Computational Modeling of Bubbles Growth Using the Coupled Level Set—Volume of Fluid Method
Abstract
:1. Introduction
2. Computational Model
2.1. Simple Combined Level Set and Volume of Fluid Method
2.2. Transport of Species Based on One-Fluid Approach
3. Bubble Hydrodynamic Verification
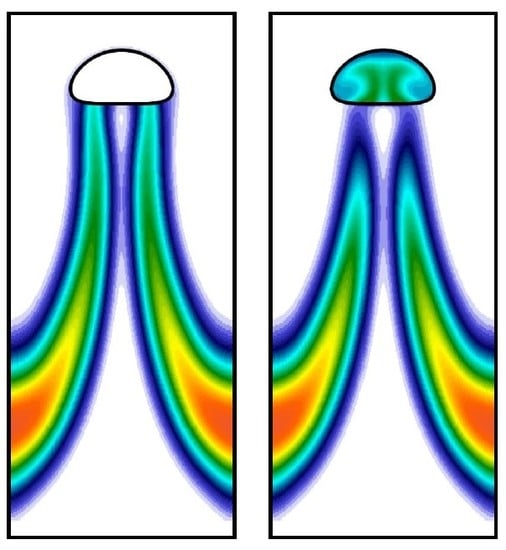
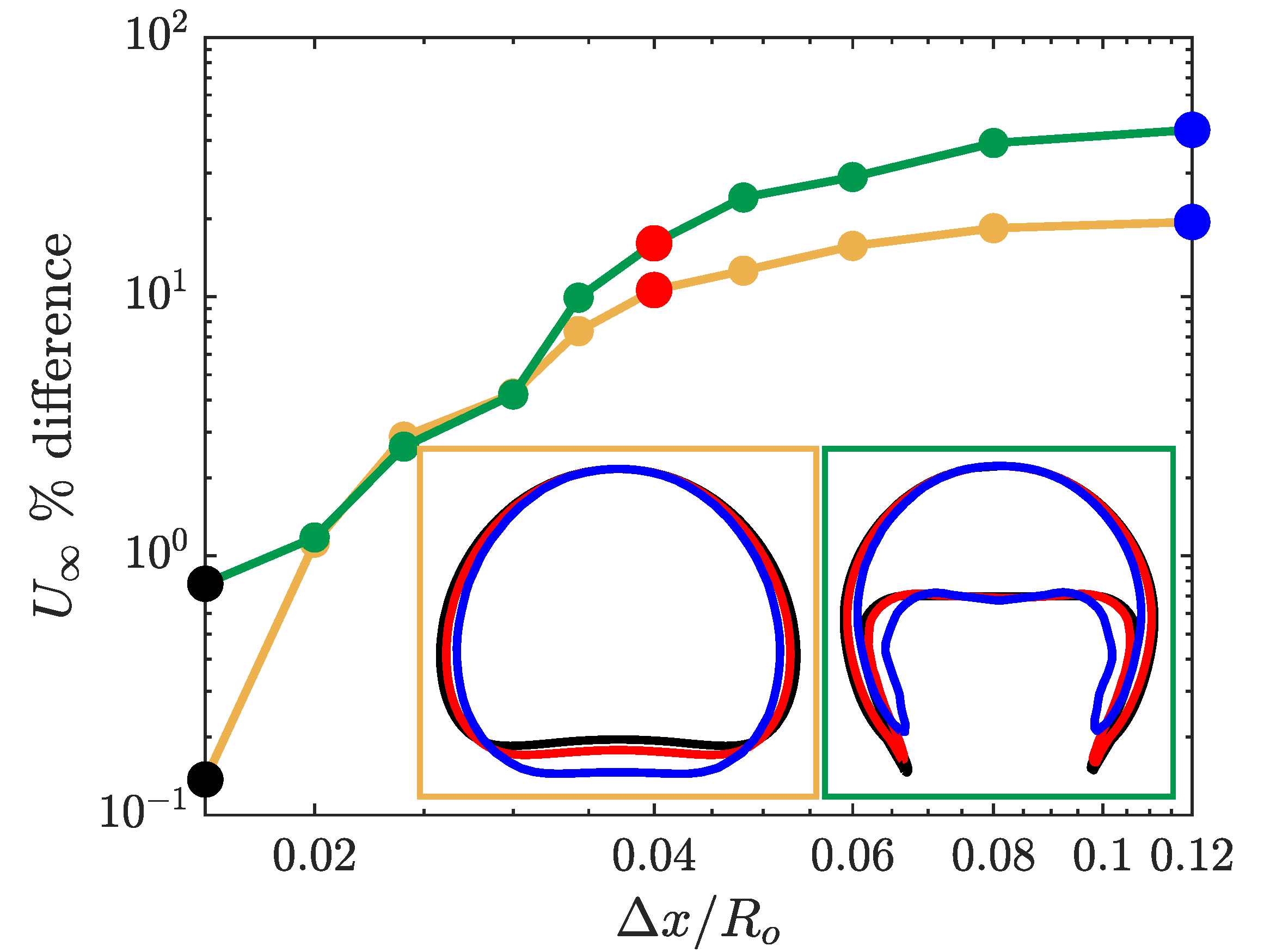
3.1. Grid Convergence Study
3.2. Volume Conservation of Rising Bubbles
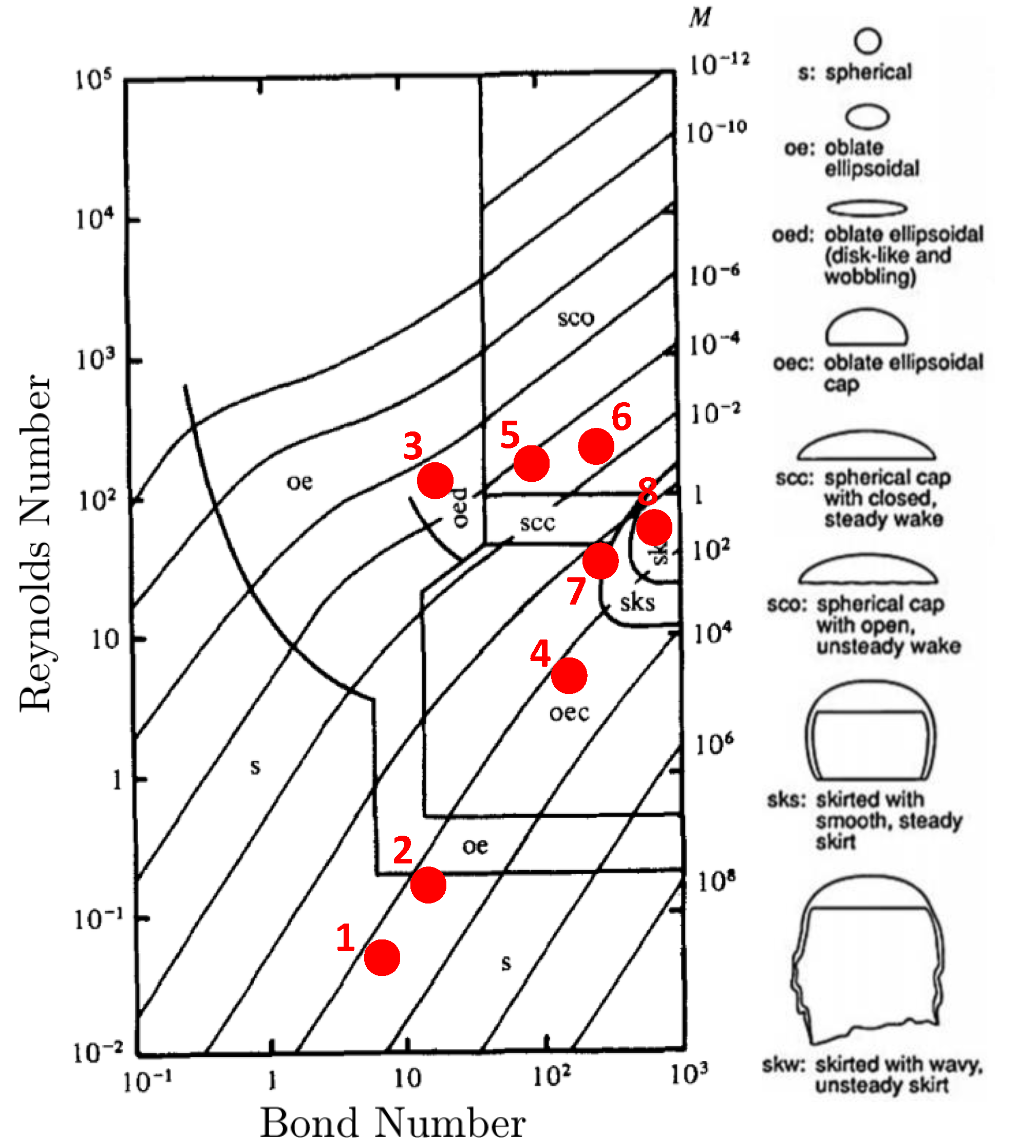
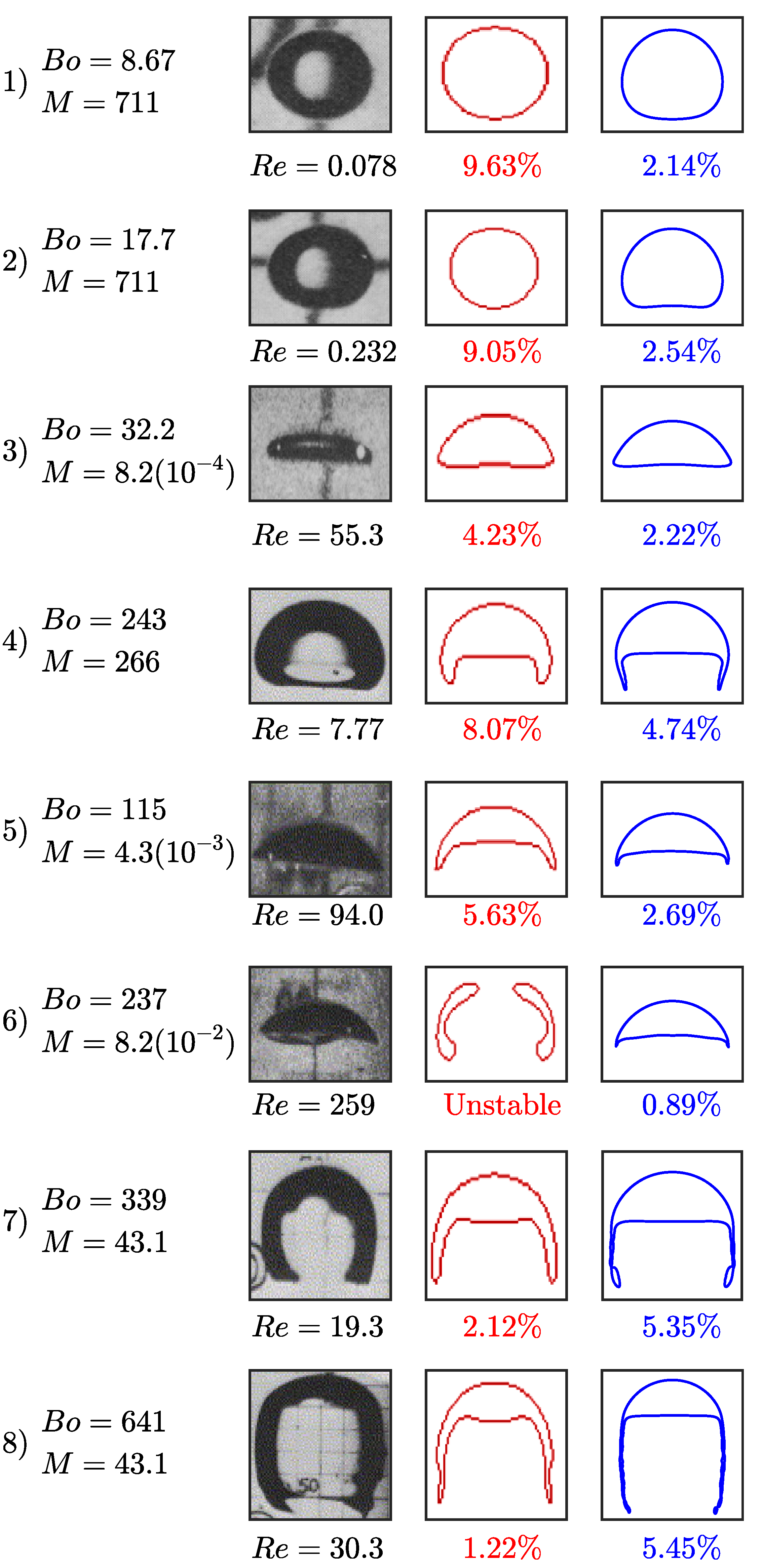
3.3. Bubble Rising in Stagnant Liquid
4. Impact of Transport of Species
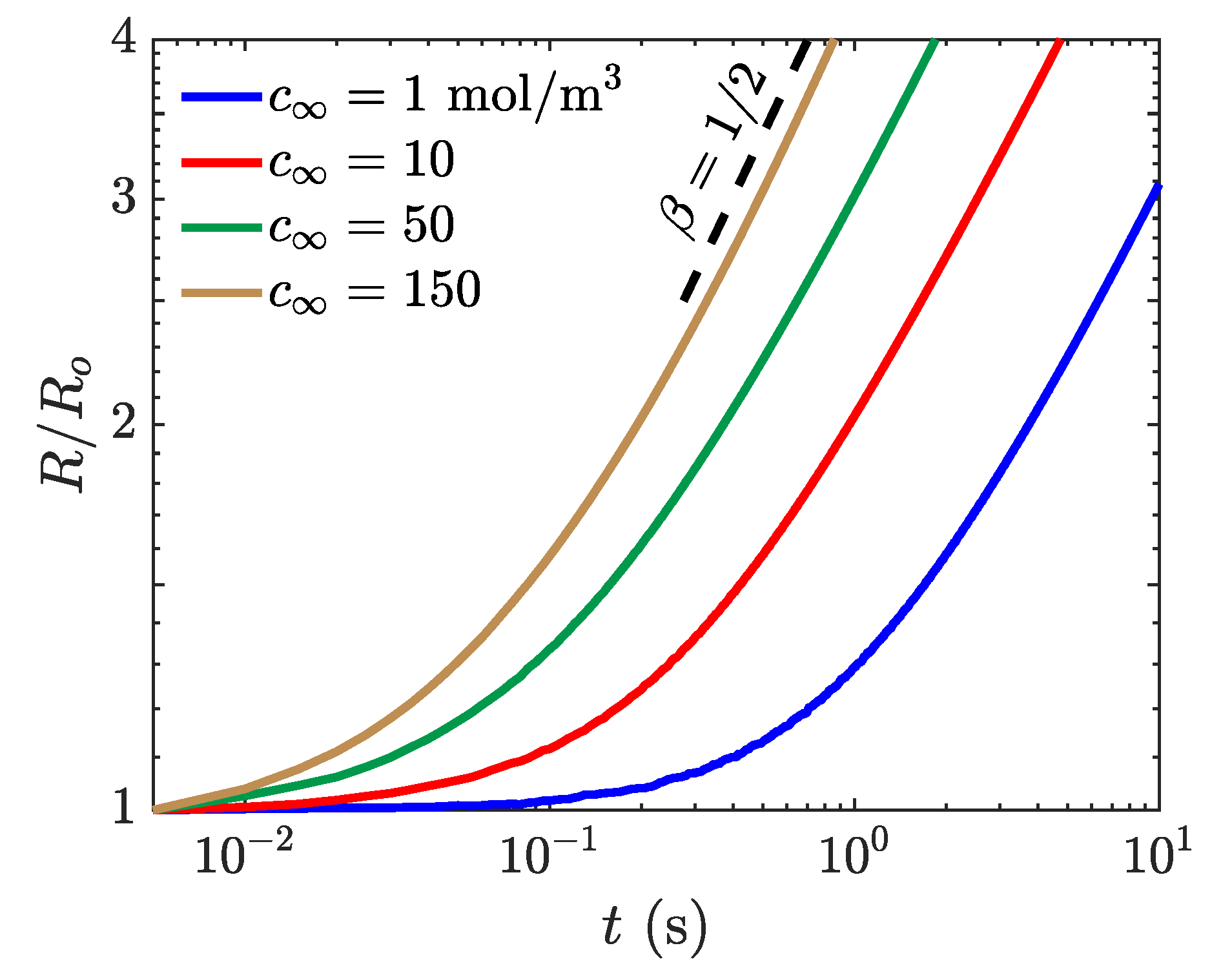
4.1. Bubble Growth Rate
4.2. Velocity of a Rising Bubble in a Reactive Flow
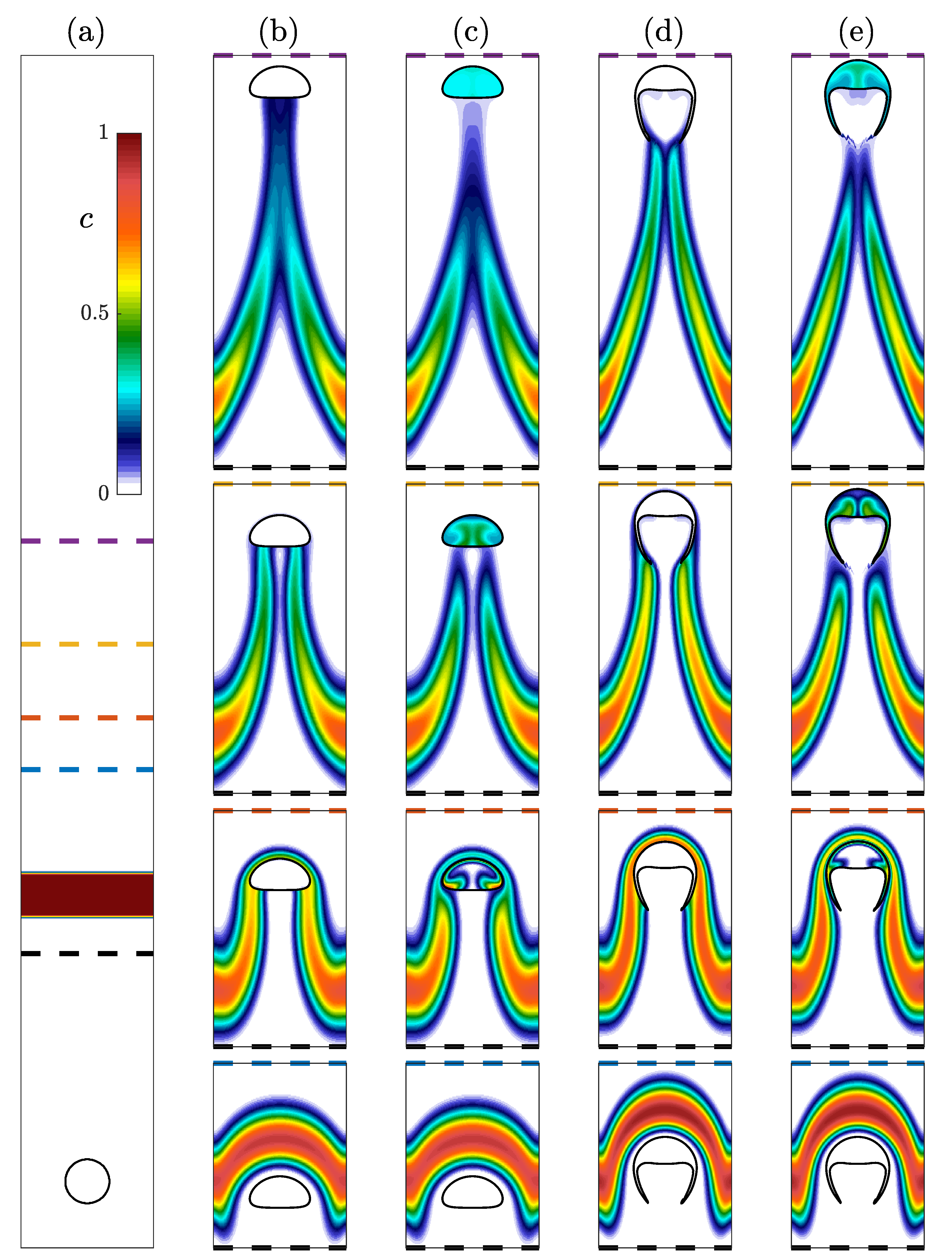
4.3. Shape of a Rising Bubble in a Reactive Flow
4.4. Transport of Species Impact on Concentration Field
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Reveillon, J.; Vervish, L. Analysis of weakly turbulent dilute-spray flames and spray combustion regimes. J. Fluid Mech. 2005, 537, 317–347. [Google Scholar] [CrossRef]
- Gómez, A.; Fueyo, N.; Díez, L.I. Modelling and simulation of fluid flow and heat transfer in the convective zone of a power-generation boiler. Appl. Therm. Eng. 2008, 28, 532–546. [Google Scholar] [CrossRef] [Green Version]
