Optimizing PCL/PLGA Scaffold Biocompatibility Using Gelatin from Bovine, Porcine, and Fish Origin
Abstract
:1. Introduction
2. Results and Discussion
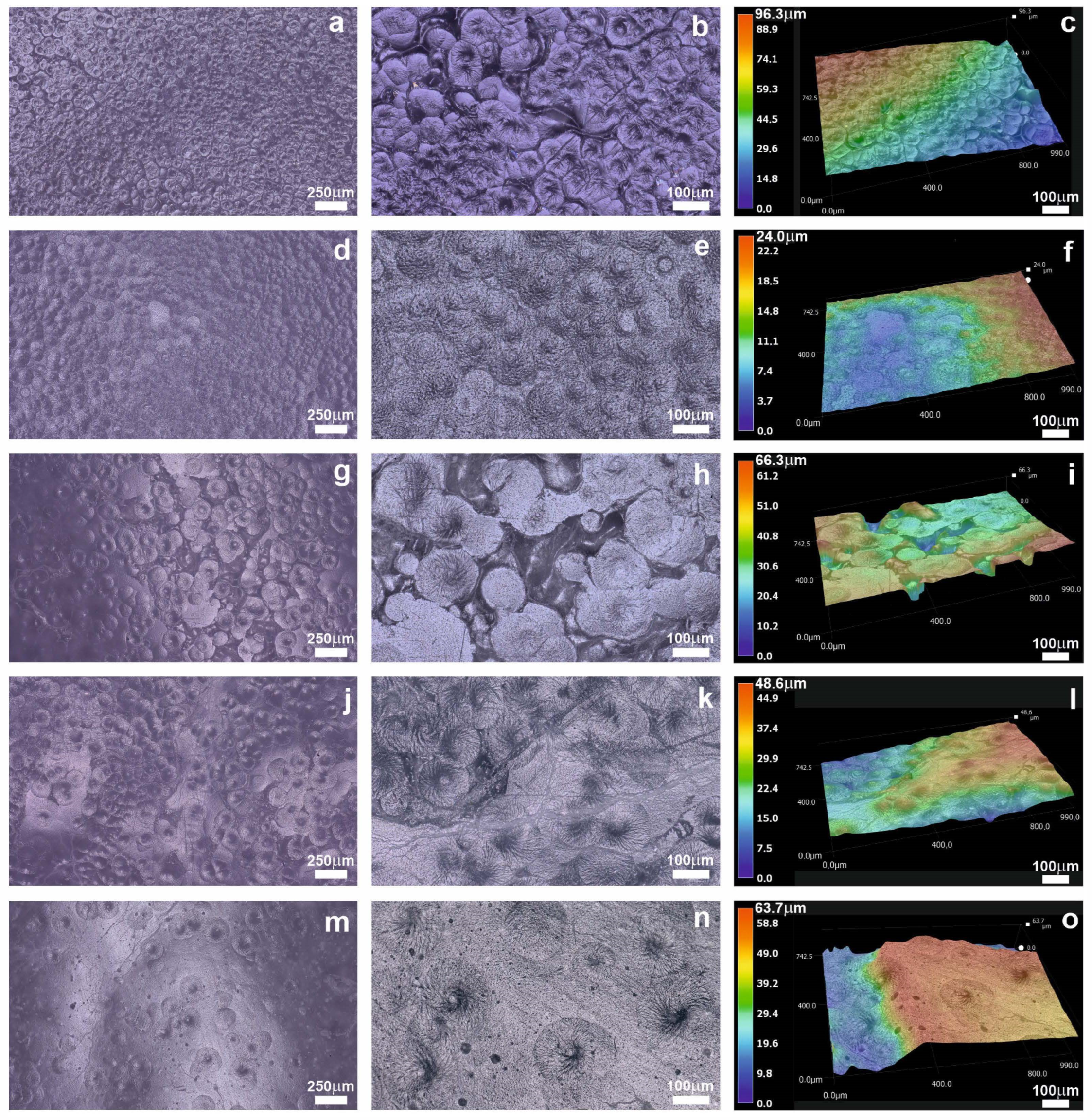
2.1. Morphology
2.2. Mechanical Properties
2.3. Thermal Properties
2.4. Biodegradability Test
2.5. Hemolytic Test
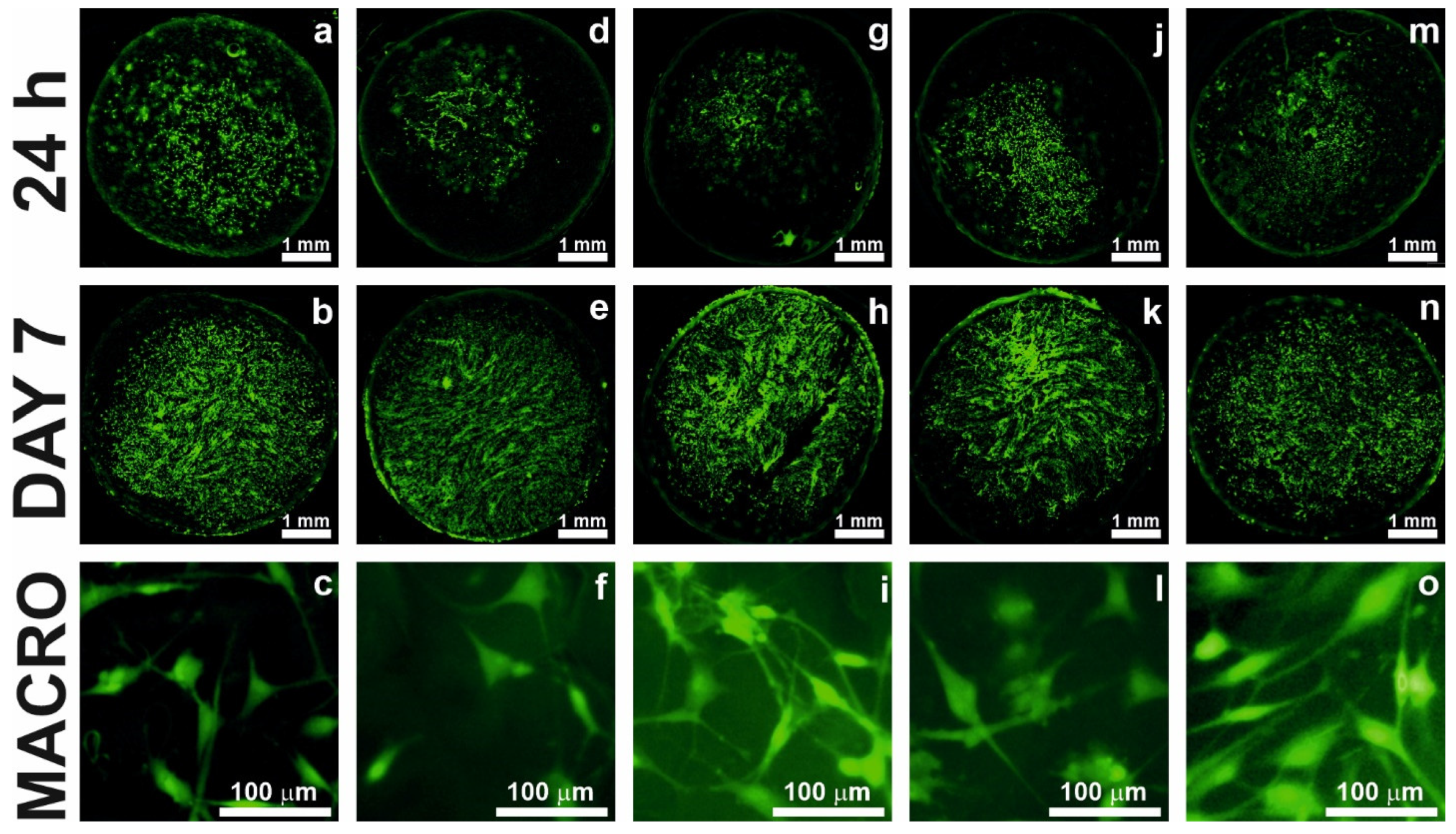
2.6. Cell Adhesion and Proliferation Test
2.7. Cell Staining
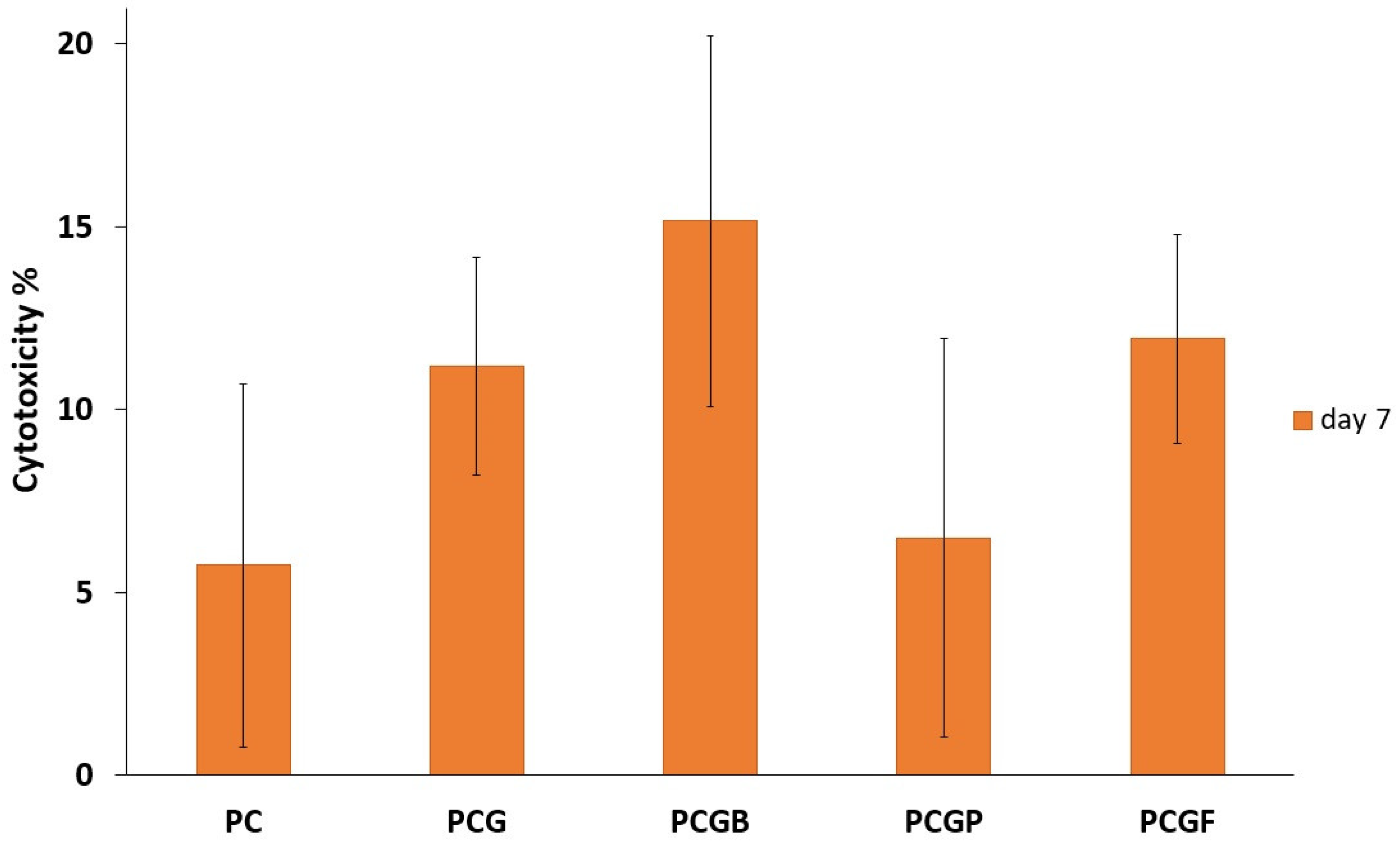
2.8. Cytotoxicity
3. Conclusions
4. Materials and Methods
4.1. Materials
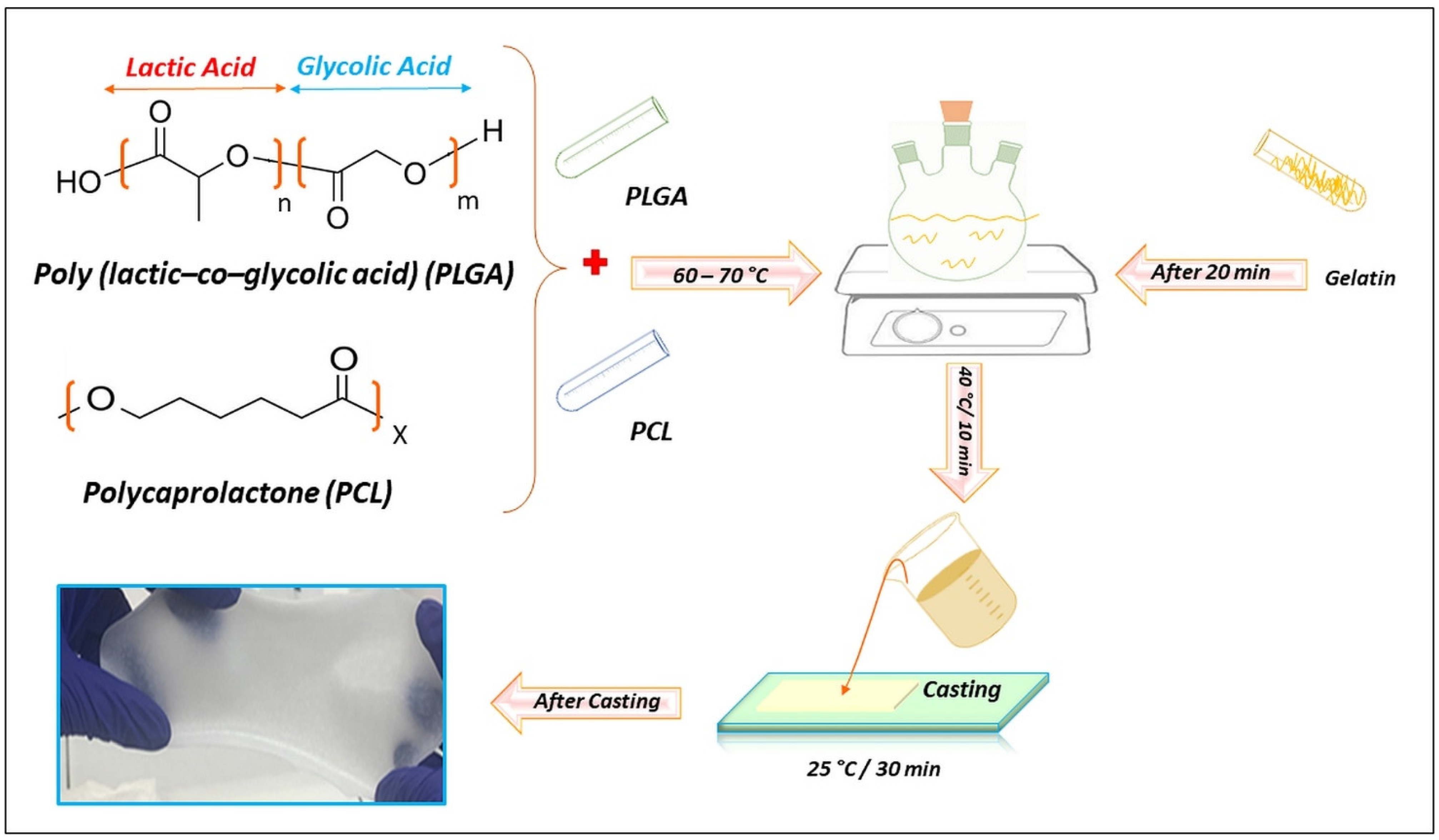
4.2. Preparation of Composite Films
4.3. Material Characterization
4.4. Analytic Methods
4.4.1. Biodegradability Test
4.4.2. Hemolytic Test
4.4.3. Human Dermal Fibroblasts Culture Conditions
4.4.4. Cell Adhesion and Proliferation
4.4.5. Cell Staining
4.4.6. Cytotoxicity Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulkarni, N.; Shinde, S.D.; Jadhav, G.S.; Adsare, D.R.; Rao, K.; Kachhia, M.; Maingle, M.; Patil, S.P.; Arya, N.; Sahu, B. Peptide-Chitosan Engineered Scaffolds for Biomedical Applications. Bioconjug. Chem. 2021, 32, 448–465. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Su, Y.; Pi, G.; Ma, P.X.; Lei, B. Biodegradable, Biomimetic Elastomeric, Photoluminescent, and Broad-Spectrum Antibacterial Polycitrate-Polypeptide-Based Membrane toward Multifunctional Biomedical Implants. ACS Biomater. Sci. Eng. 2018, 4, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Bhardwaj, N.K. Fabrication of Natural-Origin Antibacterial Nanocellulose Films Using Bio-Extracts for Potential Use in Biomedical Industry. Int. J. Biol. Macromol. 2020, 145, 914–925. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Huang, Z.; Liu, W.; Liu, Y.; Qian, H.; Lei, T.; Hua, L.; Hu, Y.; Zhang, Y.; Lei, P. Electrospun Polycaprolactone/Hydroxyapatite/ZnO Films as Potential Biomaterials for Application in Bone-Tendon Interface Repair. Colloids Surf. B Biointerfaces 2021, 204, 111825. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, F.; Zhai, W.; Cheng, S.; Li, J.; Wang, Y. Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers 2022, 14, 566. [Google Scholar] [CrossRef]
