Emerging Advances in Microfluidic Hydrogel Droplets for Tissue Engineering and STEM Cell Mechanobiology
Abstract
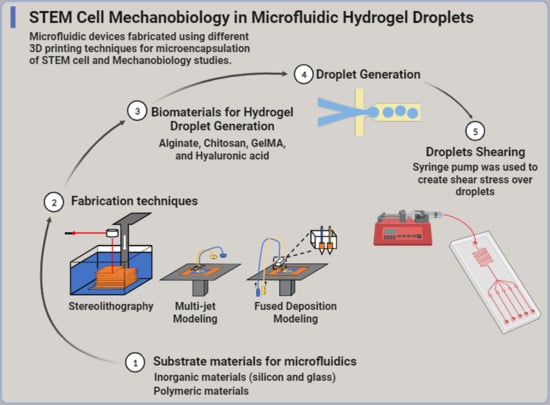
:1. Introduction
1.1. The Promise of 3D Tissue Regeneration
1.2. Employing Hydrogel Droplets in Tissue Engineering
1.3. Studying the Interfacial Adhesivity of Hydrogels
1.4. Microfluidic Control of Hydrogel Properties Promotes Cell Growth and Differentiation
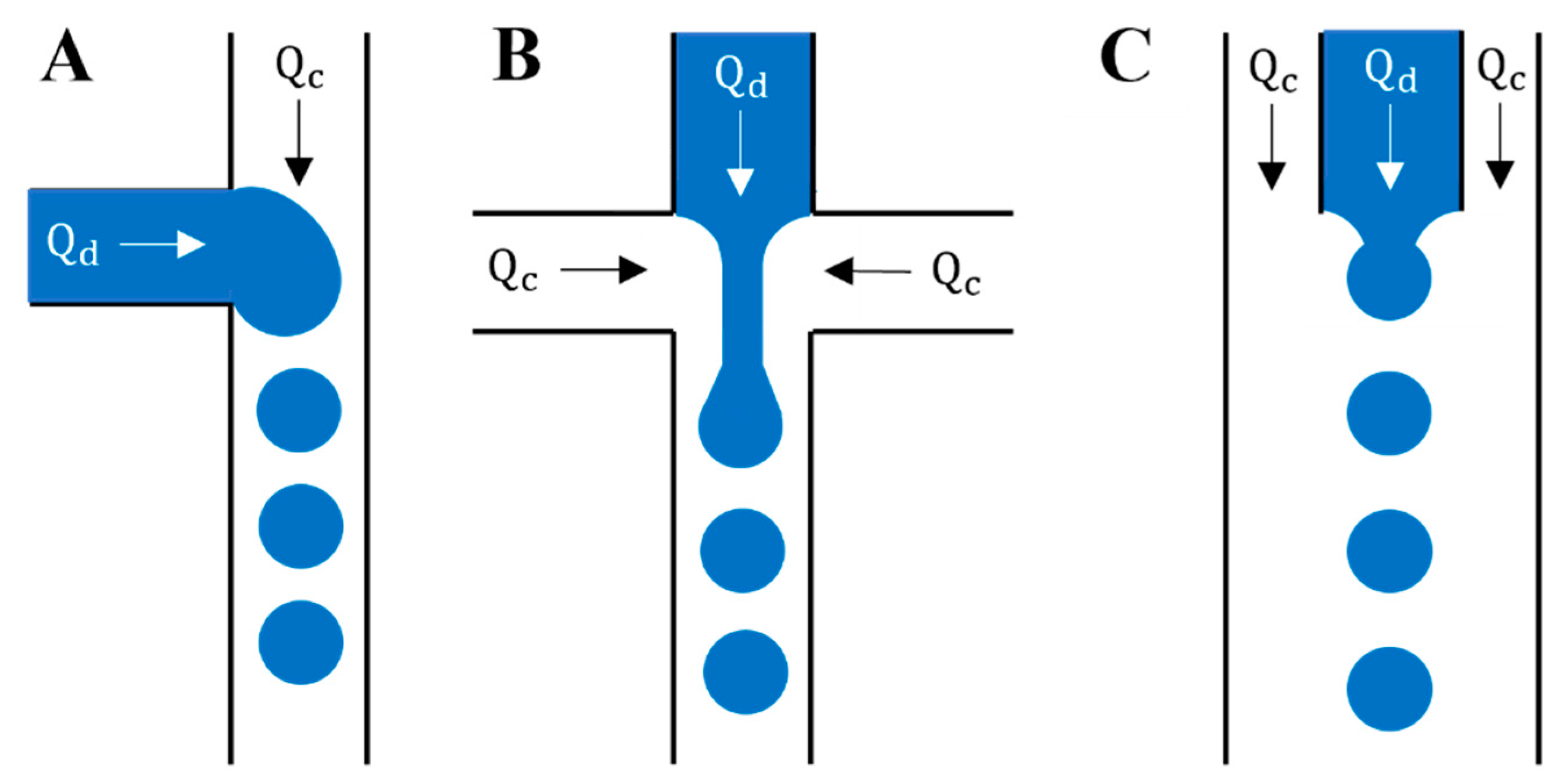
2. Short Treatise on Microfluidics Principles
3. Substrate Materials for Microfluidics
3.1. Inorganic Materials
3.1.1. Silicon
3.1.2. Glass
3.2. Polymeric Materials
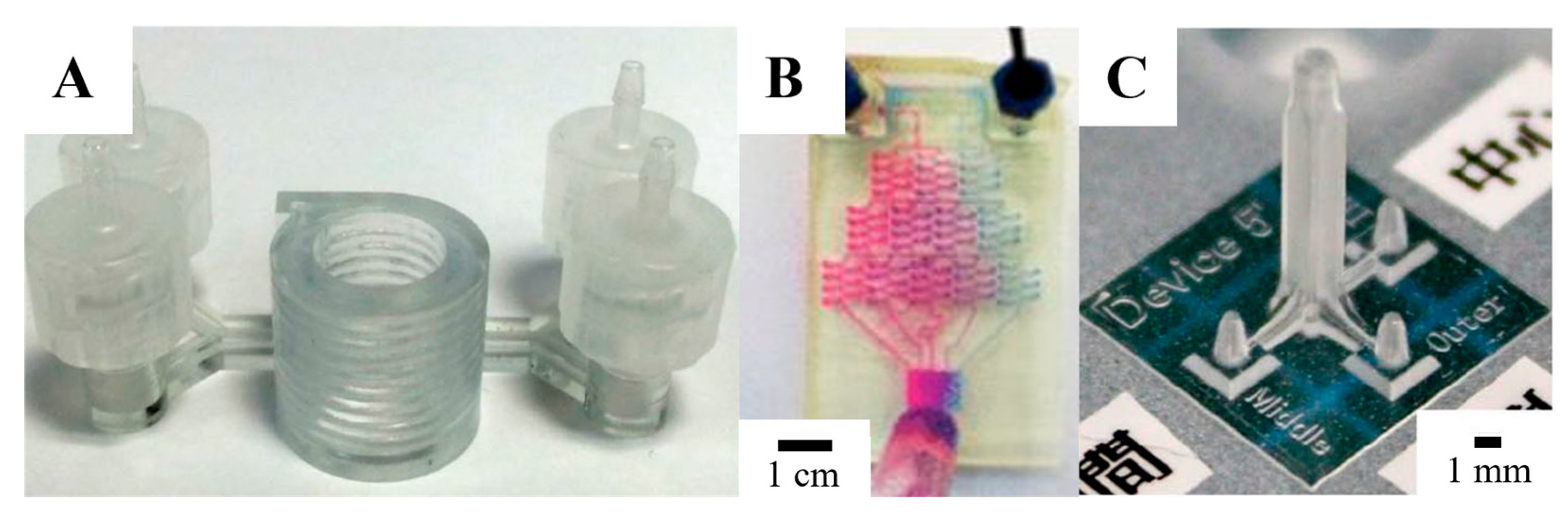
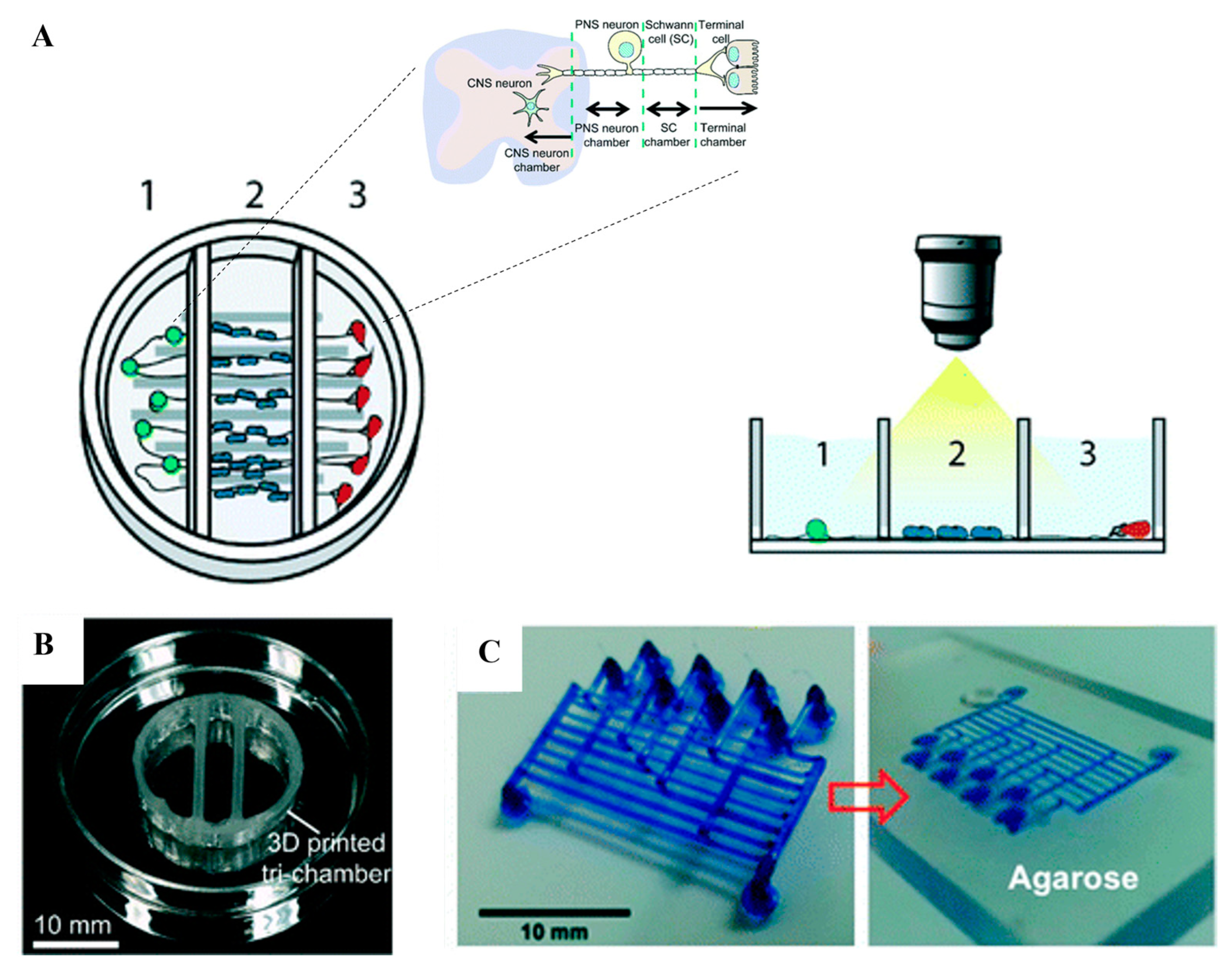