- Balakin, B.V.; Hoffmann, A.C.; Kosinski, P.; Rhyne, L.D. Eulerian-Eulerian CFD Model for the Sedimentation of Spherical Particles in Suspension with High Particle Concentrations. Eng. Appl. Comput. Fluid Mech. 2010, 4, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Soloviev, A.; Fujimura, A.; Matt, S. Air-sea interface in hurricane conditions. J. Geophys. Res. Oceans 2010, 117. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.L.; Sun, Z.; Lu, G.M.; Yu, J.G. Hydrodynamic characteristics of the two-phase flow field at gas-evolving electrodes: Numerical and experimental studies. R. Soc. Open Sci. 2018, 5, 171255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Ren, H.; Luo, L. Gas Bubbles in Electrochemical Gas Evolution Reactions. Langmuir 2019, 35, 5392–5408. [Google Scholar] [CrossRef]
- Tryggvason, G.; Scardovelli, R.; Zaleski, S. Direct Numerical Simulations of Gas–Liquid Multiphase Flows; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Hine, F.; Murakami, K. Bubble Effects on the Solution IR Drop in a Vertical Electrolyzer Under Free and Forced Convection. J. Electrochem. Soc. 1980, 127, 292–297. [Google Scholar] [CrossRef]
- Taqieddin, A.; Nazari, R.; Rajic, L.; Alshawabkeh, A. Review Physicochemical Hydrodynamics of Gas Bubbles in Two Phase Electrochemical Systems. J. Electrochem. Soc. 2017, 164, E448–E459. [Google Scholar] [CrossRef]