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG Macromolecule Crosslinked Chitosan Hydrogels as Antibacterial Wound Dressing. Carbohydr. Polym. 2022, 277, 118871. [Google Scholar] [CrossRef]
- Li, H.; Si, S.; Yang, K.; Mao, Z.; Sun, Y.; Cao, X.; Yu, H.; Zhang, J.; Ding, C.; Liang, H.; et al. Hexafluoroisopropanol Based Silk Fibroin Coatings on AZ31 Biometals with Enhanced Adhesion, Corrosion Resistance and Biocompatibility. Prog. Org. Coat. 2023, 184, 107881. [Google Scholar] [CrossRef]
- Dewle, A.; Rakshasmare, P.; Srivastava, A. A Polycaprolactone (PCL)-Supported Electrocompacted Aligned Collagen Type-I Patch for Annulus Fibrosus Repair and Regeneration. ACS Appl. Bio Mater. 2021, 4, 1238–1251. [Google Scholar] [CrossRef]
- Asadpour, S.; Yeganeh, H.; Ai, J.; Kargozar, S.; Rashtbar, M.; Seifalian, A.; Ghanbari, H. Polyurethane-Polycaprolactone Blend Patches: Scaffold Characterization and Cardiomyoblast Adhesion, Proliferation, and Function. ACS Biomater. Sci. Eng. 2018, 4, 4299–4310. [Google Scholar] [CrossRef]
- Dodero, A.; Alloisio, M.; Castellano, M.; Vicini, S. Multilayer Alginate–Polycaprolactone Electrospun Membranes as Skin Wound Patches with Drug Delivery Abilities. ACS Appl. Mater. Interfaces 2020, 12, 31162–31171. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Uyama, H.; Kadokawa, J. Enzymatic Polymerization towards Green Polymer Chemistry. In Green Chemistry and Sustainable Technology, 1st ed.; He, L., Tundo, P., Zhang, Z., Eds.; Springer: Singapore, 2019; Volume 2019, ISBN 9789811338120. [Google Scholar] [CrossRef]
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W. Reinforcement of Polycaprolactone/Chitosan with Nanoclay and Controlled Release of Curcumin for Wound Dressing. ACS Omega 2019, 4, 22292–22301. [Google Scholar] [CrossRef] [PubMed]
