CO2 Injection Effect on Geomechanical and Flow Properties of Calcite-Rich Reservoirs
Abstract
:1. Introduction
2. Numerical Model
3. Experimental Methods
3.1. Material
3.2. Hydraulic Properties
3.3. CO2 Saturation and Treatment
3.4. Geomechanical Properties
4. Results
4.1. Permeability and Porosity
4.2. Relative Permeability and Saturation
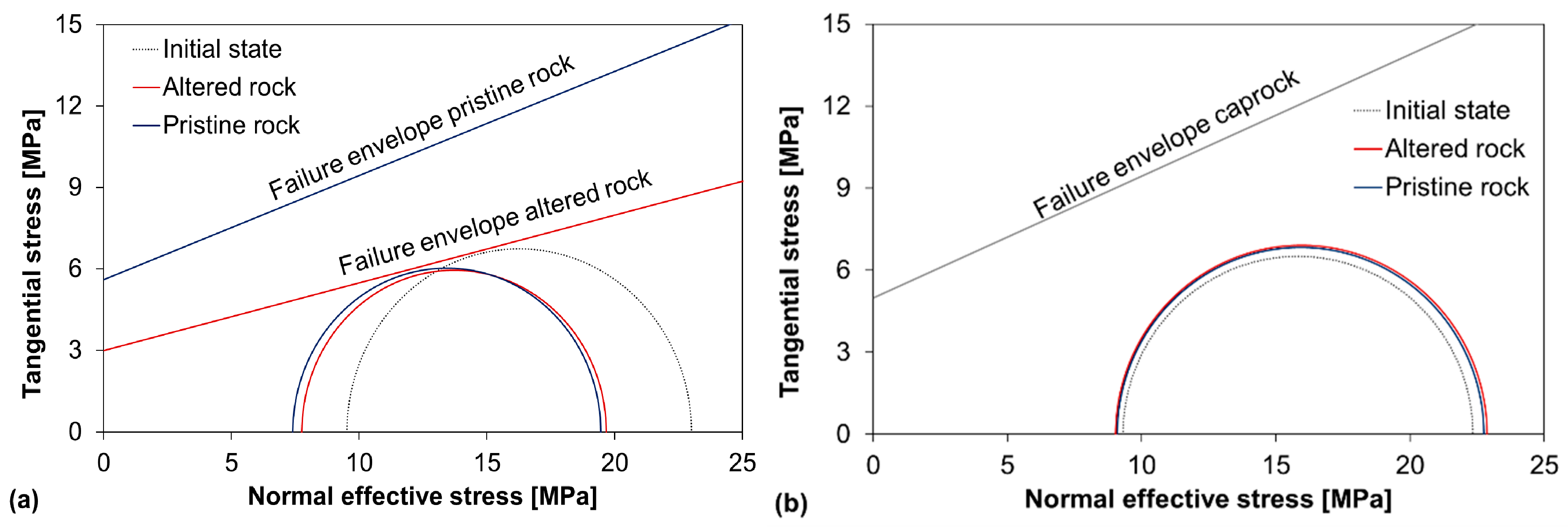
4.3. Geomechanical Properties
4.4. Numerical Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, K.; Peters, G. The trouble with negative emissions. Science 2016, 354, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Nordbotten, J.M.; Celia, M.A. Geological Storage of CO2; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- IPCC. Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Zhang, W.; Li, Y.; Xu, T.; Cheng, H.; Zheng, Y.; Xiong, P. Long-term variations of CO2 trapped in different mechanisms in deep saline formations: A case study of the Songliao Basin, China. Int. J. Greenh. Gas Control 2009, 3, 161–180. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Rohmer, J.; Pluymakers, A.; Renard, F. Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth-Sci. Rev. 2016, 157, 86–110. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Makhnenko, R.Y.; Rutqvist, J. Field and laboratory studies of geomechanical response to the injection of CO2. In Science of Carbon Storage in Deep Saline Formations: Process Coupling across Time and Spatial Scales; Elsevier: New York, NY, USA, 2018. [Google Scholar]
- Bemer, E.; Lombard, J.M. From Injectivity to Integrity Studies of CO2 Geological Storage: Chemical Alteration Effects on Carbonates Petrophysical and Geomechanical Properties. Oil Gas Sci. Technol.—Rev. Inst. Fr. Pétrol. 2010, 65, 445–459. [Google Scholar] [CrossRef]
- Alam, M.M.; Hjuler, M.L.; Foged, H.; Lykke, I.; Christensen, H.F.; Fabricius, I.L. Petrophysical and rock-mechanics effects of CO2 injection for Enhanced oil recovery: Experimental study on chalk from south Arne field, North Sea. J. Pet. Sci. Eng. 2014, 122, 468–487. [Google Scholar] [CrossRef]
- Vialle, S.; Vanorio, T. Laboratory measurements of elastic properties of carbonate rocks during injection of reactive CO2-saturated water. Geophys. Res. Lett. 2011, 38, 1–5. [Google Scholar] [CrossRef]
- Grombacher, D.; Vanorio, T.; Ebert, Y. Time-lapse acoustic, transport, and NMR measurements to characterize microstructural changes of carbonate rocks during injection of CO2-rich water. Geophysics 2012, 77, WA169–WA179. [Google Scholar] [CrossRef]
- Vanorio, T.; Nur, A.; Ebert, Y. Rock physics analysis and time-lapse rock imaging of geochemical effects due to the injection of CO2 into reservoir rocks. Geophysics 2011, 76, O23–O33. [Google Scholar] [CrossRef]
- Sterpenich, J.; Sausse, J.; Pironon, J.; Géhin, A.; Hubert, G.; Perfetti, E.; Grgic, D. Experimental ageing of oolitic limestones under CO2 storage conditions. Petrographical and chemical evidence. Chem. Geol. 2009, 265, 99–112. [Google Scholar] [CrossRef]