4. Fabrication Techniques for 3D-Printed Microfluidics
4.1. Stereolithography
4.2. Multi-Jet Modeling
4.3. Fused Deposition Modeling
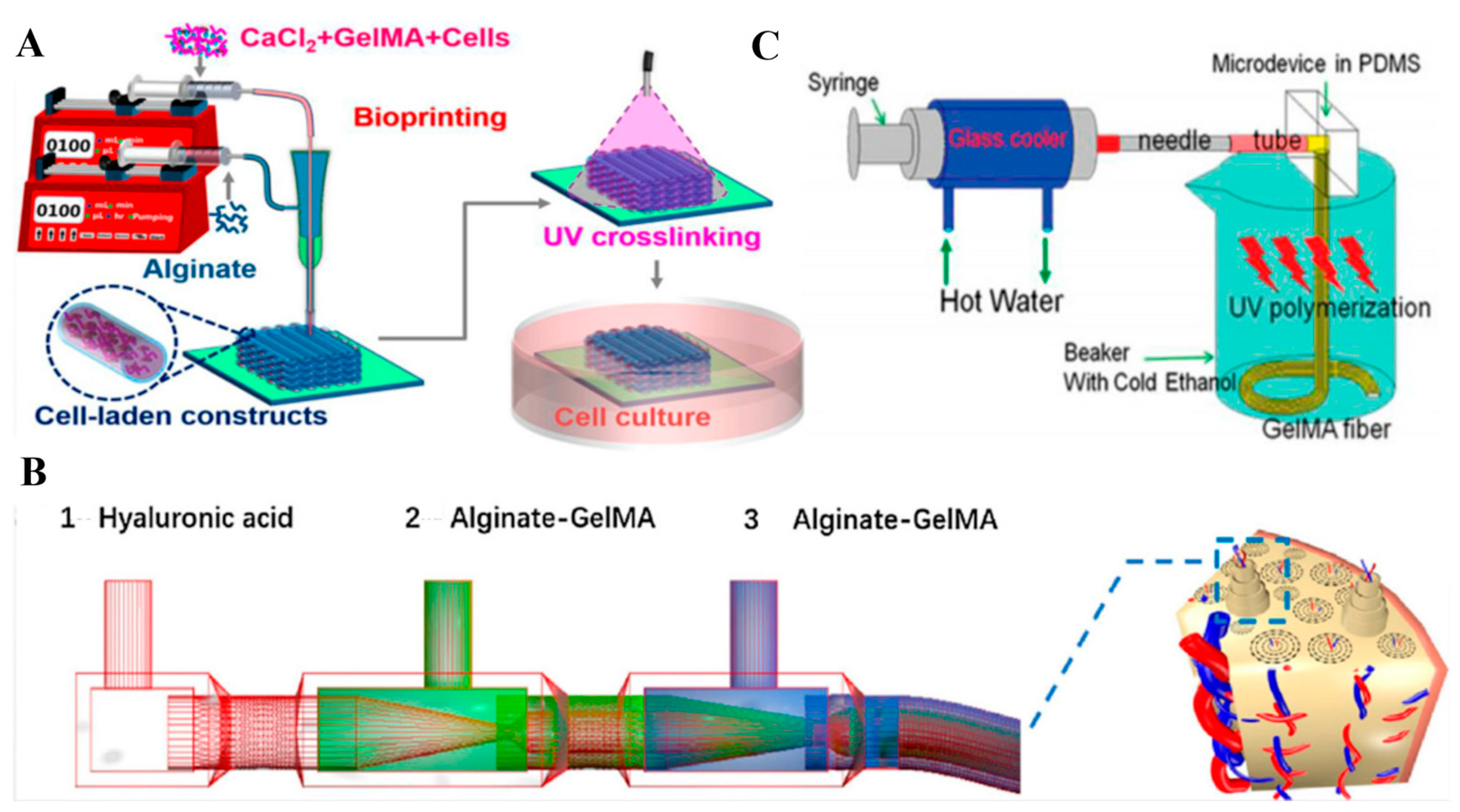
5. Biomaterials for Hydrogel Droplet Generation
5.1. Alginate
5.2. Chitosan
5.3. GelMA
5.4. Hyaluronic Acid
6. Generating Hydrogel Droplets in Microfluidics
6.1. Mechanics of Droplet Generation
6.2. Droplet Manipulation
6.3. Surfactants
6.4. Hydrogel Rheology in Droplet Microfluidics
7. Droplets Shearing and Potential Impact on Cell Physiology and Differentiation
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Organ Procurement and Transplantation Network (OPTN). Available online: https://optn.transplant.hrsa.gov/data/ (accessed on 21 February 2023).