- Khil, M.-S.; Bhattarai, S.R.; Kim, H.-Y.; Kim, S.-Z.; Lee, K.-H. Novel Fabricated Matrix via Electrospinning for Tissue Engineering. J. Biomed. Mater. Res. 2005, 72B, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rampichová, M.; Chvojka, J.; Buzgo, M.; Prosecká, E.; Mikeš, P.; Vysloužilová, L.; Tvrdík, D.; Kochová, P.; Gregor, T.; Lukáš, D.; et al. Elastic Three-Dimensional Poly (ε-Caprolactone) Nanofibre Scaffold Enhances Migration, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Cell Prolif. 2013, 46, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Sogut, E.; Seydim, A.C. Development of Chitosan and Polycaprolactone Based Active Bilayer Films Enhanced with Nanocellulose and Grape Seed Extract. Carbohydr. Polym. 2018, 195, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of Chitosan/Chitosan Nanoparticles/Polycaprolactone Transparent Membrane for Corneal Endothelial Tissue Engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Campos, A.; Teodoro, K.B.R.; Teixeira, E.M.; Corrêa, A.C.; Marconcini, J.M.; Wood, D.F.; Williams, T.G.; Mattoso, L.H.C. Properties of Thermoplastic Starch and TPS/Polycaprolactone Blend Reinforced with Sisal Whiskers Using Extrusion Processing. Polym. Eng. Sci. 2013, 53, 800–808. [Google Scholar] [CrossRef]
- Kong, J.; Yu, Y.; Pei, X.; Han, C.; Tan, Y.; Dong, L. Polycaprolactone Nanocomposite Reinforced by Bioresource Starch-Based Nanoparticles. Int. J. Biol. Macromol. 2017, 102, 1304–1311. [Google Scholar] [CrossRef]
- Talebi, A.; Labbaf, S.; Karimzadeh, F. A Conductive Film of Chitosan-Polycaprolcatone-Polypyrrole with Potential in Heart Patch Application. Polym. Test. 2019, 75, 254–261. [Google Scholar] [CrossRef]
- Agarwal, Y.; Rajinikanth, P.S.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin Loaded Polycaprolactone-/Polyvinyl Alcohol-Silk Fibroin Based Electrospun Nanofibrous Mat for Rapid Healing of Diabetic Wound: An In-Vitro and In-Vivo Studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of Sandwich-like Chitosan/Polycaprolactone/Gelatin Scaffolds for Guided Tissue Regeneration Membrane. Mater. Sci. Eng. C 2020, 109, 110618. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Rodrigues, G.; Martins, G.; Henriques, C.; Silva, J.C. Evaluation of Nanofibrous Scaffolds Obtained from Blends of Chitosan, Gelatin and Polycaprolactone for Skin Tissue Engineering. Int. J. Biol. Macromol. 2017, 102, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Gunatillake, P.; Adhikari, R. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cell. Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Briceño, M.; Torres, A.; Caballero-George, C. New Curcumin-Loaded Chitosan Nanocapsules: In Vivo Evaluation. Planta Med. 2017, 83, 877–883. [Google Scholar] [CrossRef]
- Ouyang, H.W.; Goh, J.C.H.; Mo, X.M.; Teoh, S.H.; Lee, E.H. Characterization of Anterior Cruciate Ligament Cells and Bone Marrow Stromal Cells on Various Biodegradable Polymeric Films. Mater. Sci. Eng. C 2002, 20, 63–69. [Google Scholar] [CrossRef]
- Lu, X.; Miao, L.; Gao, W.; Chen, Z.; McHugh, K.J.; Sun, Y.; Tochka, Z.; Tomasic, S.; Sadtler, K.; Hyacinthe, A.; et al. Engineered PLGA Microparticles for Long-Term, Pulsatile Release of STING Agonist for Cancer Immunotherapy. Sci. Transl. Med. 2020, 12, eaaz6606. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Herrmann, V.L.; MacKerracher, A.; Gander, B.; Yagita, H.; Rohayem, J.; Groettrup, M. PLGA-Particle Vaccine Carrying TLR3/RIG-I Ligand Riboxxim Synergizes with Immune Checkpoint Blockade for Effective Anti-Cancer Immunotherapy. Nat. Commun. 2021, 12, 2935. [Google Scholar] [CrossRef]
- East, B.; Plencner, M.; Kralovic, M.; Rampichova, M.; Sovkova, V.; Vocetkova, K.; Otahal, M.; Tonar, Z.; Kolinko, Y.; Amler, E.; et al. A Polypropylene Mesh Modified with Poly-ε-Caprolactone Nanofibers in Hernia Repair: Large Animal Experiment. Int. J. Nanomed. 2018, 13, 3129–3143. [Google Scholar] [CrossRef]
- Franco, R.A.; Nguyen, T.H.; Lee, B.-T. Preparation and Characterization of Electrospun PCL/PLGA Membranes and Chitosan/Gelatin Hydrogels for Skin Bioengineering Applications. J. Mater. Sci. Mater. Med. 2011, 22, 2207–2218. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, D.-W. Blended PCL/PLGA Scaffold Fabrication Using Multi-Head Deposition System. Microelectron. Eng. 2009, 86, 1447–1450. [Google Scholar] [CrossRef]
