigan, L.J. Recent developments in sustainably sourced protein-based biomaterials. Biochem. Soc. Trans. 2021, 49, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Schloss, A.C.; Williams, D.M.; Regan, L.J. Protein-Based Hydrogels for Tissue Engineering BT. In Protein-Based Engineered Nanostructures; Cortajarena, A., Grove, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 167–177. ISBN 978-3-319-39196-0. [Google Scholar]
- Ribeiro, A.J.A.M.; Gomes, A.C.; Cavaco-Paulo, A.M. Developing scaffolds for tissue engineering using the Ca2+-induced cold gelation by an experimental design approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 2269–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, S.; Singh, A.K.; Behera, S.P.; Gupta, S. Thermoresponsive BSA hydrogels with phase tunability. Mater. Sci. Eng. C 2021, 119, 111590. [Google Scholar] [CrossRef] [PubMed]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Böcker, L.; Hostettler, T.; Diener, M.; Eder, S.; Demuth, T.; Adamcik, J.; Reineke, K.; Leeb, E.; Nyström, L.; Mathys, A. Time-temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira platensis/Spirulina. Food Chem. 2020, 316, 126374. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, P.; Böcker, L.; Mathys, A.; Fischer, P. Proteins from microalgae for the stabilization of fluid interfaces, emulsions, and foams. Trends Food Sci. Technol. 2021, 108, 326–342. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Z.; Chen, L.; Chou, A.; Yan, H.; Zuo, Y.Y.; Zhang, X. Influence of cell properties on rheological characterization of microalgae suspensions. Bioresour. Technol. 2013, 139, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, M.; Garrido-Fernández, A.; Mijlkovic, A.; Krona, A.; Martínez-Abad, A.; Coll-Marqués, J.M.; López-Rubio, A.; Lopez-Sanchez, P. Composition and rheological properties of microalgae suspensions: Impact of ultrasound processing. Algal Res. 2020, 49, 101960. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Goff, H.D.; Weiss, J. Heat-induced gel formation of a protein-rich extract from the microalga Chlorella sorokiniana. Innov. Food Sci. Emerg. Technol. 2019, 56, 102176. [Google Scholar] [CrossRef]
- Chronakis, I.S. Gelation of Edible Blue-Green Algae Protein Isolate (Spirulina platensis Strain Pacifica): Thermal Transitions, Rheological Properties, and Molecular Forces Involved. J. Agric. Food Chem. 2001, 49, 888–898. [Google Scholar] [CrossRef]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef]
- Böcker, L.; Bertsch, P.; Wenner, D.; Teixeira, S.; Bergfreund, J.; Eder, S.; Fischer, P.; Mathys, A. Effect of Arthrospira platensis microalgae protein purification on emulsification mechanism and efficiency. J. Colloid Interface Sci. 2021, 584, 344–353. [Google Scholar] [CrossRef]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries. Sustainbility 2021, 13, 3063. [Google Scholar] [CrossRef]
- Bayu, A.; Handayani, T. High-value chemicals from marine macroalgae: Opportunities and challenges for marine-based bioenergy development. IOP Conf. Ser. Earth Environ. Sci. 2018, 209, 12046. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Panozzo, A.; Doumen, V.; Foubert, I.; Gheysen, L.; Goiris, K.; Moldenaers, P.; Hendrickx, M.E.; Van Loey, A.M. Microalgal biomass as a (multi)functional ingredient in food products: Rheological properties of microalgal suspensions as affected by mechanical and thermal processing. Algal Res. 2017, 25, 452–463. [Google Scholar] [CrossRef]
- Ebert, S.; Grossmann, L.; Hinrichs, J.; Weiss, J. Emulsifying properties of water-soluble proteins extracted from the microalgae Chlorella sorokiniana and Phaeodactylum tricornutum. Food Funct. 2019, 10, 754–764. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Functional and Bioactive Properties of Protein Extracts Generated from Spirulina platensis and Isochrysis galbana T-Iso. Appl. Sci. 2021, 11, 3964. [Google Scholar] [CrossRef]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and function-al properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef]