- Weier, T.; Landgraf, S. The two-phase flow at gas-evolving electrodes: Bubble-driven and Lorentz-force-driven convection. Eur. Phys. J. Spec. Top. 2013, 220, 313–322. [Google Scholar] [CrossRef]
- Peñas, P.; van der Linde, P.; Vijselaar, W.; van der Meer, D.; Lohse, D.; Huskens, J.; Gardeniers, H.; Modestino, M.A.; Rivas, D.F. Decoupling Gas Evolution from Water-Splitting Electrodes. J. Electrochem. Soc. 2019, 166, H769–H776. [Google Scholar] [CrossRef] [Green Version]
- Taqieddin, A.; Allshouse, M.R.; Alshawabkeh, A.N. Editors’ Choice—Critical Review—Mathematical Formulations of Electrochemically Gas-Evolving Systems. J. Electrochem. Soc. 2018, 165, E694–E711. [Google Scholar] [CrossRef] [PubMed]
- Nichita, B.A. An Improved CFD Tool to Simulate Adiabatic and Diabatic Two-Phase Flows; EPFL: Lausanne, Switzerland, 2010. [Google Scholar] [CrossRef]
- Sethian, J.A.; Smereka, P. Level Set Methods for Fluid Interfaces. Annu. Rev. Fluid Mech. 2003, 35, 341–372. [Google Scholar] [CrossRef] [Green Version]
- Tryggvason, G.; Bunner, B.; Esmaeeli, A.; Juric, D.; Al-Rawahi, N.; Tauber, W.; Han, J.; Nas, S.; Jan, Y.J. A Front-Tracking Method for the Computations of Multiphase Flow. J. Comput. Phys. 2001, 169, 708–759. [Google Scholar] [CrossRef] [Green Version]