- Jayaraman, P.; Gandhimathi, C.; Venugopal, J.R.; Becker, D.L.; Ramakrishna, S.; Srinivasan, D.K. Controlled Release of Drugs in Electrosprayed Nanoparticles for Bone Tissue Engineering. Adv. Drug Deliv. Rev. 2015, 94, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.P.; Dasgupta, S. Gelatin-Based Electrospun and Lyophilized Scaffolds with Nano Scale Feature for Bone Tissue Engineering Application: Review. J. Biomater. Sci. Polym. Ed. 2022, 33, 1704–1758. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Atma, Y. Amino Acid and Proximate Composition of Fish Bone Gelatin from Different Warm-Water Species: A Comparative Study. IOP Conf. Ser. Earth Environ. Sci. 2017, 58, 012008. [Google Scholar] [CrossRef]
- Alfaro, A.d.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Raspa, A.; Marchini, A.; Pugliese, R.; Mauri, M.; Maleki, M.; Vasita, R.; Gelain, F. A Biocompatibility Study of New Nanofibrous Scaffolds for Nervous System Regeneration. Nanoscale 2016, 8, 253–265. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yun, Y.-P.; Park, Y.-E.; Lee, S.-H.; Yong, W.; Kundu, J.; Jung, J.W.; Shim, J.-H.; Cho, D.-W.; Kim, S.E.; et al. In Vitro and in Vivo Evaluation of Bone Formation Using Solid Freeform Fabrication-Based Bone Morphogenic Protein-2 Releasing PCL/PLGA Scaffolds. Biomed. Mater. 2014, 9, 025008. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, W.; Li, J.; Liu, H.; Li, C.; Li, J. Friction Behavior of Biodegradable Electrospun Polyester Nanofibrous Membranes. Tribol. Int. 2023, 188, 108891. [Google Scholar] [CrossRef]
- Zong, C.; Wang, M.; Yang, F.; Chen, G.; Chen, J.; Tang, Z.; Liu, Q.; Gao, C.; Ma, L.; Wang, J. A Novel Therapy Strategy for Bile Duct Repair Using Tissue Engineering Technique: PCL/PLGA Bilayered Scaffold with hMSCs: Effect of Scaffold with MSC on Bile Duct Repair. J. Tissue Eng. Regen. Med. 2017, 11, 966–976. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Olsen, C.W.; Olson, D.A.; Chiou, B.; Yee, E.; Bechtel, P.J.; McHugh, T.H. Water Vapor Permeability of Mammalian and Fish Gelatin Films. J. Food Sci. 2006, 71, E202–E207. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Avena-Bustillos, R.J.; Bechtel, P.J.; Jafri, H.; Narayan, R.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Cold Water Fish Gelatin Films: Effects of Cross-Linking on Thermal, Mechanical, Barrier, and Biodegradation Properties. Eur. Polym. J. 2008, 44, 3748–3753. [Google Scholar] [CrossRef]
- Bigi, A. Drawn Gelatin Films with Improved Mechanical Properties. Biomaterials 1998, 19, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.; Albano, C.; González, G.; Castillo, R.V.; Karam, A.; Covis, M. Characterization and Thermal Degradation of Poly(d,l-Lactide-Co-Glycolide) Composites with Nanofillers. Polym. Eng. Sci. 2013, 53, 1414–1429. [Google Scholar] [CrossRef]
- Dai, C.-A.; Chen, Y.-F.; Liu, M.-W. Thermal Properties Measurements of Renatured Gelatin Using Conventional and Temperature Modulated Differential Scanning Calorimetry. J. Appl. Polym. Sci. 2006, 99, 1795–1801. [Google Scholar] [CrossRef]
- Cen, R.; Wang, L.; He, Y.; Yue, C.; Tan, Y.; Li, L.; Lei, X. Dermal Fibroblast Migration and Proliferation Upon Wounding or Lipopolysaccharide Exposure Is Mediated by Stathmin. Front. Pharmacol. 2022, 12, 781282. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun Gelatin/PCL and Collagen/PLCL Scaffolds for Vascular Tissue Engineering. Int. J. Nanomed. 2014, 2335. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Pandey, V.K.; Yadav, N.; Mishra, B. PLGA/Gelatin-Based Electrospun Nanofiber Scaffold Encapsulating Antibacterial and Antioxidant Molecules for Accelerated Tissue Regeneration. Mater. Today Commun. 2023, 35, 105633. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Supova, M.; Zaloudkova, M.; Babuska, V. In Situ Hydroxyapatite Synthesis Enhances Biocompatibility of PVA/HA Hydrogels. Int. J. Mol. Sci. 2021, 22, 9335. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.-H.; Ramakrishna, S. Electrospun Poly(ε-Caprolactone)/Gelatin Nanofibrous Scaffolds for Nerve Tissue Engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Guler, Z.; Silva, J.C.; Sezai Sarac, A. RGD Functionalized Poly( ε -Caprolactone)/Poly(m-Anthranilic Acid) Electrospun Nanofibers as High-Performing Scaffolds for Bone Tissue Engineering RGD Functionalized PCL/P3ANA Nanofibers. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 139–148. [Google Scholar] [CrossRef]
- Hassan, A.A.; Radwan, H.A.; Abdelaal, S.A.; Al-Radadi, N.S.; Ahmed, M.K.; Shoueir, K.R.; Hady, M.A. Polycaprolactone Based Electrospun Matrices Loaded with Ag/Hydroxyapatite as Wound Dressings: Morphology, Cell Adhesion, and Antibacterial Activity. Int. J. Pharm. 2021, 593, 120143. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, H.; Jiang, L.; Zhang, K.; Liu, W.; Zhu, Y.; Fangteng, J.; Shi, C.; Zhao, L.; Sun, H.; et al. Enhanced Biocompatibility of PLGA Nanofibers with Gelatin/Nano-Hydroxyapatite Bone Biomimetics Incorporation. ACS Appl. Mater. Interfaces 2014, 6, 9402–9410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Electrospinning of Gelatin Fibers and Gelatin/PCL Composite Fibrous Scaffolds. J. Biomed. Mater. Res. 2005, 72B, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Aldemir Dikici, B.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A Novel Bilayer Polycaprolactone Membrane for Guided Bone Regeneration: Combining Electrospinning and Emulsion Templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef]
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Role of Lignosulphonate in Properties of Fish Gelatin Films. Food Hydrocoll. 2012, 27, 60–71. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; De Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-Based Biomaterials for Accelerated Diabetic Wound Healing: A Critical Review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproducein Vivo Surface-Structure Changes in Bioactive Glass-Ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Kolaja Dobra, J.; Babuska, V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. Int. J. Mol. Sci. 2020, 21, 5719. [Google Scholar] [CrossRef] [PubMed]