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Taboada, G.M.; Yang, K.; Pereira, M.J.N.; Liu, S.S.; Hu, Y.; Karp, J.M.; Artzi, N.; Lee, Y. Overcoming the Translational Barriers of Tissue Adhesives. Nat. Rev. Mater. 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Berg, A. Microfabrication and Microfluidics for Tissue Engineering: State of the Art and Future Opportunities. Lab Chip 2004, 4, 98–103. [Google Scholar] [CrossRef]
- Marler, J.; Upton, J.; Langer, R.; Vacanti, J. Transplantation of cells in matrices for tissue generation. Adv. Drug Deliv. Rev. 1998, 33, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite Hydrogels for Tissue Engineering Applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Clarke, D.E.; Pashuck, E.T.; Bertazzo, S.; Weaver, J.V.M.; Weaver, M.; Stevens, M. Self-Healing, Self-Assembled β-Sheet Peptide-Poly(γ-glutamic acid) Hybrid Hydrogels. J. Am. Chem. Soc. 2017, 139, 7250–7255. [Google Scholar] [CrossRef]
- Wragg, J.W.; Durant, S.; McGettrick, H.M.; Sample, K.M.; Egginton, S.; Bicknell, R. Shear Stress Regulated Gene Expression and Angiogenesis in Vascular Endothelium. Microcirculation 2014, 21, 290–300. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Park, J.; Lee, K.-P.; Lee, S.; Lee, D.-M.; Kim, K.H.; Kim, H.K.; Cho, D.-W. Shear-Induced Alignment of Collagen Fibrils Using 3D Cell Printing for Corneal Stroma Tissue Engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogels From Soft Contact Lenses and Implants to Self-Assembled Nanomaterials. J. Polym. Sci. A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Guan, Q.-F.; Yang, H.-B.; Han, Z.-M.; Ling, Z.-C.; Yin, C.-H.; Yang, K.-P.; Zhao, Y.-X.; Zhao, S.-H. Sustainable Cellulose-Nanofiber-Based Hydrogels. ACS Nano 2021, 15, 7889–7898. [Google Scholar] [CrossRef]
- Davidson, M.D.; Ban, E.; Schoonen, A.C.M.; Lee, M.-H.; D’Este, M.; Shenoy, V.B.; Burdick, J.A. Mechanochemical Adhesion and Plasticity in Multifiber Hydrogel Networks. Adv. Mater. 2020, 32, 1905719. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, C.; Teo, W.L.; Qian, C.; Zhao, Y. Self-Sorting Double-Network Hydrogels with Tunable Supramolecular Handedness and Mechanical Properties. Angew. Chem. Int. Ed. 2019, 58, 9366–9372. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Ma, Y.; Bruekers, S.M.C.; Ma, S.; Huck, W.T.S. Designer Hydrogels for Cell Cultures: A Materials Selection Guide. Adv. Mater. 2014, 26, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, 500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel Machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Liu, G.; Sheng, J.; Wu, H.; Yang, C.; Yang, G.; Li, Y.; Ganguly, R.; Zhu, L.; Zhao, Y. Controlling Supramolecular Chirality of Two-Component Hydrogels by J- and H-Aggregation of Building Blocks. J. Am. Chem. Soc. 2018, 140, 6467–6473. [Google Scholar] [CrossRef]
- Wirthl, D.; Pichler, R.; Drack, M.; Kettlguber, G.; Moser, R.; Gerstmayr, R.; Hartmann, F.; Bradt, E.; Kaltseis, R.; Siket, C.M.; et al. Instant Tough Bonding of Hydrogels for Soft Machines and Electronics. Sci. Adv. 2017, 3, e1700053. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the Clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xue, Y.; Xu, M.; Zhao, W.; Zong, C.; Liu, X.; Zhang, Q. Viscosity based droplet size controlling in negative pressure driven droplets generator for large-scale particle synthesis. Electrophoresis 2017, 38, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Peretzki, A.J.; Schmidt, S.; Flachowsky, E.; Das, A.; Gerhardt, R.F.; Belder, D. How electrospray potentials can disrupt droplet microfluidics and how to prevent this. Lab Chip 2020, 20, 4456–4465. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, G.; Wang, H.; Ting, D.S.K.; Ahamed, M.J. Development of a rapid manufacturable microdroplet generator with pneumatic control. Microsyst. Technol. 2021, 27, 3095–3103. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Sato, A.C.K. Sonication technique to produce emulsions: The impact of ultrasonic power and gelatin concentration. Ultrason. Sonochemistry 2019, 52, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Hofman, A.H.; Hees, I.A.V.; Yang, J.; Kamperman, M. Bioinspired Underwater Adhesives by Using the Supramolecular Toolbox. Adv. Mater. 2018, 30, 1704640. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in Biomaterials for Tissue Engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.; Zhou, Y.; Zhou, F.; Liu, W.; Wang, Z. Mussel-Inspired Hydrogels: From Design Principles to Promising Applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough Bonding of Hydrogels to Diverse Non-Porous Surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R.; et al. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [PubMed]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel Bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Bian, S.; Zheng, Z.; Liu, Y.; Ruan, C.; Pan, H.; Zhao, X. A Shear-Thinning Adhesive Hydrogel Reinforced by Photo-Initiated Crosslinking as a Fit-to-Shape Tissue Sealant. J. Mater. Chem. B 2019, 7, 6488–6499. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Zhou, M.; Wang, Y.; Oberholzer, J.; Lo, J.F. Visualizing hypoxic modulation of beta cell secretions via a sensor augmented oxygen gradient. Microsyst. Nanoeng. 2023, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-W.; Su, W.-T.; Liu, C.-Y.; Huang, C.-C. Highly organized porous gelatin-based scaffold by microfluidic 3D-Foaming technology and dynamic culture for cartilage tissue engineering. Int. J. Mol. Sci. 2022, 23, 8449. [Google Scholar] [CrossRef]
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110578. [Google Scholar] [CrossRef]
- Katsakouli, C.; Jiang, X.; Lau, W.K.; Rohn, J.L.; Edirisinghe, M. Generating antibacterial microporous structures using microfluidic Processing. ACS Omega 2019, 4, 2225–2233. [Google Scholar] [CrossRef]
- Wang, C.-C.; Yang, K.-C.; Lin, K.-H.; Liu, H.-C.; Lin, F.-H. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials 2011, 32, 7118–7126. [Google Scholar] [CrossRef]
- Zhan, X. Effect of matrix stiffness and adhesion ligand density on chondrogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. 2020, 108, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Orabi, M.; Ghosh, G. Investigating the interplay between matrix compliance and passaging history on chondrogenic differentiation of mesenchymal stem cells encapsulated within alginate-gelatin hybrid hydrogels. Ann. Biomed. Eng. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Dong, C.; Yang, L.; Lv, Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 15790–15802. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Park, T.J.; Soo, S.Y.; Wang, K.W.; Kim, B.I.I.; Park, J.H.; Lee, C.-S.; Kim, D.H.; Lee, S.J. Synthesis and utilization of E coliencapsulated PEG-based microdroplet using a microfuidic chip for biological application. Biotechnol. Bioeng. 2010, 107, 747–751. [Google Scholar] [CrossRef]
- Al-Ameen, M.A.; Ghosh, G. Sensitive quantification of vascular endothelial growth factor (VEGF) using porosity induced hydrogel microspheres. Biosens. Bioelectron. 2013, 49, 105–110. [Google Scholar] [CrossRef]
- Moreira, A.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J. Production of hydrogel microparticles in microfluidic devices: A review. Microfluid. Nanofluidics 2021, 25, 10. [Google Scholar]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977. [Google Scholar]
- Shao, C.; Chi, J.; Shang, L.; Fan, Q.; Ye, F. Droplet microfluidics-based biomedical microcarriers. Acta Biomater. 2022, 138, 21–33. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Z.; Lin, L.; Zheng, X.; Hou, Y.; Lin, J.-M. Microfluidic droplet-based functional materials for cell manipulation. Lab Chip 2021, 21, 4311–4329. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, J.; Yuan, D.; Li, W. Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 2017, 38, 238–249. [Google Scholar] [CrossRef]
- Maleki, M.; Soltani, M.; Kashaninejad, N.; Nguyen, N.-T. Effects of magnetic nanoparticles on mixing in droplet-based microfluidics. Phys. Fluids 2019, 31, 032001. [Google Scholar] [CrossRef]
- Lao, Z.; Zheng, Y.; Dai, Y.; Hu, Y.; Ni, J.; Ji, S.; Cai, Z.; Smith, Z.; Li, J.; Zhang, L.; et al. Nanogap plasmonic structures fabricated by switchable capillary-force driven self-assembly for localized sensing of anticancer medicines with microfluidic SERS. Adv. Funct. Mater. 2020, 30, 1909467. [Google Scholar] [CrossRef]