- Liteanu, E.; Spiers, C.J.; De Bresser, J.H.P. The influence of water and supercritical CO2 on the failure behavior of chalk. Tectonophysics 2013, 599, 157–169. [Google Scholar] [CrossRef]
- Grgic, D. Influence of CO2 on the long-term chemomechanical behavior of an oolitic limestone. J. Geophys. Res. Solid Earth 2011, 116, B07201. [Google Scholar] [CrossRef]
- Saaltink, M.W.; Vilarrasa, V.; De Gaspari, F.; Silva, O.; Carrera, J.; Rötting, T.S. A method for incorporating equilibrium chemical reactions into multiphase flow models for CO2 storage. Adv. Water Resour. 2013, 62, 431–441. [Google Scholar] [CrossRef]
- Juanes, R.; Spiteri, E.J.; Orr, F.M., Jr.; Blunt, M.J. Impact of relative permeability hysteresis on geological CO2 storage. Water Resour. Res. 2006, 42, W12418. [Google Scholar] [CrossRef]
- Bennion, D.B.; Bachu, S. Drainage and Imbibition Relative Permeability Relationships for Supercritical CO2/Brine and H2S/Brine Systems in Intergranular Sandstone, Carbonate, Shale, and Anhydrite Rocks. Soc. Pet. Eng. 2008, 11. [Google Scholar] [CrossRef]
- Zekri, A.Y.; Shedid, S.A.; Almehaideb, R.A. Possible Alteration of Tight Limestone Rocks Properties and the Effect of Water Shelding on the Performance of SC CO2 Flooding for Carbonate Formation; Society of Petroleum Engineers: Richardson, TX, USA, 2007. [Google Scholar]
- André, L.; Audigane, P.; Azaroual, M.; Menjoz, A. Numerical modeling of fluid-rock chemical interactions at the supercritical CO2-liquid interface during CO2 injection into a carbonate reservoir, the Dogger aquifer (Paris Basin, France). Energy Convers. Manag. 2007, 48, 1782–1797. [Google Scholar] [CrossRef]
- Bear, J. Dynamics of Fluids in Porous Media; Elsevier: New York, NY, USA, 1972. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Bolster, D.; Olivella, S.; Carrera, J. Coupled hydromechanical modeling of CO2 sequestration in deep saline aquifers. Int. J. Greenh. Gas Control 2010, 4, 910–919. [Google Scholar] [CrossRef] [Green Version]
- Vilarrasa, V.; Makhnenko, R.; Gheibi, S. Geomechanical analysis of the influence of CO2 injection location on fault stability. J. Rock Mech. Geotech. Eng. 2016, 8, 805–818. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Makhnenko, R.Y. Caprock integrity and induced seismicity from laboratory and numerical experiments. Energy Procedia 2017, 125, 494–503. [Google Scholar] [CrossRef]
- Dentz, M.; Tartakovsky, D.M. Abrupt-interface solution for carbon dioxide injection into porous media. Transp. Porous Media 2009, 79, 15. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Bolster, D.; Dentz, M.; Olivella, S.; Carrera, J. Effects of CO2 compressibility on CO2 storage in deep saline aquifers. Transp. Porous Media 2010, 85, 619–639. [Google Scholar] [CrossRef] [Green Version]
- Olivella, S.; Gens, A.; Carrera, J.; Alonso, E.E. Numerical formulation for a simulator (CODE_BRIGHT) for the coupled analysis of saline media. Eng. Comput. 1996, 13, 87–112. [Google Scholar] [CrossRef] [Green Version]