- Hirt, C.; Nichols, B. Volume of fluid (VOF) method for the dynamics of free boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Diggs, A.; Balachandar, S. Modeling and simulation challenges in Eulerian-Lagrangian computations of multiphase flows. AIP Conf. Proc. 2017, 1793, 150008. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk, W.P.; Klimanek, A.; Białecki, R.A.; Wȩcel, G.; Kozołub, P.; Czakiert, T. Comparison of the standard Euler–Euler and hybrid Euler–Lagrange approaches for modeling particle transport in a pilot–scale circulating fluidized bed. Particuology 2014, 15, 129–137. [Google Scholar] [CrossRef]
- Pianet, G.; Ten Cate, A.; Derksen, J.J.; Arquis, E. Assessment of the one-fluid method for DNS of particulate flows: Sedimentation of a single sphere at moderate to high Reynolds numbers. Comput. Fluids 2007, 36, 359–375. [Google Scholar] [CrossRef]
- Bonometti, T.; Magnaudet, J. An interface-capturing method for incompressible two-phase flows. Validation and application to bubble dynamics. Int. J. Multiph. Flow 2007, 33, 109–133. [Google Scholar] [CrossRef] [Green Version]
- Sussman, M.; Smereka, P.; Osher, S. A Level Set Approach for Computing Solutions to Incompressible Two-Phase Flow. J. Comput. Phys. 1994, 114, 146–159. [Google Scholar] [CrossRef]