- Bethi, C.M.S.; Narayan, B.; Martin, A.; Kudre, T.G. Recovery, physicochemical and functional characteristics of proteins from different meat processing wastewater streams. Environ. Sci. Pollut. Res. 2020, 27, 25119–25131. [Google Scholar] [CrossRef]
- de Melo, A.N.F.; de Souza, E.L.; da Silva Araujo, V.B.; Magnani, M. Stability, nutritional and sensory characteristics of French salad dressing made with mannoprotein from spent brewer’s yeast. LWT Food Sci. Technol. 2015, 62, 771–774. [Google Scholar] [CrossRef]
- Avelar, Z.; Vicente, A.A.; Saraiva, J.A.; Rodrigues, R.M. The role of emergent processing technologies in tailoring plant protein functionality: New insights. Trends Food Sci. Technol. 2021, 113, 219–231. [Google Scholar] [CrossRef]
- Subaşı, B.G.; Jahromi, M.; Casanova, F.; Capanoglu, E.; Ajalloueian, F.; Mohammadifar, M.A. Effect of moderate electric field on structural and thermo-physical properties of sunflower protein and sodium caseinate. Innov. Food Sci. Emerg. Technol. 2021, 67, 102593. [Google Scholar] [CrossRef]
- Tapal, A.; Vegarud, G.E.; Sreedhara, A.; Kaul Tiku, P. Nutraceutical protein isolate from pigeon pea (Cajanus cajan) milling waste by-product: Functional aspects and digestibility. Food Funct. 2019, 10, 2710–2719. [Google Scholar] [CrossRef] [PubMed]


| Source | Functional Properties | Application | Reference |
|---|---|---|---|
| Milk protein concentrate and whey protein concentrate | 3D printing of food simulant | Food | [81] |
| Casein nanocarrier | Glyceraldehyde (GAL) used as a crosslinking agent for the entrapment of Nile Red | Biomedical | [82] |
| Casein-based nanocomposite | Favorable for drug loading and release using ibuprofen (IBU), docetaxel (Dtxl), and vitamin B5 (B5) as model | Biomedical | [83] |
| WPI nanofibrils | Complexation with curcumin | Food | [84] |
| WPI-based nanocomposites | Controlled delivery of antimalarials | Biomedical | [85] |
| BSA/casein | Production of biodegradable scaffolds | Biomedical | [4] |
| Source | Functional Properties | Application | Reference |
|---|---|---|---|
| Tetraselmis suecica | Improved gelation and surface activity (foaming and emulsification) when compared with WPI | Food | [33] |
| Chlorella sorokiniana | Protein heat-induced gel governed by electrostatic interactions | Food | [100] |
| Chlorella sorokiniana and Phaeodactylum tricornutum | Emulsifying properties of water-soluble proteins | Food | [107] |
| Spirulina sp. and Isochrysis galbana | Water- and oil-holding capacities, foaming, emulsifying activities, and stabilities of protein extracts | Food | [108] |
| Arthrospira platensis | Protein isolate with emulsifying, foaming, gelling, and film-forming properties favored at pH 10 | Food | [102] |
| Arthrospira platensis | Interfacial viscoelastic network was faster, and final network strength increased with the degree of purification | Food | [103] |
| Chlorella vulgaris | Enhanced emulsifying capacity and stability | Food | [109] |
| Porphyridium cruentum, Chlorella vulgaris and Odontella aurita | Shear thinning behavior at 8% w/w, showing aelastic-like behavior (G′ > G″) | Food | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.N.; Rodrigues, R.M. Emergent Proteins-Based Structures—Prospects towards Sustainable Nutrition and Functionality. Gels 2021, 7, 161. https://doi.org/10.3390/gels7040161
Pereira RN, Rodrigues RM. Emergent Proteins-Based Structures—Prospects towards Sustainable Nutrition and Functionality. Gels. 2021; 7(4):161. https://doi.org/10.3390/gels7040161
Chicago/Turabian StylePereira, Ricardo N., and Rui M. Rodrigues. 2021. "Emergent Proteins-Based Structures—Prospects towards Sustainable Nutrition and Functionality" Gels 7, no. 4: 161. https://doi.org/10.3390/gels7040161