- Vilarrasa, V.; Silva, O.; Carrera, J.; Olivella, S. Liquid CO2 injection for geological storage in deep saline aquifers. Int. J. Greenh. Gas Control 2013, 14, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Ciantia, M.O.; Hueckel, T. Weathering of submerged stressed calcarenites: Chemo-mechanical coupling mechanisms. Geotechnique 2013, 63, 768–785. [Google Scholar] [CrossRef]
- Makhnenko, R.Y.; Labuz, J. Calcarenite as a possible host rock for geologic CO2. In Proceedings of the 48th U.S. Rock Mechanics/Geomechanics Symposium, Minneapolis, MN, USA, 1–4 June 2014. No. 7559. [Google Scholar]
- Espinoza, D.N.; Santamarina, J.C. Water-CO2-mineral systems: Interfacial tension, contact angle, and diffusion—Implications to CO2 geological storage. Water Resour. Res. 2010, 46, W07537. [Google Scholar] [CrossRef]
- Krevor, S.C.M.; Pini, R.; Zuo, L.; Benson, S.M. Relative permeability and trapping of CO2 and water in sandstone rocks at reservoir conditions. Water Resour. Res. 2012, 48, W02532. [Google Scholar] [CrossRef]
- Andrew, M.; Bijeljic, B.; Blunt, M.J. Pore-scale imaging of trapped supercritical carbon dioxide in sandstones and carbonates. Int. J. Greenh. Gas Control 2014, 22, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Huo, D.; Benson, S.M. Experimental Investigation of Stress-Dependency of Relative Permeability in Rock Fractures. Transp. Porous Media 2016, 113, 567–590. [Google Scholar] [CrossRef]
- Skempton, A.W. The pore-pressure coefficients A and B. Geotechnique 1954, 4, 143–147. [Google Scholar] [CrossRef]
- Bishop, A.W. The influence of an undrained change in stress on the pore pressure in porous media of low compressibility. Géotechnique 1976, 26, 371–375. [Google Scholar] [CrossRef]
- Detournay, E.; Cheng, A.H.-D. Fundamentals of Poroelasticity. In Comprehensive Rock Engineering: Principles, Practice and Projects, Vol. II, Analysis and Design Methods; Fairhurst, C., Ed.; Pergamon Press: Oxford, UK, 1993; Chapter 5. [Google Scholar]
- Achenbach, J.D. Wave Propagation in Elastic Solids; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Wood, A.W. A Textbook of Sound; McMillan Co.: New York, NY, USA, 1955. [Google Scholar]
- Makhnenko, R.Y.; Labuz, J.F. Elastic and inelastic deformation of fluid-saturated rock. Philos. Trans. R. Soc. A 2016, 374, 20150422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, R.H.; Corey, A.T. Hydraulic Properties of Porous Media and Their Relation to Drainage Design. Trans. ASAE 1964, 7, 26–28. [Google Scholar]
- Meier, P.M.; Carrera, J.; Sánchez-Vila, X. An evaluation of Jacob’s method for the interpretation of pumping tests in heterogeneous formations. Water Resour. Res. 1998, 34, 1011–1025. [Google Scholar] [CrossRef]
- Butler, J.J., Jr. Pumping tests in nonuniform aquifers—The radially symmetric case. J. Hydrol. 1988, 101, 15–30. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Carrera, J.; Jurado, A.; Pujades, E.; Vázquez-Suné, E. A methodology for characterizing the hydraulic effectiveness of an annular low-permeability barrier. Eng. Geol. 2011, 120, 68–80. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Carrera, J.; Olivella, S. Hydromechanical characterization of CO2 injection sites. Int. J. Greenh. Gas Control 2013, 19, 665–677. [Google Scholar] [CrossRef]
