Interaction of TX-100 and Antidepressant Imipramine Hydrochloride Drug Mixture: Surface Tension, 1H NMR, and FT-IR Investigation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics at the Air-Interfacial Surfaces of Pure and Mixed System
2.2. Composition of Component and Interaction Parameters at the Air-Interfacial Surfaces
2.3. Thermodynamic Parameters
2.4. Packing Parameters
2.5. 1H NMR Study
2.6. FT-IR Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Measurement of Surface Tension
4.2.2. 1H NMR Study
4.2.3. FTIR Spectroscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biswal, N.R.; Paria, S. Interfacial and wetting behavior of natural–synthetic mixed surfactant systems. RSC Adv. 2014, 4, 9182–9188. [Google Scholar] [CrossRef]
- Sheng, Y.; Yan, C.; Li, Y.; Peng, Y.; Ma, L.; Wang, Q. Thermal stability of gel foams stabilized by xanthan gum, silica nanoparticles and surfactants. Gels 2021, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Kumar, D.; Rub, M.A. Kinetic and mechanistic investigations of [Zn (II)-Trp]+ and ninhydrin in aqueous and cationic CTAB surfactant. J. Phys. Org. Chem. 2019, 32, e3997. [Google Scholar] [CrossRef]
- Kumar, D.; Rub, M.A. Kinetic study of ninhydrin with chromium (III)-glycylleucine in aqueous–alkanediyl-α,ω-bis (dimethylcetylammonium bromide) gemini surfactants. J. Phys. Org. Chem. 2019, 32, e3946. [Google Scholar] [CrossRef]
- de Molina, P.M.; Gradzielski, M. Gels obtained by colloidal self-assembly of amphiphilic molecules. Gels 2017, 3, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attwood, D.; Florence, A.T. Surfactant systems. In Their Chemistry, Pharmacy and Biology; Chapman and Hall: New York, NY, USA, 1983. [Google Scholar]
- Sachin, K.M.; Karpe, S.A.; Kumar, D.; Singh, M.; Dominguez, H.; Ríos-López, M.; Bhattarai, A. A simulation study of self-assembly behaviors and micellization properties of mixed ionic surfactants. J. Mol. Liq. 2021, 336, 116003. [Google Scholar] [CrossRef]
- Yin, B.; Ni, J.; Witherel, C.E.; Yang, M.; Burdick, J.A.; Wen, C.; Wong, S.H.D. Harnessing Tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics 2022, 12, 207–231. [Google Scholar] [CrossRef]