- Kimmel, R. The Osher-Sethian Level Set Method. In Numerical Geometry of Images: Theory, Algorithms, and Applications; Springer: New York, NY, USA, 2004; pp. 50–60. [Google Scholar] [CrossRef]
- Dijkhuizen, W. Deriving Closures for Bubbly Flows Using Direct Numerical Simulations. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2008. [Google Scholar]
- Wang, X.; Klaasen, B.; Degréve, J.; Mahulkar, A.; Heynderickx, G.; Reyniers, M.F.; Blanpain, B.; Verhaeghe, F. Volume-of-fluid simulations of bubble dynamics in a vertical Hele-Shaw cell. Phys. Fluids 2016, 28, 053304. [Google Scholar] [CrossRef]
- Niethammer, M.U.; Brenn, G.; Marschall, H.; Bothe, D. An Extended Volume of Fluid Method and Its Application to Single Bubbles Rising in a Viscoelastic Liquid. J. Comput. Phys. 2019, 387, 326–355. [Google Scholar] [CrossRef] [Green Version]
- Liovic, P.; Liow, J.L.; Rudman, M. A Volume of Fluid (VOF) Method for the Simulation of Metallurgical Flows. ISIJ Int. 2001, 41, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Ye, Z.; Sheu, T.W.; Lin, Y.; Zhao, X. An improved interface preserving level set method for simulating three dimensional rising bubble. Int. J. Heat Mass Transf. 2016, 103, 753–772. [Google Scholar] [CrossRef]