| Samples | Composition Materials (PCL/PLGA/Gelatin Type) | Biodegradability [%] | Hemolysis * [%] |
|---|---|---|---|
| PC | (PCL/PLGA/-) | 11.70 ± 0.07 | 4.6 ± 0.2 (+++) |
| PCG | (PCL/PLGA/-) | 15.15 ± 0.08 | 4.3 ± 0.3 (+++) |
| PCGB | (PCL/PLGA/Bovine) | 16.22 ± 0.07 | 4.7± 0.5 (+++) |
| PCGP | (PCL/PLGA/Porcine) | 21.50 ± 0.09 | 4.3 ± 0.2 (+++) |
| PCGF | (PCL/PLGA/ Fish) | 28.40 ± 0.10 | 4.2 ± 0.3(+++) |
| Samples | PCL [wt.%] | PLGA [wt.%] | Bovine Gelatin [wt.%] | Porcine Gelatin [wt.%] | Fish Gelatin [wt.%] |
|---|---|---|---|---|---|
| PC | 100 | - | - | - | - |
| PCG | 80 | 20 | - | - | - |
| PCGB | 80 | 10 | 10 | - | - |
| PCGP | 80 | 10 | - | 10 | |
| PCGF | 80 | 10 | - | - | 10 |
| Order | Reagent | Amount |
|---|---|---|
| 1 | NaCl | 4.018 g |
| 2 | NaHCO3 | 0.178 g |
| 3 | KCl | 0.114 g |
| 4 | K2HPO4 3H2O | 0.116 g |
| 5 | MgCl2 6H2O | 0.159 g |
| 6 | 1M HCl | 19.5 mL |
| 7 | CaCl2 | 0.146 g |
| 8 | Na2SO4 | 0.036 g |
| 9 | Tris | 3.059 g |
| 10 | 1M HCl | 1.84 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafouri Azar, M.; Wiesnerova, L.; Dvorakova, J.; Chocholata, P.; Moztarzadeh, O.; Dejmek, J.; Babuska, V. Optimizing PCL/PLGA Scaffold Biocompatibility Using Gelatin from Bovine, Porcine, and Fish Origin. Gels 2023, 9, 900. https://doi.org/10.3390/gels9110900
Ghafouri Azar M, Wiesnerova L, Dvorakova J, Chocholata P, Moztarzadeh O, Dejmek J, Babuska V. Optimizing PCL/PLGA Scaffold Biocompatibility Using Gelatin from Bovine, Porcine, and Fish Origin. Gels. 2023; 9(11):900. https://doi.org/10.3390/gels9110900
Chicago/Turabian StyleGhafouri Azar, Mina, Lucie Wiesnerova, Jana Dvorakova, Petra Chocholata, Omid Moztarzadeh, Jiri Dejmek, and Vaclav Babuska. 2023. "Optimizing PCL/PLGA Scaffold Biocompatibility Using Gelatin from Bovine, Porcine, and Fish Origin" Gels 9, no. 11: 900. https://doi.org/10.3390/gels9110900