- Hamed, F.A.; Zoveidavianpoor, M. The Foaming Behavior and Synergistic Effect in Aqueous CO2 Foam by In Situ Physisorption of Alpha Olefin Sulfonate and Triton X-100 Surfactants and Their Mixture. Petroleum Sci. Technol. 2014, 32, 2376–2386. [Google Scholar]
- Farcet, J.-B.; Kindermann, J.; Karbiener, M.; Kreil, T.R. Development of a Triton X-100 replacement for effective virus inactivation in biotechnology processes. Eng. Rep. 2019, 1, e12078. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, K.; Bharatiya, B.; Ray, D.; Aswal, V.; Bahadur, P. Molecular interactions involving aqueous Triton X-100 micelles and anionic surfactants: Investigations on, surface activity and morphological transitions. J. Mol. Liq. 2016, 223, 611–620. [Google Scholar] [CrossRef]
- Dharaiya, N.; Bahadur, P.; Singh, K.; Marangoni, D.; Bahadur, P. Light scattering and NMR studies of Triton X-100 micelles in the presence of short chain alcohols and ethoxylates. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 252–259. [Google Scholar] [CrossRef]
- Ćirin, D.M.; Posa, M.M.; Krstonosic, V.S. Interactions between selected bile salts and Triton X-100 or sodium lauryl ether sulfate. Chem. Central J. 2011, 5, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreier, S.; Malheiros, S.V.; de Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta 2000, 1508, 210–234. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Hidayathulla, S.; Rub, M.A. Association behavior of a mixed system of the antidepressant drug imipramine hydrochloride and dioctyl sulfosuccinate sodium salt: Effect of temperature and salt. J. Mol. Liq. 2018, 271, 254–264. [Google Scholar] [CrossRef]
- Alomar, M.J. Factors affecting the development of adverse drug reactions. Saudi Pharm. J. 2014, 22, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Rub, M.A.; Azum, N.; Alotaibi, M.M.; Asiri, A.M. Solution behaviour of antidepressant imipramine hydrochloride drug and non-ionic surfactant mixture: Experimental and theoretical study. Polymers 2021, 13, 4025. [Google Scholar] [CrossRef]
- Bagheri, A. Comparison of the interaction between propranolol hydrochloride (PPL) with anionic surfactant and cationic surface active ionic liquid in micellar phase. Colloids Surf. A 2021, 615, 126183. [Google Scholar] [CrossRef]