- Can, E.; Prosperetti, A. A level set method for vapor bubble dynamics. J. Comput. Phys. 2012, 231, 1533–1552. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H. Level set method for numerical simulation of a cavitation bubble, its growth, collapse and rebound near a rigid wall. Acta Mech. Sin. 2007, 23, 645–653. [Google Scholar] [CrossRef]
- Albadawi, A.; Donoghue, D.; Robinson, A.; Murray, D.; Delauré, Y. Influence of surface tension implementation in volume of fluid and coupled volume of fluid with level set methods for bubble growth and detachment. Int. J. Multiph. Flow 2013, 53, 11–28. [Google Scholar] [CrossRef]
- Yamamoto, T.; Okano, Y.; Dost, S. Validation of the S-CLSVOF method with the density-scaled balanced continuum surface force model in multiphase systems coupled with thermocapillary flows. Int. J. Numer. Methods Fluids 2017, 83, 223–244. [Google Scholar] [CrossRef]
- Bothe, D.; Koebe, M.; Wielage, K.; Prüss, J.; Warnecke, H.J. Direct numerical simulation of mass transfer between rising gas bubbles and water. In Bubbly Flows: Analysis, Modelling and Calculation; Sommerfeld, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 159–174. [Google Scholar] [CrossRef]
- Bothe, D.; Koebe, M.; Wielage, K.; Warnecke, H.J. VOF-Simulations of Mass Transfer From Single Bubbles and Bubble Chains Rising in Aqueous Solutions. Fluids Eng. Div. Summer Meet. 2003, 2, 423–429. [Google Scholar] [CrossRef]