- Han, W.; Chen, X. A review on microdroplet generation in microfluidics. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 2385. [Google Scholar] [CrossRef]
- Maeki, M. Chapter 4–Microfluidics for Pharmaceutical Applications; Micro and Nano Technologies: Gainesville, FL, USA, 2019; pp. 101–119. [Google Scholar]
- Li, M.; Boulafentis, T.; Stathoulopoulos, A.; Liu, Z.; Balabani, S. Flows inside polymer microfluidic droplets: Role of elasticity. Chem. Eng. Sci. 2023, 278, 118887. [Google Scholar] [CrossRef]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef]
- Du, G.; Fang, Q.; Toonder, J.M.J.D. Microfluidics for cell-based high throughput screening platforms–A review. Anal. Chim. Acta 2016, 903, 36–50. [Google Scholar] [CrossRef]
- Jackson, J.M.; Witek, M.A.; Kamande, J.W.; Soper, S.A. Materials and microfluidics: Enabling the efficient isolation and analysis of circulating tumor cells. Chem. Soc. Rev. 2017, 46, 4245–4280. [Google Scholar] [CrossRef]
- Trantidou, T.; Fridden, M.S.; Salehi-Reyhani, A.; Ces, O.; Elani, Y. Droplet microfluidics for the construction of compartmentalized model mambranes. Lab Chip 2018, 18, 2488–2509. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.-A.; Manz, A. Micro total analysis systems, 1, Introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials. functions. integration. and applications. Chem Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron. Dev. 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Grover, W.H.; Skelley, A.M.; Liu, C.N.; Lagally, E.T.; Mathies, R.A. Monolithic membrane valves and diaphragm pumps for practical large-scale integration into glass microfluidic devices. Sens. Actuators B 2003, 89, 315–323. [Google Scholar] [CrossRef]
- Grover, W.H.; Ivester, R.H.C.; Jensen, E.C.; Mathies, R.A. Development and multiplexed control of latching pneumatic valves using microfluidic logical structures. Lab Chip 2006, 6, 623–631. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Zhang, Z.; Weitz, D.A.; Zhang, H.; Zhang, Y.; Cui, W. Advanced microfluidic devices for fabricating multi-structural hydrogel microsphere. Exploration 2021, 1, 20210036. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, T.; Sugioka, K.-I.; Tsutsumino, T.; Fukuyama, H.; Kobatake, H. Effect of static magnetic field on a thermal conductivity measurement of a molten droplet using an electromagnetic levitation technique. Int. J. Heat Mass Transf. 2009, 52, 5152–5157. [Google Scholar] [CrossRef]
- Pham, N.M.; Rusch, S.; Temiz, Y.; Lovchik, R.D.; Beck, H.-P.; Karlen, W.; Delamarche, E. A bead-based immuogold-silver staining assay on capillary-driven microfluidics. Biomed. Microdevices 2018, 20, 41. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Gerhardt, R.F.; Peretzki, A.J.; Piendl, S.K.; Belder, D. Seamless Combination of High-pressure Chip-HPLC and Droplet Microfluidics on an Integrated Microfluidic Glass Chip. Anal. Chem. 2017, 89, 13030–13037. [Google Scholar] [CrossRef]
- Manz, A.; Harrison, D.J.; Verpoorte, E.M.J.; Fettinger, J.C.; Paulus, A.; Ludi, H.; Widmer, H.M. Planar Chips technology of miniaturization and integration of separation techniques into monitoring systems: Capillary electrophoresis on a chip. J. Chromatogr. A 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Mathies, R.A.; Lagally, E.T.; Kamei, T.; Grover, W.H.; Liu, C.N.; Sherer, J.R. Presented in part at solid-state sensors. In Actuators, and Microsystems Workshop Hilton Head Island, South Carolina; The Foundation: Cleveland, Ohio, 2002. [Google Scholar]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 2006, 442, 387–393. [Google Scholar] [CrossRef]
- Moreira, R.C.; Costa, B.M.C.; Marra, M.C.; Santana, M.H.P.; Maldaner, A.O.; Botelho, E.D.; Paixao, T.R.L.C.; Richter, E.M.; Coltro, W.K.T. Screening of seized cocaine samples using electrophoresis microchips with integrated contactless conductivity detection. Electrophoresis 2018, 39, 2188–2194. [Google Scholar] [CrossRef]
- Italia, V.; Giakoumaki, A.N.; Bonfadini, S.; Bharadwaj, V.; Phu, T.L.; Eaton, S.M.; Ramponi, R.; Bergamini, G.; Lanzani, G.; Criante, L. Laser-Inscribed Glass Microfluidic Device for Non-Mixing Flow of Miscible Solvents. Micromachines 2019, 10, 23. [Google Scholar] [CrossRef]
- Shan, C.; Chen, F.; Yang, Q.; Jiang, Z.; Hou, X. 3D Multi-Microchannel Helical Mixer Fabricated by Femtosecond Laser inside Fused Silica. Micromachines 2018, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Calmo, R.; Lovera, A.; Stassi, S.; Chiadò, A.; Scaiola, D.; Bosco, F.; Ricciardi, C. Monolithic glass suspended microchannel resonators for enhanced mass sensing of liquids. Sens. Actuators B Chem. 2019, 283, 298–303. [Google Scholar] [CrossRef]
- Kazoe, Y.; Pihosh, Y.; Takahashi, H.; Ohyama, T.; Sano, H.; Morikawa, K.; Mawatari, K.; Kitamori, T. Femtoliter nanofluidic valve utilizing glass deformation. Lab Chip 2019, 19, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Gal-Or, E.; Gershoni, Y.; Scotti, G.; Nilsson, S.M.; Saarinen, J.; Jokinen, V.; Kotiaho, T. Chemical analysis using 3D printed glass microfluidics. Anal. Methods 2019, 11, 1802–1810. [Google Scholar] [CrossRef]
- Kopparthy, V.L.; Crews, N.D. Microfab in a Microwave Oven: Simultaneous Patterning and Bonding of Glass Microfluidic Devices. J. Microelectromechanical Syst. 2018, 27, 434–439. [Google Scholar] [CrossRef]
- Hirama, H.; Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Kanamori, T.; Inoue, T. Glass-based organ-on-a-chip device for restricting small molecular absorption. J. Biosci. Bioeng. 2019, 127, 641–646. [Google Scholar] [CrossRef]
- Heidenhain. Available online: https://microtasconferences.org/microtas2020/images/current_support_files/imt/Application_Note_Droplet_generator.pdf (accessed on 21 February 2023).
- Microfluidic Chipshop. Available online: https://www.microfluidic-chipshop.com/microfluidics/materials-in-microfluidics/glass-versus-polymers/ (accessed on 21 February 2023).
- Lokensgard, E. Industrial Plastics: Theory and Applications, 6th ed.; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Wu, Z.; Chen, X.; Wu, Z.; Zhang, Q.; Gao, Q. Experimental study of fabricating a four-layers Cantor fractal microfluidic chip by CO2 laser system. Microsyst. Technol. 2019, 25, 1251–1256. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Pfeiffer, T.T.; Lillehoj, P.B. Stability of UV/ozone-treated thermoplastics under different storage conditions for microfluidic analytical devices. RSC Adv. 2017, 7, 37374–37379. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, S.; Zhang, Y. Direct bonding of polymer/glass-based microfluidic chips with dry film photoresist. Microsyst. Technol. 2018, 24, 1659–1665. [Google Scholar] [CrossRef]
- Su, S.; Jing, G.; Zhang, M.; Liu, B.; Zhu, X.; Wang, B.; Fu, M.; Zhu, L.; Cheng, J.; Guo, Y. One-step bonding and hydrophobic surface modification method for rapid fabrication of polycarbonate-based droplet microfluidic chips. Sens. Actuators B Chem. 2019, 282, 60–68. [Google Scholar] [CrossRef]
- Ganser, P.; Baum, C.; Chargin, D.; Sauer-Budge, A.F.; Sharon, A. A practical approach for the optimization of channel integrity in the sealing of shallow microfluidic devices made from cyclic olefin polymer. Biomed. Microdevices 2018, 20, 24. [Google Scholar] [CrossRef]
- Voicu, D.; Lestari, G.; Wang, Y.; DeBono, M.; Seo, M.; Cho, S.; Kumacheva, E. Thermoplastic microfluidic devices for targeted chemical and biological applications. RSC Adv. 2017, 7, 2884–2889. [Google Scholar] [CrossRef]
- Sun, H.; Chan, C.-W.; Wang, Y.; Yao, X.; Mu, X.; Lu, X.; Zhou, J.; Cai, Z.; Ren, K. Reliable and reusable whole polypropylene plastic microfluidic devices for a rapid. low-cost antimicrobial susceptibility test. Lab Chip 2019, 19, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Qiao, Y.; Duan, M.; Ju, A.; Lu, N.; Li, J.; Tu, J.; Lu, Z. Fabrication of a microfluidic chip based on the pure polypropylene material. RSC Adv. 2018, 8, 8732–8738. [Google Scholar] [CrossRef] [PubMed]
- Dolomite. Available online: https://www.dolomite-microfluidics.com/product/2-reagent-droplet-chip/ (accessed on 21 February 2023).
- Dolomite. Available online: https://www.dolomite-microfluidics.com/product/pdms-chip-slide/ (accessed on 21 February 2023).
- Elveflow. Available online: https://www.elveflow.com/microfluidic-reviews/general-microfluidics/the-polydimethylsiloxane-pdms-and-microfluidics/ (accessed on 21 February 2023).