- Alam, M.S.; Siddiq, A.M. Self-association and mixed micellization of an amphiphilic antidepressant drug, 5-[3-(Dimethylamino)propyl]-10,11-dihydro-5H-dibenz[b,f]azepine hydrochloride and a nonionic surfactant, poly(ethylene glycol) t-octylphenyl ether: Evaluation of thermodynamics. J. Mol. Liq. 2018, 252, 321–328. [Google Scholar] [CrossRef]
- Kumar, D.; Azum, N.; Rub, M.A.; Asiri, A.M. Aggregation behavior of sodium salt of ibuprofen with conventional and gemini surfactant. J. Mol. Liq. 2018, 262, 86–96. [Google Scholar] [CrossRef]
- Pal, A.; Pillania, A. Modulations in surface and aggregation properties of non-ionic surfactant Triton X-45 on addition of ionic liquids in aqueous media. J. Mol. Liq. 2017, 233, 243–250. [Google Scholar] [CrossRef]
- Kabir-ud-Din, K.A.; Naqvi, A.Z. Mixed micellization and interfacial properties of nonionic surfactants with the phenothiazine drug promazine hydrochloride at 30 °C. J. Solut. Chem. 2012, 41, 1587–1599. [Google Scholar] [CrossRef]
- Rub, M.A.; Khan, F.; Sheikh, M.S.; Azum, N.; Asiri, A.M. Tensiometric, fluorescence and 1H NMR study of mixed micellization of non-steroidal anti-inflammatory drug sodium salt of ibuprofen in the presence of non-ionic surfactant in aqueous/urea solutions. J. Chem. Thermodyn. 2016, 96, 196–207. [Google Scholar] [CrossRef]
- Zhou, Q.; Rosen, M.J. Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: The regular solution approach. Langmuir 2003, 19, 4555–4562. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Khan, F.; Asiri, A.M. Aggregation of sodium salt of ibuprofen and sodium taurocholate mixture in different media: A tensiometry and fluorometry study. J. Chem. Thermodyn. 2018, 121, 199–210. [Google Scholar] [CrossRef]
- Dar, A.A.; Chatterjee, B.; Rather, G.M.; Das, A.R. Mixed micellization and interfacial properties of dodecyltrimethylammonium bromide and tetraethyleneglycol mono-n-dodecyl ether in absence and presence of sodium propionate. J. Colloid Interface Sci. 2006, 298, 395–405. [Google Scholar] [CrossRef]
- Sharma, R.; Mahajan, R.K. An investigation of binding ability of ionic surfactants with trifluoperazine dihydrochloride: Insights from surface tension, electronic absorption and fluorescence measurements. RSC Adv. 2012, 2, 9571–9583. [Google Scholar] [CrossRef]
- Rosen, M.J.; Aronson, S. Standard free energies of adsorption of surfactants at the aqueous solution/air interface from surface tension data in the vicinity of the critical micelle concentration. Colloids Surf. 1981, 13, 201–208. [Google Scholar] [CrossRef]