- Deising, D.; Bothe, D.; Marschall, H. Direct Numerical Simulation of Mass Transfer in Bubbly Flows. Comput. Fluids 2018. [Google Scholar] [CrossRef]
- Haroun, Y.; Legendre, D.; Raynal, L. Volume of fluid method for interfacial reactive mass transfer: Application to stable liquid film. Chem. Eng. Sci. 2010, 65, 2896–2909. [Google Scholar] [CrossRef]
- Marschall, H.; Hinterberger, K.; Schüler, C.; Habla, F.; Hinrichsen, O. Numerical simulation of species transfer across fluid interfaces in free-surface flows using OpenFOAM. Chem. Eng. Sci. 2012, 78, 111–127. [Google Scholar] [CrossRef]
- Nieves-Remacha, M.J.; Yang, L.; Jensen, K.F. OpenFOAM computational fluid dynamic simulations of two-phase flow and mass transfer in an Advanced-Flow Reactor. Ind. Eng. Chem. Res. 2015, 54, 6649–6659. [Google Scholar] [CrossRef]
- Dianat, M.; Skarysz, M.; Garmory, A. A Coupled Level Set and Volume of Fluid method for automotive exterior water management applications. Int. J. Multiph. Flow 2017, 91, 19–38. [Google Scholar] [CrossRef] [Green Version]
- Kharangate, C.R.; Mudawar, I. Review of computational studies on boiling and condensation. Int. J. Heat Mass Transf. 2017, 108, 1164–1196. [Google Scholar] [CrossRef]
- Fedkiw, S.O.R.; Osher, S. Level set methods and dynamic implicit surfaces. Surfaces 2002, 44, 77. [Google Scholar] [CrossRef]