- Hsieh, P.A. Deformation-induced changes in hydraulic head during ground-water withdrawal. Groundwater 1996, 34, 1082–1089. [Google Scholar] [CrossRef]
- Gouze, P.; Edlmann, K.; McDermott, C.I.; Luquot, L. Laboratory Experiments. In Geological Storage of CO2 in Deep Saline Formations; Niemi, A., Bear, J., Bensabat, J., Eds.; Theory and Applications of Transport in Porous Media, Vol. 29; Springer: Dordrecht, The Netherlands, 2017; Chapter 6. [Google Scholar]
- Ciantia, M.O.; Castellanza, R.; di Prisco, C. Experimental Study on the Water-Induced Weakening of Calcarenites. Rock Mech Rock Eng. 2015, 48, 441–461. [Google Scholar] [CrossRef]








| Property | Pristine Rock |
|---|---|
| Permeability, k [m2] | 4 × 10−21 |
| Porosity, φ [−] | 0.12 |
| Relative water permeability, krw [−] | Sw6 |
| Relative CO2 permeability, krc [−] | Sc6 |
| Gas entry pressure, p0 [MPa] | 6.0 |
| van Genuchten shape parameter m [−] | 0.3 |
| Undrained Young’s modulus, E [GPa] | 2.8 |
| Undrained Poisson’s ratio, νu [−] | 0.40 |
| Cohesion, c′ [MPa] | 5.0 |
| Friction angle, ϕ [°] | 24 |
| Pristine | CO2-Treated | ||||
|---|---|---|---|---|---|
| Calc-0 | Calc-1 | Calc-2 | Calc-3 | Calc-4 | |
| Min. principal stress, σlat [MPa] | 2.0 | 3.5 | 3.5 | 3.5 | 3.5 |
| Pore pressure p, [MPa] | 1.8 | 3.4 | 2.0 | 0.5 | 1.0 |
| Permeability, k [m2] (at P’ = 1 MPa) | 9 × 10−15 | 3.5 × 10−15 | 5.1 × 10−15 | 5.6 × 10−15 | 5.8 × 10−15 |
| Porosity, φ [−] | 0.35 | 0.34 | 0.34 | 0.32 | 0.34 |
| Young’s modulus, E [GPa] | 7.1 | 3.4 | 4.4 | 4.7 | 4.5 |
| Poisson’s ratio, ν [−] | 0.25 | - | - | 0.25 | 0.25 |
| Max. principal stress at failure, σax [MPa] | 15.6 | 7.7 | 10.7 | 12.5 | 12.6 |
| Property | Pristine Rock | CO2-Treated Rock |
|---|---|---|
| Permeability, k [m2] | 9 × 10−15 | 5–6 × 10−15 |
| Porosity, φ [−] | 0.35 | 0.34 |
| Relative water permeability, krw [−] | Sw2.1 | Sw1.2 |
| Relative CO2 permeability, krc [−] | Sc6 | Sc5.5 |
| Gas entry pressure, p0 [MPa] | 0.02 | 0.02 |
| van Genuchten shape parameter m [−] | 0.42 | 0.42 |
| Young’s modulus, E [GPa] | 7.1 | 4.4 |
| Poisson ratio, ν [−] | 0.25 | 0.25 |
| Cohesion, c′ [MPa] | 5.6 | 3.0 |
| Friction angle, ϕ′ [°] | 21 | 14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Vilarrasa, V.; Makhnenko, R.Y. CO2 Injection Effect on Geomechanical and Flow Properties of Calcite-Rich Reservoirs. Fluids 2018, 3, 66. https://doi.org/10.3390/fluids3030066
Kim K, Vilarrasa V, Makhnenko RY. CO2 Injection Effect on Geomechanical and Flow Properties of Calcite-Rich Reservoirs. Fluids. 2018; 3(3):66. https://doi.org/10.3390/fluids3030066
Chicago/Turabian StyleKim, Kiseok, Victor Vilarrasa, and Roman Y. Makhnenko. 2018. "CO2 Injection Effect on Geomechanical and Flow Properties of Calcite-Rich Reservoirs" Fluids 3, no. 3: 66. https://doi.org/10.3390/fluids3030066