- Fatma, N.; Panda, M.; Ansari, W.H. Environment-friendly ester bonded gemini surfactant: Mixed micellization of 14-E2-14 with ionic and nonionic conventional surfactants. J. Mol. Liq. 2015, 211, 247–255. [Google Scholar] [CrossRef]
- Sugihara, G.; Miyazono, A.M.; Nagadome, S.; Oida, T.; Hayashi, Y.; Ko, J.S. Adsorption and micelle formation of mixed surfactant systems in water. II a combination of cationic gemini-type with MEGA-10. J. Oleo Sci. 2003, 52, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Azum, N.; Rub, M.A.; Asiri, A.M. Interfacial and spectroscopic behavior of phenothiazine drug/bile salt mixture in urea solution. Chem. Pap. 2021, 75, 3949–3956. [Google Scholar] [CrossRef]
- Rodenas, E.; Valiente, M.; Villafruela, M.S. Different theoretical approaches for the study of the mixed tetraethylene glycol mono-n-dodecyl ether/hexadecyltrimethylammonium bromide micelles. J. Phys. Chem. B 1999, 103, 4549–4554. [Google Scholar] [CrossRef]
- Khan, F.; Rub, M.A.; Azum, N.; Asiri, A.M. Mixtures of antidepressant amphiphilic drug imipramine hydrochloride and anionic surfactant: Micellar and thermodynamic investigation. J. Phys. Org. Chem. 2018, 31, e3812. [Google Scholar] [CrossRef]
- Kumar, D.; Rub, M.A.; Azum, N.; Asiri, A.M. Mixed micellization study of ibuprofen (sodium salt) and cationic surfactant (conventional as well as gemini). J. Phys. Org. Chem. 2018, 31, e3730. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Khan, F.; Asiri, A.M. Surface, micellar, and thermodynamic properties of antidepressant drug nortriptyline hydrochloride with TX-114 in aqueous/urea solutions. J. Phys. Org. Chem. 2017, 30, e3676. [Google Scholar] [CrossRef]
- Israelashvili, J.N. Intermolecular and Surface Forces; Academic Press: London, UK, 1991. [Google Scholar]
- Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Azum, N.; Rub, M.A.; Asiri, A.M.; Bawazeer, W.A. Micellar and interfacial properties of amphiphilic drug–non-ionic surfactants mixed systems: Surface tension, fluorescence and UV–vis studies. Colloids Surf. A 2017, 522, 183–192. [Google Scholar] [CrossRef]