- Piruska, A.; Nikcevic, I.; Lee, S.H.; Ahn, C.; Heineman, W.R.; Limbach, P.A.; Seliskar, C.J. The autofluorescence of plastic materials and chips measured under laser irradiation. Lab Chip 2005, 5, 1348–1354. [Google Scholar] [CrossRef]
- Lo, J.F.; Wang, Y.; Blake, A.; Yu, G.; Harvat, T.A.; Jeon, H.; Oberholzer, J.; Eddington, D.T. Islet Preconditioning via Multimodal Microfluidic Modulation of Intermittent Hypoxia. Anal. Chem. 2012, 84, 1987–1993. [Google Scholar] [CrossRef]
- Rao, H.-X.; Liu, F.-N.; Zhang, Z.-Y. Preparation and oxygen/nitrogen permeability of PDMS crosslinked membrane and PDMS/tetraethoxysilicone hybrid membrane. J. Membr. Sci. 2007, 303, 132–139. [Google Scholar] [CrossRef]
- Lo, J.F.; Wang, Y.; Li, Z.; Zhao, Z.; Hu, D.; Eddington, D.T.; Oberholzer, J. Quantitative and Temporal Control of Oxygen Microenvironment at the Single Islet Level. J. Vis. Exp. 2013, 81, e50616. [Google Scholar]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent progress in fabrication and application of polydimethylsiloxane sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H. Automatic method for fabricating a three dimensional plastic model with photo-hardening polymer. Rev. Sci. Instrum. 1981, 52, 1770–1773. [Google Scholar] [CrossRef]
- Zissi, S.; Bertsch, A.; Jezequel, J.-Y.; Corbel, S.; Lougnot, D.J.; Stereolithography, J.C.A.; Andre, J.C. Stereolithography and microtechniques. Microsyst. Technol. 1996, 2, 97–102. [Google Scholar] [CrossRef]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip-towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; DeSimone, J.M. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, I.; Cho, D. Development of an Assembly-free Process Based on Virtual Environment for Fabricating 3D Microfluidic Systems Using Microstereolithography Technology. J. Manuf. Sci. Eng. 2004, 126, 766–771. [Google Scholar] [CrossRef]
- Lee, W.; Kwon, D.; Chung, B.; Jung, G.Y.; Au, A.; Folch, A.; Jeon, S. Ultrapid detection of pathogenic bacteria using a 3D immunomagnetic flow assay. Anal. Chem. 2014, 86, 6683–6688. [Google Scholar] [CrossRef]
- Lee, W.; Kwon, D.; Choi, W.; Jung, G.Y.; Jeon, S. 3D-printed microfluidic device for the detection of pathogenic bacteria using size-based separation in helical channel with trapezoid cross-section. Sci. Rep. 2015, 5, 7717. [Google Scholar] [CrossRef] [PubMed]
- Shallan, A.I.; Smejkal, P.; Corban, M.; Guijt, R.M.; Breadmore, M.C. Cost-effective three-dimensional printing of visibly transparent microchips within minutes. Anal. Chem. 2014, 86, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Tsuchiya, M. Microfluidic devices fabricated using stereolithography for preparation of monodisperse double emulsions. Chem. Eng. J. 2016, 290, 400–404. [Google Scholar] [CrossRef]
- Patrick, W.G.; Nielsen, A.A.K.; Keating, S.J.; Levy, T.J.; Wang, C.-W.; Rivera, J.J.; Mondragón-Palomino, O.; Carr, P.A.; Voigt, C.A.; Oxman, N.; et al. DNA Assembly in 3D Printed Fluidics. PLoS ONE 2015, 10, e0143636. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Lee, W.; Folch, A. Mail-order microfluidics: Evaluation of stereolithography for the production of microfluidic devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Martin, N.; Hini, D.; Mills, B.; Kim, K. Rapid Fabrication of Multilayer Microfluidic Devices Using the Liquid Crystal Display-Based Stereolithography 3D Printing System. 3d Print. Addit. Manuf. 2017, 4, 28. [Google Scholar] [CrossRef]
- Kamperman, T.; Teixeira, L.M.; Salehi, S.S.; Kerckhofs, G.; Guyot, Y.; Geven, M.; Geris, L.; Grijpma, D.; Blanquer, S.; Leijten, J. Engineering 3D parallelized microfluidic droplet generators with equal flow profiles by computational fluid dynamics and stereolithography printing. Lab Chip 2020, 20, 490–495. [Google Scholar] [CrossRef]
- Pilipović, A.; Raos, P.; Šercer, M. Experimental analysis of properties of materials for rapid prototyping. Int. J. Adv. Des. Manuf. Technol. 2009, 40, 105–115. [Google Scholar] [CrossRef]
- Ahn, D.-G.; Lee, J.-Y.; Yang, D.-Y. Rapid Prototyping and Reverse Engineering Application for Orthopedic Surgery Planning. J. Mech. Sci. Technol. 2006, 20, 19–28. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chen, S.J. Manufacturing of Cardiac Models Through Rapid Prototyping Technology for Surgery Planning. Mater. Sci. Forum 2006, 505–507, 1063–1068. [Google Scholar] [CrossRef]
- Erbano, B.O.; Opolski, A.C.; Olandoski, M.; Foggiatto, J.A.; Kubrusly, L.F.; Dietz, U.A.; Ramina, R. Rapid prototyping of three-dimensional biomodels as an adjuvant in the surgical planning for intracranial aneurysms. Acta Cir. Bras. 2013, 28, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.B.; Lockwood, S.Y.; Martin, R.S.; Spence, D.M. A 3D Printed Fluidic Device that Enables Integrated Features. Anal. Chem. 2013, 85, 5622–5626. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, S.Y.; Meisel, J.E.; Monsma, F.J.; Spence, D.M. A Diffuion-Based and Dynamic 3D-Printed Device That Enables Parallel in Vitro Pharmacokinetic Profiling of Molecules. Anal. Chem. 2016, 88, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Bonyár, A.; Sántha, H.; Ring, B.; Varga, M.; Kovács, J.G.; Harsányi, G. 3D Rapid Prototyping Technology (RPT) as a powerful tool in microfluidic development. Procedia Eng. 2010, 5, 291–294. [Google Scholar] [CrossRef]
- Munshi, A.S.; Martin, R.S. Microchip-based electrochemical detection using a 3-D printed wall-jet electrode device. Analyst 2016, 141, 862–869. [Google Scholar] [CrossRef]
- Chen, X.; Mo, D.; Gong, M. 3D Printed Reconfigurable Modular Microfluidic System for Generating Gel Microspheres. Micromachines 2020, 11, 224. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D Printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Shaffer, S.; Yang, K.; Vargas, J.; Di Prima, M.A.; Voit, W. On reducing anisotropy in 3D printed polymers via ionizing radiation. Polymer 2014, 55, 5969–5979. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.-S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D Printing of Interdigitated Li-Ion Microbattery Architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef]
- Lopes, A.J.; MacDonald, E.; Wicker, R.B. Integrating Stereolithography and Direct Print Technologies for 3D Structural Electronics Fabrication. Rapid Prototyp. J. 2012, 18, 129–143. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D printed bionic ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Quero, R.F.; Da Silveira, G.D.; Da Silva, J.A.F.; De Jesus, D.P. Understanding and improving FDM 3D printing to fabricate high-resolution and optically transparent microfluidic devices. Lab Chip 2021, 21, 3715–3729. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Lancaster, K.Z.; Hogue, I.B.; Meng, F.; Kong, Y.L.; Enquist, L.W.; McAlpine, M.C. 3D printed nervous system on a chip. Lab Chip 2016, 16, 1393–1400. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid Casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Gelber, M.K.; Bhargava, R. Monolithic multilayer microfluidics via sacrificial molding of 3D-printed isomalt. Lab Chip 2015, 15, 1736–1741. [Google Scholar] [CrossRef]
- Zhang, J.M.; Ji, Q.; Duan, H. Three-Dimensional Printed Devices in Droplet Microfluidics. Micromachines 2019, 10, 754. [Google Scholar] [CrossRef]
- Yazdi, A.A.; Popma, A.; Wong, W.S.Y.; Nguyen, T.; Pan, Y.; Xu, J. 3D printing: An emerging tool for novel microfluidics and lab-on-a-chip applications. Microfluid. Nanofluidics 2016, 20, 50. [Google Scholar] [CrossRef]
- Morgan, A.J.L.; Jose, L.H.S.; Jamieson, W.D.; Wymant, J.M.; Song, B.; Stephens, P.; Barrow, D.A.; Castell, O.K. Simple and versatile 3D printed microfluidics using fused filament fabrication. PLoS ONE 2016, 11, e0152023. [Google Scholar] [CrossRef]
- Romanov, V.; Samuel, R.; Chaharlang, M.; Jafek, A.R.; Frost, A.; Gale, B.K. FDM 3D Printing of High-Pressure. Heat-Resistant. Transparent Microfluidic Devices. Anal. Chem 2018, 90, 10450–10456. [Google Scholar] [CrossRef]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible. transparent. sub-100 mm microfluidic channels with FDM 3D-printed thermoplastic polyurethane. J. Micromech. Microeng. 2019, 29, 095010. [Google Scholar] [CrossRef]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D printer and resin for 18 µm × 20 µm microfluidic flow channels. Lab Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993. [Google Scholar] [CrossRef]