- Khan, S.A.; Ullah, Q.; Almalki, A.S.A.; Kumar, S.; Obaid, R.J.; Alsharif, M.A.; Alfaifi, S.Y.; Hashmi, A.A. Synthesis and photophysical investigation of (BTHN) Schiff base as off-on Cd2+ fluorescent chemosensor and its live cell imaging. J. Mol. Liq. 2021, 328, 115407. [Google Scholar] [CrossRef]
- Asad, M.; Khan, S.A.; Arshad, M.N.; Asiri, A.M.; Rehan, M. Design and synthesis of novel pyrazoline derivatives for their spectroscopic, single crystal X-ray and biological studies. J. Mol. Struct. 2021, 1234, 130131. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Mahajan, S.; Bhadani, A.; Singh, S. Physicochemical studies of pyridinium gemini surfactants with promethazine hydrochloride in aqueous solution. Phys. Chem. Chem. Phys. 2012, 14, 887–898. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, X.-Y.; Chen, H.; Mao, S.-Z.; Liu, M.-L.; Luo, P.-Y.; Du, Y.-R. NMR study of the dynamics of cationic gemini surfactant 14-2-14 in mixed solution with conventional surfactants. J. Phys. Chem. B 2009, 113, 8357–8361. [Google Scholar] [CrossRef]
- Rub, M.A.; Khan, F.; Azum, N.; Asiri, A.M.; Marwani, H.M. Micellization phenomena of amphiphilic drug and TX-100 mixtures: Fluorescence, UV-visible and 1 H NMR study. J. Taiwan Inst. Chem. Eng. 2016, 60, 32–43. [Google Scholar] [CrossRef]
- Vautier-Giongo, C.; Bakshi, M.S.; Singh, J.; Ranganathan, R.; Joseph, H.; Bales, C.B.L. Effects of interactions on the formation of mixed micelles of 1,2-diheptanoyl-sn-glycero-3-phosphocholine with sodium dodecyl sulfate and dodecyltrimethylammonium bromide. J. Colloid Interface Sci. 2005, 282, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Tseng, M.-Y.; Chen, S.-H.; Roberts, M.F. Temperature dependence of the growth of diheptanoylphosphatidylcholine micelles studied by small-angle neutron scattering. J. Phys. Chem. 1990, 94, 7239–7243. [Google Scholar] [CrossRef]
- Siddiqui, U.S.; Khan, F.; Khan, I.A.; Dar, A.A. Role of added counterions in the micellar growth of bisquaternary ammonium halide surfactant (14-s-14): 1H NMR and viscometric studies. J. Colloid Interface Sci. 2011, 355, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rub, M.A. Effect of additives (TX-114) on micellization and microstructural phenomena of amphiphilic ibuprofen drug (sodium salt): Multi-technique approach. J. Lumin. 2018, 197, 252–265. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Wang, H.; Kou, Y.; Wen, Q.H.; Fu, Z.F.; Chang, H.X. Response surface optimization and physicochemical properties of polysaccharides from Nelumbo nucifera leaves. Int. J. Biol. Macromol. 2015, 74, 103–110. [Google Scholar] [CrossRef]
- Gaikar, V.G.; Padalkar, K.V.; Aswal, V.K. Characterization of mixed micelles of structural isomers of sodium butyl benzene sulfonate and sodium dodecyl sulfate by SANS, FTIR spectroscopy and NMR spectroscopy. J. Mol. Liq. 2008, 138, 155–167. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N. Association behavior of amphiphlic drug promethazine hydrochloride and sodium p-toluenesulfonate mixtures: Effect of additives. J. Mol. Liq. 2021, 325, 114654. [Google Scholar] [CrossRef]
- Banjare, M.K.; Kurrey, R.; Yadav, T.; Sinha, S.; Satnami, M.L.; Ghosh, K.K. A comparative study on the effect of imidazolium-based ionic liquid on self-aggregation of cationic, anionic and nonionic surfactants studied by surface tension, conductivity, fluorescence and FTIR spectroscopy. J. Mol. Liq. 2017, 241, 622–632. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, N.; Katal, A. Aggregation behaviour of cationic (cetyltrimethylammonium bromide) and anionic (sodium dodecylsulphate) surfactants in aqueous solution of synthesized ionic liquid [1-pentyl-3-methylimidazolium bromide] -Conductivity and FT-IR spectroscopic studies. J. Mol. Liq. 2018, 258, 285–294. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Kumar, D.; Alotaibi, M.M.; Asiri, A.M. Impact of numerous media on association, interfacial, and thermodynamic properties of promethazine hydrochloride (PMT) + benzethonium chloride (BTC) mixture of various composition. J. Mol. Liq. 2022, 346, 118287. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Kumar, D.; Khan, A.; Arshad, M.N.; Asiri, A.M.; Alotaibi, M.M. Aggregational behaviour of promethazine hydrochloride and TX-45 surfactant mixtures: A multi-techniques approach. J. Mol. Liq. 2021, 342, 117558. [Google Scholar]
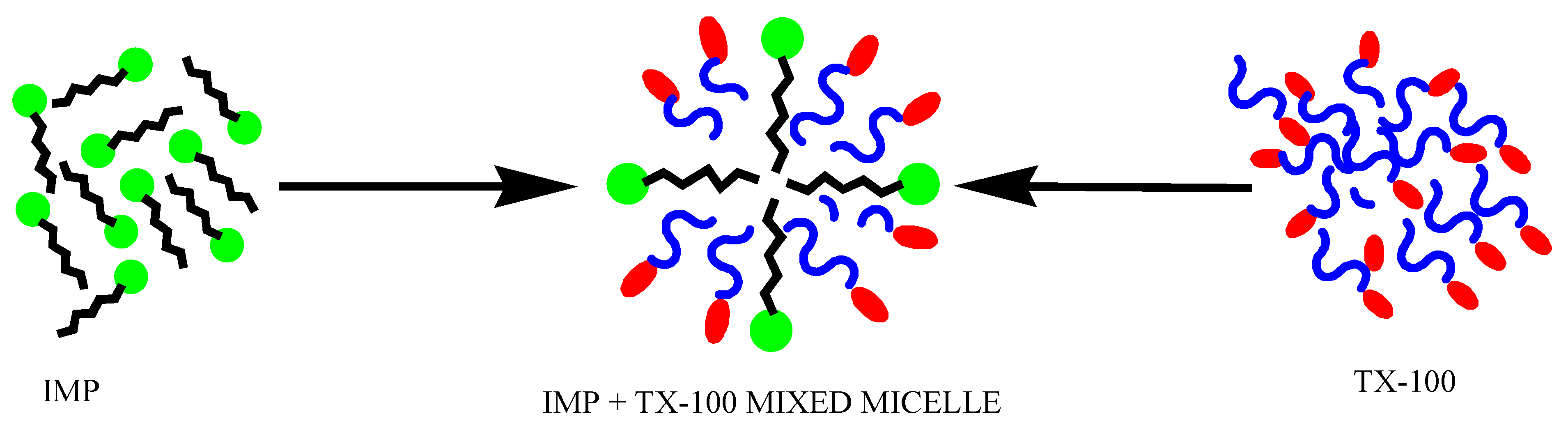




| α1 | X1σ | βσ | f1σ | f2σ | Γmax 107 (mol·m−2) | Amin/Aid (Ǻ2) | γcmc (mN·m−1) | πcmc (mN·m−1) | pC20 | ln(C1/C2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous solution | ||||||||||
| 0 | 12.78 | 129.95 | 42.58 | 28.42 | 1.95 | |||||