- Duan, K.; Orabi, M.; Warchock, A.; Al-Akraa, Z.; Ajami, Z.; Chun, T.-H.; Lo, J.F. Monolithically 3D-Printed Microfluidics with Embedded µTesla Pump. Micromachines 2023, 14, 237. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Machado, D.I.; Lopez-Cervantes, J.; Correa-Murrieta, M.A.; Sanchez-Duarte, R.G.; Cruz-Flores, P.; De La Mora-Lopez, G.S. Nonvitamin and Nonmineral Nutritional Supplements; Academic Express: Cambridge, MA, USA, 2019; pp. 485–493. [Google Scholar]
- Antunes, J.; Gaspar, V.M.; Ferreira, L.; Monteiro, M.; Henrique, R.; Jerónimo, C.; Mano, J.F. In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening. Acta Biomater. 2019, 94, 392–409. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. -Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Pullagura, B.K.; Amarapalli, S.; Gundabala, V. Coupling electrohydrodynamics with photopolymerization for microfluidics-based generation of polyethylene glycol diacrylate (PEGDA) microparticles and hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125586. [Google Scholar] [CrossRef]
- Chen, Z.; Kheiri, S.; Young, E.W.K.; Kumacheva, E. Trends in Droplet Microfluidics: From Droplet Generation to Biomedical Applications. Langmuir 2022, 38, 6233–6248. [Google Scholar] [CrossRef]
- Wee, S.; Gombotz, W.R. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar]
- Cleetus, C.M.; Primo, F.A.; Fregoso, G.; Raveendran, N.L.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate hydrogels with embedded ZnO nanoparticles for wound healing therapy. Int. J. Nanomed. 2020, 15, 5097–5111. [Google Scholar] [CrossRef]
- Lai, W.-F. Development of hydrogels with self-healing properties for delivery of bioactive agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- LeRoux, M.A.; Guilak, F.; Setton, L.A. Compressive and shear properties of alginate gel: Effects of sodium ions and alginate concentration. J. Biomed. Mater. Res. 1999, 47, 46–53. [Google Scholar] [CrossRef]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, L.M.C.; Duchi, S.; Onofrillo, C.; O’Connell, C.D.; Bella, C.D.; Moulton, S.E. Formation of alginate microspheres prepared by optimized microfluidics parameters for high encapsulation of bioactive molecules. J. Colloid Interface Sci. 2021, 587, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Lacko, C.S.; Zhao, Z.; Schmidt, C.E.; Rinaldi, C. Preparation and evaluation of microfluidic magnetic alginate microparticles for magnetically templated hydrogels. J. Colloid Interface Sci. 2020, 561, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Cain, B.; Soman, P. Gelatin methacrylate-alginate hydrogel with tunable viscoelastic properties. AIMS Mater. Sci. 2017, 4, 363–369. [Google Scholar] [CrossRef]
- Felt, O.; Furrer, P.; Mayer, J.M.; Plazonnet, B.; Buri, P.; Gurny, R. Topical use of chitosan in ophthalmology: Tolerance assessment and evaluation of precorneal retention. Int. J. Pharm. 1999, 180, 185–193. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Kumar, M.N.V.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Suh, J.K.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Elmizadeh, H.; Khanmohammadi, M.; Ghasemi, K.; Hassanzadeh, G.; Nassiri-Asl, M.; Garmarudi, A.B. Preparation and optimization of chitosan nanoparticles and magnetic chitosan nanoparticles as delivery systems using Box–Behnken statistical design. J. Pharm. Biomed. Anal. 2013, 80, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, Q.; Cao, J.; Wang, Y.; Yang, X.; Xu, Q.; Liang, Q.; Sun, Y. Recent advances in microfluidic aided chitosan based multifunctional materials for biomedical applications. Int. J. Pharm. 2021, 600, 120465. [Google Scholar] [CrossRef]
- Wang, B.; Bai, Z.; Jiang, H.; Prinsen, P.; Luque, R.; Zhao, S.; Xuan, J. Selective heavy metal removal and water purification by microfluidically generated chitosan microspheres: Characteristics. modeling. and application. J. Hazard Mater. 2019, 364, 192–205. [Google Scholar] [CrossRef]
- Shi, L.; Yang, L.; Chen, J.; Pei, Y.; Chen, M.; Hui, B.; Li, J. Preparation and characterization of pH-sensitive hydrogel of chitosan/poly(acrylic acid) co-polymer. J. Biomater. Sci. Polym. Ed. 2004, 15, 465–474. [Google Scholar] [CrossRef]
- Vian, A.; Favrod, V.; Amstad, E. Reducing the shell thickness of double emulsions using microfluidics. Microfluid. Nanofluidics 2016, 20, 159. [Google Scholar] [CrossRef]
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Takeuchi, I.; Takeshita, T.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for positively charged drugs. Colloid Surf B Biointerfaces 2017, 160, 520–526. [Google Scholar] [CrossRef]
- Oh, J.; Kim, K.; Won, S.W.; Cha, C.; Gaharwar, A.K.; Selimovic, S.; Bae, H.; Lee, K.H.; Lee, D.H.; Lee, S.-H.; et al. Microfluidic fabrication of cell adhesive chitosan microtubes. Biomed. Microdev. 2013, 15, 465–472. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yu, Y.; Chen, W.; Jiang, L.; Qin, J. Simple fabrication of inner chitosan-coated alginate hollow microfiber with higher stability. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2527–2536. [Google Scholar] [CrossRef]
- Roman-Hidalgo, C.; Lopez-Perez, G.; Martin-Valero, M.J.; Bello-Lopez, M.A. Chitosan tailor-made membranes as biopolymeric support for electromembrane extraction. Talanta 2019, 199, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-B.; Hao, Y.-F.; He, B.-Q.; Song, Y.-F.; Ji, Y.-H.; Zhang, Y.-H.; Bo, L.; Li, J.-X. A chitosan-separation-layer nanofiltration membrane prepared through homogeneous hybrid and copper ion enhancement. Sep. Purif. Technol. 2020, 234, 116084. [Google Scholar] [CrossRef]
- Huang, Q.; Li, G.; Chen, M.; Dong, S. Graphene oxide functionalized O-(carboxymethyl)-chitosan membranes: Fabrication using dialysis and applications in water purification. Colloids Surf. A Physiochemical Eng. Asp. 2018, 554, 27–33. [Google Scholar] [CrossRef]
- Islam, M.R.; Tumbarello, M.; Lyon, L.A. Deswelling induced morphological changes in dual pH- and temperature-responsive ultra-low cross-linked poly(N-isopropyl acrylamide)-co-acrylic acid microgels. Colloid Polym. Sci. 2019, 297, 667–676. [Google Scholar] [CrossRef]
- Chalard, A.E.; Dixon, A.W.; Taberner, A.J.; Malmstrom, J. Visible light stiffness patterning of GelMA hydrogels towards in vitro scar tissue models. Front. Cell Dev. Biol. 2022, 10, 976754. [Google Scholar] [CrossRef]
- Yue, K.; Santiago, G.T.-D.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis. properties. and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable. porous. and cell responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef]
- Vlierberghe, S.V.; Dubruel, P.; Schacht, E. Effect of cryogenic treatment on the rheological properties of gelatin hydrogels. J. Bioact. Compat. Polym. 2010, 25, 498–512. [Google Scholar] [CrossRef]
- Vlierberghe, S.V.; Cnudde, V.; Dubruel, P.; Masschaele, B.; Cosijns, A.; De Paepe, I.; Jacobs, P.J.S.; Hoorebeke, L.V.; Remon, J.P.; Schacht, E. Porous gelatin hydrogels: 1. Cryogenic formation and structure analysis. Biomacromolecules 2007, 8, 331–337. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, R.-Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Liu, T.; Weng, W.; Zhang, Y.; Sun, X.; Yang, H. Applications of gelatin methacryloyl (GelMA) hydrogels in microfluidic technique assisted tissue engineering. Molecules 2020, 25, 5305. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.C.; Sharifi, F.; Wrede, A.H.; Kimlinger, D.F.; Thomas, D.-G.; Wiel, J.B.V.; Chen, Y.; Montazami, R.; Hashemi, N.N. Microfibers as Physiologically Relevant Platforms for Creation of 3D Cell Cultures. Macromol. Biosci. 2017, 17, 279. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Takeuchi, S. Cell-laden microfibers for bottom-up tissue engineering. Drug Discov. Today 2015, 20, 236–246. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, Z.; Hu, N.; Zhou, Y.; Maggio, L.; Miri, A.K.; Fragasso, A.; Jin, X.; Khademhosseini, A.; Zhang, Y.S. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 2018, 10, 024102. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zuo, Y.; Sun, J.; Guo, Z.; Fan, H.; Zhang, X. Degradation regulated bioactive hydrogel as the bioink with desirable moldability for microfluidic biofabrication. Carbohydr. Polym. 2017, 178, 8–17. [Google Scholar] [CrossRef]