| 0.1 | 0.7334 | −6.07 | 0.6496 | 0.0382 | 23.09 | 71.89/137.77 | 30.35 | 40.65 | 3.90 | −6.04 |
| 0.3 | 0.7621 | −7.77 | 0.6443 | 0.0110 | 23.22 | 71.51/139.94 | 29.94 | 41.06 | 4.36 | −6.04 |
| 0.5 | 0.7741 | −8.79 | 0.6385 | 0.0052 | 24.16 | 68.72/140.84 | 29.67 | 41.33 | 4.58 | −6.04 |
| 0.7 | 0.7969 | −9.34 | 0.6803 | 0.0027 | 25.73 | 64.54/142.56 | 29.36 | 41.64 | 4.68 | −6.04 |
| 0.9 | 0.8421 | −9.62 | 0.7868 | 0.0011 | 27.90 | 59.52/145.97 | 29.40 | 41.60 | 4.71 | −6.04 |
| 1 | 36.02 | 46.10 | 29.31 | 41.69 | 4.57 | |||||
| 50 mmol∙kg−1 NaCl | ||||||||||
| 0 | 8.86 | 187.41 | 44.69 | 26.31 | 2.07 | |||||
| 0.1 | 0.8828 | −2.76 | 0.9629 | 0.1168 | 27.25 | 60.93/149.04 | 30.66 | 40.34 | 3.88 | −6.32 |
| 0.3 | 0.8712 | −4.80 | 0.9235 | 0.0262 | 26.04 | 63.77/148.16 | 29.91 | 41.09 | 4.38 | −6.32 |
| 0.5 | 0.9149 | −4.76 | 0.9661 | 0.0186 | 28.65 | 57.95/151.46 | 29.83 | 41.17 | 4.57 | −6.32 |
| 0.7 | 0.8819 | −6.75 | 0.9101 | 0.0052 | 28.01 | 59.27/148.97 | 29.53 | 41.47 | 4.75 | −6.32 |
| 0.9 | 0.9210 | −7.23 | 0.9559 | 0.0022 | 31.30 | 53.05/151.91 | 29.45 | 41.55 | 4.82 | −6.32 |
| 1 | 27.60 | 60.15 | 29.65 | 41.35 | 4.96 | |||||
| 250 mmol∙kg−1 U | ||||||||||
| 0 | 12.46 | 133.24 | 44.03 | 26.97 | 1.86 | |||||
| 0.1 | 0.7776 | −4.69 | 0.7930 | 0.0587 | 25.19 | 65.91/141.11 | 30.21 | 40.79 | 3.70 | −6.05 |
| 0.3 | 0.8085 | −6.12 | 0.7989 | 0.0183 | 25.92 | 64.06/143.43 | 29.59 | 41.41 | 4.15 | −6.05 |
| 0.5 | 0.8149 | −7.26 | 0.7797 | 0.0080 | 25.42 | 65.31/143.92 | 29.31 | 41.69 | 4.38 | −6.05 |
| 0.7 | 0.8189 | −8.50 | 0.7567 | 0.0033 | 25.57 | 64.94/144.22 | 29.80 | 41.20 | 4.54 | −6.05 |
| 0.9 | 0.8096 | −10.99 | 0.6715 | 0.0007 | 24.60 | 67.49/143.52 | 29.71 | 41.29 | 4.71 | −6.05 |
| 1 | 33.18 | 50.04 | 30.17 | 40.83 | 4.49 | |||||
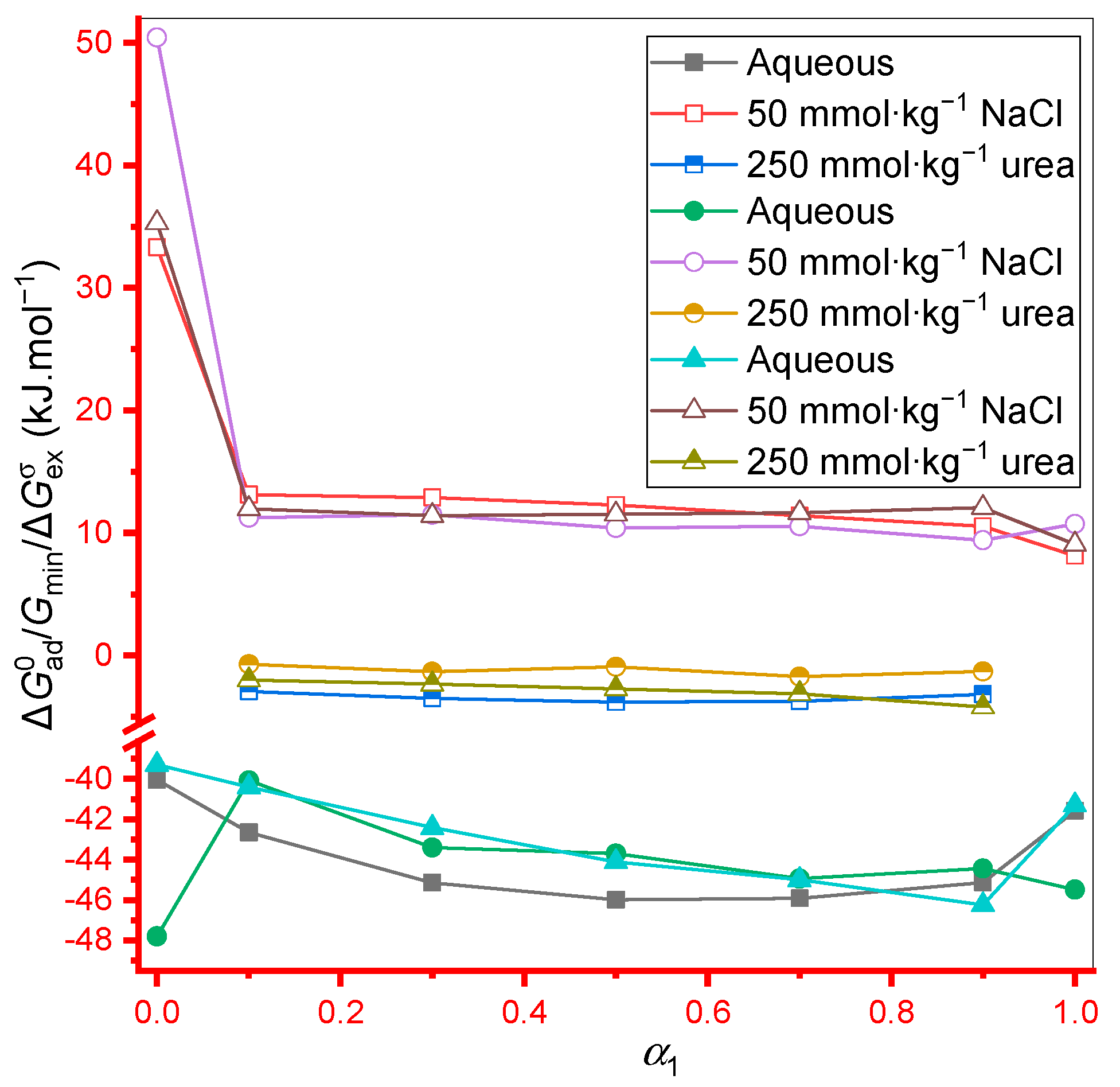
| α1 | (kJ·mol−1) | Gmin (kJ·mol−1) | (kJ·mol−1) | P |
|---|---|---|---|---|
| Aqueous system | ||||
| 0 | −40.06 | 33.33 | 0.34 | |
| 0.1 | −42.66 | 13.14 | −2.94 | 0.60 |
| 0.3 | −45.15 | 12.89 | −3.49 | 0.61 |
| 0.5 | −45.99 | 12.28 | −3.81 | 0.63 |
| 0.7 | −45.92 | 11.41 | −3.74 | 0.67 |
| 0.9 | −45.13 | 10.54 | −3.17 | 0.73 |
| 1 | −41.58 | 8.14 | 0.95 | |
| 50 mmol∙kg−1 NaCl | ||||
| 0 | −47.80 | 50.44 | 0.24 | |
| 0.1 | −40.08 | 11.25 | −0.71 | 0.71 |
| 0.3 | −43.40 | 11.49 | −1.33 | 0.68 |