- Shi, X.; Ostrovidov, S.; Zhao, Y.; Liang, X.; Kasuya, M.; Kurihara, K.; Nakajima, K.; Bae, H.; Khademhosseini, A. Microfluidic Spinning of Cell-Responsive Grooved Microfibers. Adv. Funct. Mater. 2015, 25, 2250–2259. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef]
- Cha, C.; Oh, J.; Kim, K.; Qiu, Y.; Joh, M.; Shin, S.R.; Wang, X.; Camci-Unal, G.; Wan, K.-T.; Liao, R.; et al. Microfluidics-Assisted Fabrication of Gelatin-Silica Core–Shell Microgels for Injectable Tissue Constructs. Biomacromolecules 2014, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, D.; Wang, L.; Hou, J.; Zhang, H.; Li, Y.; Zhong, S.; Wang, Y.; Wu, Y.; Huang, W. 3D bioprinted multiscale composite sca_olds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J. Colloid Interface Sci. 2020, 574, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Gao, Q.; Wang, Y.; Zeng, J.; Zhao, H.; Sun, Y.; Shen, J.; Ramezani, H.; Fu, Z.; Liu, Z.; et al. Vessel-on-a-chip with Hydrogel-based Microfluidics. Small 2018, 14, e1802368. [Google Scholar] [CrossRef]
- Xie, M.; Gao, Q.; Zhao, H.; Nie, J.; Hu, Z.; Wang, H.; Chen, L.; Shao, L.; Fu, J.; Chen, Z.; et al. Electro-assisted bioprinting of low-concentration GelMA Microdroplets. Small 2019, 15, 1804216. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Kheiri, S.; Islam, S.; Kumar, H.; Yang, A.; Kim, K. An integrated microfluidic flow focusing platfdorm for on chip fabrication and filtration of cell laden microgels. Lab Chip 2019, 19, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, B.; Meyer, K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. J. Am. Chem. Soc. 1954, 76, 1753–1757. [Google Scholar] [CrossRef]
- Weissmann, B.; Meyer, K.; Sampson, P.; Linker, A. Isolation of oligosaccharides enzymatically produced from hyaluronic acid. J. Biol. Chem. 1954, 208, 417–429. [Google Scholar] [CrossRef]
- Wiley, J. The Biology of Hyaluronan; A Wiley-Interscience Publication: New York, NY, USA, 1989. [Google Scholar]
- Bates, E.J.; Harper, G.S.; Lowther, D.A.; Preston, B.N. Effect of oxygen-derived reactive species on cartilage proteoglycan- hyaluronate aggregates. Biochem. Int. 1984, 8, 629–637. [Google Scholar]
- Turino, G.M.; Cantor, J.O. Hyaluronan in respiratory injury and repair. Am. J. Respir. Crit. Care Med. 2003, 167, 1169–1175. [Google Scholar] [CrossRef]
- Armstrong, S.E.; Bell, D.R. Relationship between lymph and tissue hyaluronan in skin and skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2485–H2494. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar]
- Papakonstantinou, E.; Karakiulakis, G.; Roth, M.; Block, L.H. Platelet-derived growth factor stimulates the secretion of hyaluronic acid by proliferating human vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA 1995, 92, 9881–9885. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Karakiulakis, G. The ‘sweet’ and ‘bitter’ involvement of glycosaminoglycans in lung diseases: Pharmacotherapeutic relevance. Br. J. Pharmacol. 2009, 157, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, L. Hyaluronan in skin. Minisymp. Hyaluronic 1997, 242, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Teriete, P.; Banerji, S.; Noble, M.; Blundell, C.D.; Wright, A.J.; Pickford, A.R.; Lowe, E.; Mahoney, D.J.; Tammi, M.I.; Kahmann, J.D.; et al. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Mol. Cell 2004, 13, 483–496. [Google Scholar] [CrossRef]
- Knudson, W. Tumor-associated hyaluronan. Providing an extracellular matrix that facilitates invasion. Am. J. Pathol. 1996, 148, 1721–1726. [Google Scholar]
- Lobasova, A.S.; Minakov, A.V.; Rudyak, V.Y. Viscosity Effect on the Flow Patterns in T-Type Micromixers. Fluid Dyn. 2016, 51, 381–388. [Google Scholar] [CrossRef]
- Chiesa, E.; Dorati, R.; Pisani, S.; Conti, B.; Bergamini, G.; Modena, T.; Genta, I. The microfluidic technique and the manufacturing of polysaccharide nanoparticles. Pharmaceutics 2018, 10, 267. [Google Scholar] [CrossRef]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A.; et al. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Greco, A.; Riva, F.; Dorati, R.; Conti, B.; Modena, T.; Genta, I. Hyaluronic acid-based nanoparticles for protein delivery: Systematic examination of microfluidic production conditions. Pharmaceutics 2021, 13, 1565. [Google Scholar] [CrossRef]
- Russo, M.; Bevilacqua, P.; Netti, P.A.; Torino, E. A microfluidic platform to design crosslinked hyaluronic acid nanoparticles (cHANPs) for enhanced MRI. Sci. Rep. 2016, 6, 37906. [Google Scholar] [CrossRef]
- Samandari, M.; Alipanah, F.; Javanmard, S.H.; Sanati-Nezhad, A. One step wettability patterning of PDMS microchannels for generation of monodisperse alginate microbeads by in situ external gelation in double emulsion microdroplets. Sens. Actuators B Chem. 2019, 291, 418–425. [Google Scholar] [CrossRef]
- Koltsov, S.I.; Statsenko, T.G.; Morozova, S.M. Modification of commercial 3D fused deposition modeling printer for extrusion printing of hydrogels. Polymers 2022, 14, 5539. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Parker, B.; Samanipour, R.; Ahmadi, A.; Kim, K. Rapid fabrication of circular channel microfluidic flow focusing devices for hydrogel generation. Micro Nano Lett. 2016, 11, 41–45. [Google Scholar] [CrossRef]
- Zamora-Mora, V.; Velasco, D.; Hernandez, R.; Mijangos, C.; Kumacheva, E. Chitosan/agarose hydrogels: Cooperative properties and microfluidic preparation. Carbohydr. Polym. 2014, 111, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Chuah, A.M.; Kuroiwa, T.; Kobayashi, I.; Zhang, X.; Nakajima, M. Preparation of uniformly sized alginate microspheres using the novel combined methods of microchannel emulsification and external gelation. Colloids Surf. A Physicochem. Eng. Asp. 2009, 351, 9–17. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Mano, J.F. Polymer-based microparticles in tissue engineering and regenerative medicine. Biotechnol. Prog. 2011, 27, 897–912. [Google Scholar] [CrossRef]
- The, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar]
- Leng, X.; Zhang, W.; Wang, C.; Cui, L.; Yang, C.J. Agarose droplet microfluidics for highly parallel and efficient single molecule emulsion PCR. Lab Chip 2010, 10, 2841–2843. [Google Scholar] [CrossRef]
- Baret, J.-C. Surfactants in droplet-based microfluidics. Lab Chip 2012, 12, 422–433. [Google Scholar] [CrossRef]
- Sharma, S.; Srisa-Art, M.; Scott, S.; Asthana, A.; Cass, A. Droplet-Based Microfluidics. Methods Mol. Biol. 2013, 949, 207–230. [Google Scholar] [PubMed]
- Yang, C.-G.; Xu, Z.-R.; Wang, J.-H. Manipulation of droplets in microfluidic systems. Trends Anal. Chem. 2010, 29, 141–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Nguyen, N.-T. Magnetic digital microfluidics: A review. Lab Chip 2017, 17, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Destgeer, G.; Sung, H.J. Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves. Lab Chip 2015, 15, 2722–2738. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.C.; Kim, C.-J. Droplet actuation by electrowettingon- dielectric (EWOD): A review. J. Adhes. Sci. Technol. 2012, 26, 1747–1771. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Samanipour, R.; Wang, Z.; Ahmadi, A.; Kim, K. Experimental and computational study of microfluidic flow-focusing generation of gelatin methacrylate hydrogel droplets. J. Appl. Polym. Sci. 2016, 133, 43701. [Google Scholar] [CrossRef]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef]