| 0.5 | −43.70 | 10.41 | −0.92 | 0.75 |
| 0.7 | −44.94 | 10.54 | −1.74 | 0.73 |
| 0.9 | −44.45 | 9.41 | −1.30 | 0.82 |
| 1 | −45.48 | 10.74 | 0.73 | |
| 250 mmol∙kg−1 U | ||||
| 0 | −39.30 | 35.33 | 0.33 | |
| 0.1 | −40.40 | 11.99 | −2.01 | 0.66 |
| 0.3 | −42.41 | 11.42 | −2.35 | 0.68 |
| 0.5 | −44.11 | 11.53 | −2.71 | 0.66 |
| 0.7 | −45.0 | 11.66 | −3.12 | 0.67 |
| 0.9 | −46.25 | 12.08 | −4.20 | 0.64 |
| 1 | −41.30 | 9.09 | 0.87 | |
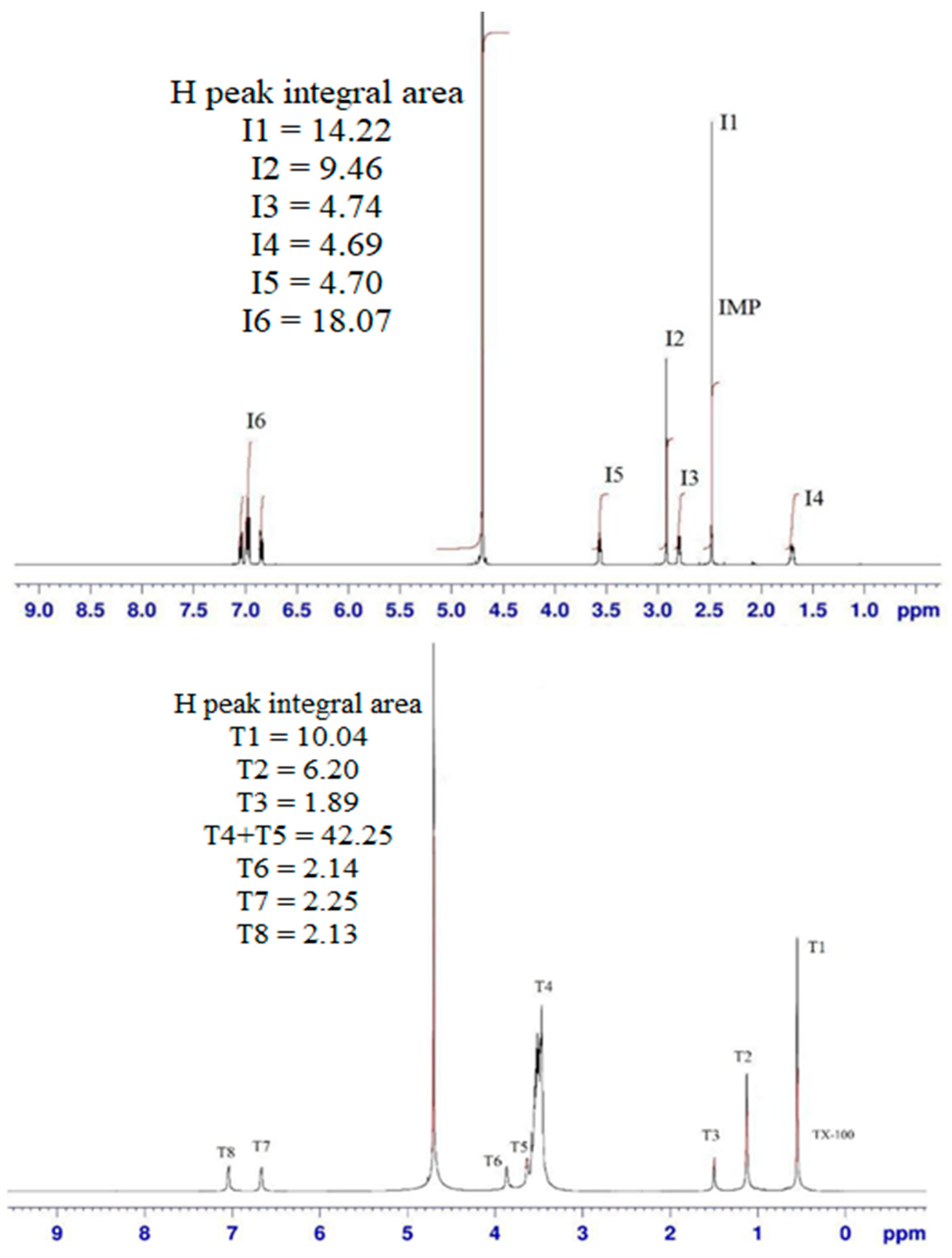
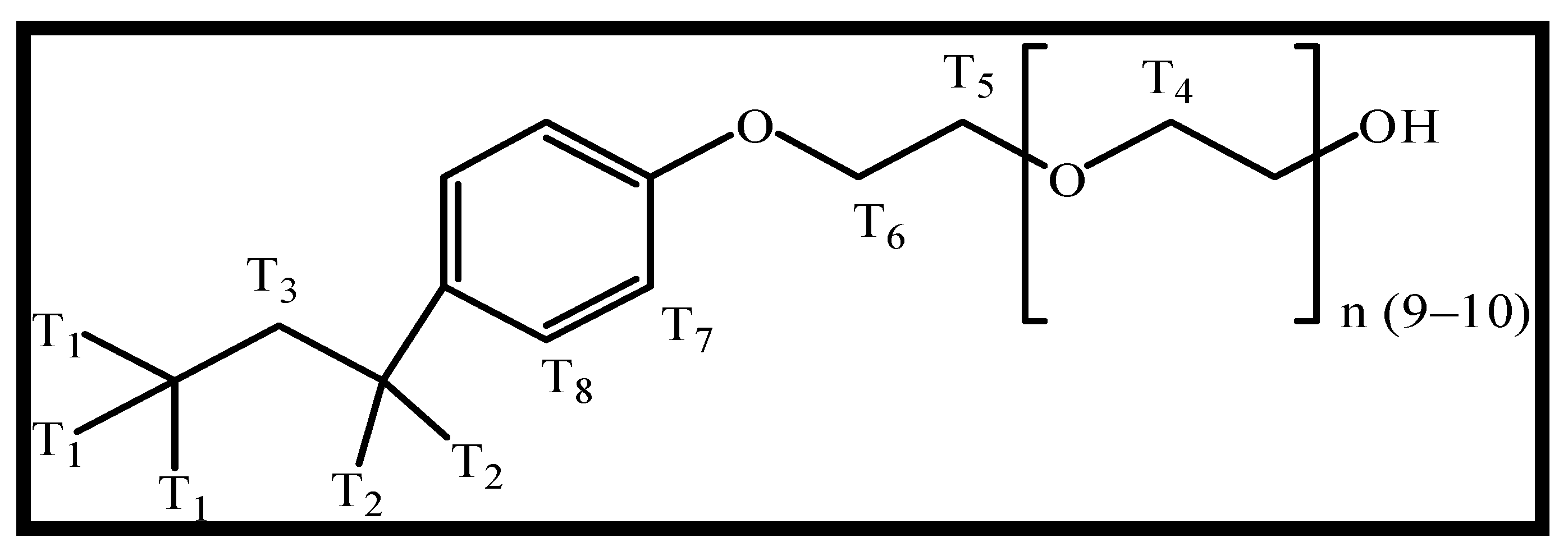
| Compound | Chemical Shifts (δ, ppm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pure IMP | I1 | I2 | I3 | I4 | I5 | I6 | ||
| 2.478 | 2.916 | 2.790 | 1.695 | 3.564 | 6.983 | |||
| Pure TX-100 a | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 |
| 0.549 | 1.124 | 1.495 | 3.591 | 3.806 | 3.865 | 7.015 | 7.097 | |
| Chemical Shifts (δ, ppm) | |||||
|---|---|---|---|---|---|
| α1 = 0.1 | α1 = 0.3 | α1 = 0.5 | α1 = 0.7 | α1 = 0.9 | |
| T1 | 0.486 | 0.517 | 0.527 | 0.534 | 0.542 |
| T2 | 1.01 | 1.06 | 1.08 | 1.096 | 1.111 |
| T3 | 1.376 | 1.389 | 1.437 | 1.464 | 1.473 |
| T4–T6 | 3.406 | 3.417 | 3.42 | 3.44 | 3.517 |
| T7 | 6.82 | 6.831 | 6.944 | 6.999 | 7.004 |
| T8 | 6.975 | 6.997 | 7.007 | 7.026 | 7.029 |
| I1 | 2.496 | 2.515 | 2.524 | 2.526 | 2.685 |
| I2 | 2.924 | 2.93 | 2.932 | 2.937 | 3.41 |
| I3 | 2.834 | 2.845 | 2.86 | 2.874 | 2.903 |
| I4 | 1.716 | 1.754 | 1.759 | 1.764 | 1.817 |
| I5 | 3.57 | 3.582 | 3.596 | 3.628 | 3.65 |
| I6 | 6.991 | 6.998 | 7.012 | 7.018 | 7.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rub, M.A.; Azum, N.; Kumar, D.; Asiri, A.M. Interaction of TX-100 and Antidepressant Imipramine Hydrochloride Drug Mixture: Surface Tension, 1H NMR, and FT-IR Investigation. Gels 2022, 8, 159. https://doi.org/10.3390/gels8030159
Rub MA, Azum N, Kumar D, Asiri AM. Interaction of TX-100 and Antidepressant Imipramine Hydrochloride Drug Mixture: Surface Tension, 1H NMR, and FT-IR Investigation. Gels. 2022; 8(3):159. https://doi.org/10.3390/gels8030159
Chicago/Turabian StyleRub, Malik Abdul, Naved Azum, Dileep Kumar, and Abdullah M. Asiri. 2022. "Interaction of TX-100 and Antidepressant Imipramine Hydrochloride Drug Mixture: Surface Tension, 1H NMR, and FT-IR Investigation" Gels 8, no. 3: 159. https://doi.org/10.3390/gels8030159