- Zhao, Z.; Al-Ameen, M.A.; Duan, K.; Ghosh, G.; Lo, J.F. On-chip porous microgel generation for microfluidic enhanced VEGF detection. Biosens. Bioelectron. 2015, 74, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Ghosh, G.; Lo, J.F. Optimizing Multiplexed Detections of Diabetes Antibodies via Quantitative Microfluidic Droplet Array. Small 2017, 13, 1702323. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Doutel, E.; Campos, B.L.; Miranda, J.M. PDMS droplet formation and characterization by hydrodynamic flow focusing technique in a PDMS square microchannel. J. Micromechics Microengineering 2016, 26, 105013. [Google Scholar] [CrossRef]
- Gwon, K.; Hong, H.J.; Gonzalez-Suarez, A.M.; Stybayeva, G.; Revzin, A. Microfluidic fabrication of core-shell microcapsules carrying human pluripotent stem cell spheroids. Bioengineering 2021, 176, e62944. [Google Scholar] [CrossRef]
- Song, Y.; Michaels, T.C.T.; Ma, Q.; Liu, Z.; Yuan, H.; Takayama, S.; Knowles, T.P.J.; Shum, H.C. Budding like division of all aqueous emulsion droplets modulated by networks of protein nanofibrils. Nat. Commun. 2018, 9, 2110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mithieux, S.M.; Weiss, A.S. Fabrication techniques for vascular and vascularized tissue engineering. Adv. Healthc. Mater. 2019, 8, 1900742. [Google Scholar] [CrossRef]
- Xu, J.H.; Dong, P.F.; Zhao, H.; Tostado, C.P.; Luo, G.S. The dynamic effects of surfactants on droplet formation in coaxial microfluidic devices. Langmuir 2012, 28, 9250–9258. [Google Scholar] [CrossRef]
- McClements, D.J.; Henson, L.; Popplewell, L.M.; Decker, E.A.; Choi, S.J. Inhibition of Ostwald ripening in model beverage emulsions by addition of poorly water soluble triglyceride oils. J. Food Sci. 2012, 77, C33–C38. [Google Scholar] [CrossRef]
- Pensado, A.; Fernandez-Pineiro, I.; Seijo, B.; Sanchez, A. Anionic nanoparticles based on Span 80 as low-cost. simple and efficient non-viral gene-transfection systems. Int. J. Pharm. 2014, 476, 23–30. [Google Scholar] [CrossRef]
- Cubaud, T.; Mason, T.G. Capillary threads and viscous droplets in square microchannels. Phys. Fluids 2008, 20, 053302. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Lee, J.W.; Durand, G.; Majumder, S.; Liu, A.P. Protein aggregation with poly(vinyl) alcohol surfactant reduces double emulsion encapsulated mammalian cell free expression. PLoS ONE 2017, 12, e0174689. [Google Scholar] [CrossRef]
- Worthington, K.S.; Green, B.J.; Rethwisch, M.; Wiley, L.A.; Tucker, B.A.; Guymon, C.A.; Salem, A.K. Neuronal differentiation of induced pluripotent stem cells on surfactant templated chitosan hydrogels. Biomacromolecules 2016, 17, 1684–1695. [Google Scholar] [CrossRef] [PubMed]
- Besanjideh, M.; Shamloo, A.; Hannani, S. Enhanced oil-in-water droplet generation in a T-junction microchannel using water-based nanofluids with shear thinning behavior: A numerical study. Phys. Fluids 2021, 33, 012007. [Google Scholar] [CrossRef]
- Chiarello, E.; Gupta, A.; Mistura, G.; Sbragaglia, M.; Pierno, M. Droplet breakup driven by shear thinning solutions in a microfluidic T-junction. Phys. Rev. Fluids 2017, 2, 12. [Google Scholar] [CrossRef]
- Shahrivar, K.; Giudice, F. Controlled viscoelastic particle encapsulation in microfluidic devices. Soft Matter. 2021, 17, 8068–8077. [Google Scholar] [CrossRef]
- Yang, F.; Carmona, A.; Stojkova, K.; Huitron, E.I.G.; Goddi, A.; Bhushan, A.; Cohen, R.N.; Brey, E.M. A 3D human adipose tissue model within a microfluidic device. Lab Chip 2021, 21, 435–446. [Google Scholar] [CrossRef]
- Siddique, A.; Meckel, T.; Stark, R.W.; Narayan, S. Improved cell adhesion under shear stress in PDMS microfluidic devices. Colloids Surf. B Biointerfaces 2017, 150, 456–464. [Google Scholar] [CrossRef]
- Yu, W.; Qu, H.; Hu, G.; Zhang, Q.; Song, K.; Guan, H.; Liu, T.; Qin, J. A Microfluidic-Based Multi-Shear Device for Investigating the Effects of Low Fluid-Induced Stresses on Osteoblasts. PLoS ONE 2014, 9, e89966. [Google Scholar] [CrossRef]
- Zhao, Y.; Richardson, K.; Yang, R.; Bousraou, Z.; Lee, Y.K.; Amoako, K.; Wang, S. Low Fluid Shear Stress Regulates Osteogenic Differentiation of Human Mesenchymal Stem Cells through Notch1-DII4 Signaling. In Proceedings of the 2021 IEEE 15th International Conference on Nano/Molecular Medicine & Engineering (NANOMED), Taipei, Taiwan, 15–18 November 2021. [Google Scholar]
- Wollborn, T.; Luhede, L.; Fritsching, U. Evaluating interfacial shear and strain strenn during droplet deformation in micro-pores. Phys. Fluids 2019, 31, 012109. [Google Scholar] [CrossRef]





| Advantages | Disadvantages | References | |
|---|---|---|---|
| Silicon |
|
| [63,65,66,67,68,69] |
|
| ||
|
| ||
|
| ||
| |||
| Glass |
|
| [48,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
| |||
| |||
| |||
| |||
| Polymeric Materials |
|
| [60,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| Printing Technology | Manufacturer | Material | Resolution (x, y) µm | Ref |
|---|---|---|---|---|
| Stereolithography (SLA) | Custom | Custom resin | 18 × 20 | [140] |
| Model | Acrylate-based resin | 56 × 56 | [141] | |
| Multi-Jet Modeling (MJM) | Custom | Full Cure 720 resin | 375 × 375 | [124] |
| Model | ABS | 68 × 68 | [141] | |
| Fused Deposition Modeling (FDM) | Custom | ABS | 100 × 50 | [142] |
| Model | ABS | 100 × 100 | [141] |
| Advantages | Disadvantages | Applications | |
|---|---|---|---|
| Stereolithography (SLA) |
|
| Immunomagnetic separation of bacteria [109], cell separation with helical channels [110], gradient generation [111], emulsion droplet generators [111,112], DNA assembly [113], microfluidic droplet generator [115], 3D parallelized microfluidic droplet generator [116] |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
| |||
| |||
| Multi-jet Modeling (MJM) |
|
| Anatomically accurate models for medical surgeries [118,119,120], microfluidic applications [102], pharmacokinetic profiling of drugs [121,122], microfluidic mixer and homogenizer [124], sodium alginate microspheres [125] |
|
| ||
|
| ||
|
| ||
| |||
| Fused Deposition Modeling (FDM) |
|
| Interconnects [129], electrodes within biological tissue [130], microchannels in microfluidics [131], 3D-printed nervous system [132], biomimetic scaffold assembly [132], 3D microfluidic molds [102], endothelial-cell-lined vascular network [133], microfluidic channel network [134], droplet microfluidics [137,138] |
|
| ||
|
| ||
|
| ||
|
| ||
| |||
| |||
| |||
|
| Advantages | Disadvantages | References | |
|---|---|---|---|
| Alginate |
|
| [143,149,156] |
|
| ||
|
| ||
|
| ||
|
| ||
| |||
| Chitosan |
|
| [157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175] |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
| |||
| GelMA |
|
| [176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194] |
|
| ||
|
| ||
|
| ||
|
| ||
| |||
| |||
| |||
| Hyaluronic acid |
|
| [195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211] |
|
| ||
| |||
| |||
|
| Biomaterials | 3D Fabrication Techniques | Droplet Size (µm) | Ref |
|---|---|---|---|
| Alginate | Stereolithography (SLA) | 70–115 | [212] |
| Multi-Jet Modeling (MJM) | 200 | [124] | |
| Fused Deposition Modeling (FDM) | 100 | [213] | |
| Chitosan | Stereolithography (SLA) | 378.2 | [161] |
| Multi-Jet Modeling (MJM) | <50 | [214] | |
| Fused Deposition Modeling (FDM) | - | - | |
| GelMA | Stereolithography (SLA) | 200 | [215] |
| Multi-Jet Modeling (MJM) | - | - | |
| Fused Deposition Modeling (FDM) | - | - | |
| Hyaluronic Acid | Stereolithography (SLA) | - | - |
| Multi-Jet Modeling (MJM) | - | - | |
| Fused Deposition Modeling (FDM) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orabi, M.; Lo, J.F. Emerging Advances in Microfluidic Hydrogel Droplets for Tissue Engineering and STEM Cell Mechanobiology. Gels 2023, 9, 790. https://doi.org/10.3390/gels9100790
Orabi M, Lo JF. Emerging Advances in Microfluidic Hydrogel Droplets for Tissue Engineering and STEM Cell Mechanobiology. Gels. 2023; 9(10):790. https://doi.org/10.3390/gels9100790
Chicago/Turabian StyleOrabi, Mohamad, and Joe F. Lo. 2023. "Emerging Advances in Microfluidic Hydrogel Droplets for Tissue Engineering and STEM Cell Mechanobiology" Gels 9, no. 10: 790. https://doi.org/10.3390/gels9100790