- Ozkan, F.; Wenka, A.; Hansjosten, E.; Pfeifer, P.; Kraushaar-Czarnetzki, B. Numerical investigation of interfacial mass transfer in two phase flows using the VOF method. Eng. Appl. Comput. Fluid Mech. 2016, 10, 100–110. [Google Scholar] [CrossRef]
- Samkhaniani, N.; Ajami, A.; Kayhani, M.H.; Dari, A.S. Direct numerical simulation of single bubble rising in viscous stagnant liquid. In Proceedings of the International Conference on Merchanical, Automobile and Robotics Engineering (ICMAR’2012), Penang, Malaysia, 11–12 February 2012. [Google Scholar]
- Clift, R.; Grace, J.R.; Weber, M.E. Bubbles, Drops, and Particles; Courier Corporation: North Chelmsford, MA, USA, 2005. [Google Scholar]
- Bhaga, D.; Weber, M.E. Bubbles in viscous liquids: Shapes, wakes and velocities. J. Fluid Mech. 1981, 105, 61–85. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.K.; Sahu, K.C.; Govindarajan, R. Dynamics of an initially spherical bubble rising in quiescent liquid. Nat. Commun. 2015, 6, 6268. [Google Scholar] [CrossRef] [Green Version]
- Hua, J.; Stene, J.F.; Lin, P. Numerical simulation of 3D bubbles rising in viscous liquids using a front tracking method. J. Comput. Phys. 2008, 227, 3358–3382. [Google Scholar] [CrossRef] [Green Version]
- Hua, J.; Lou, J. Numerical simulation of bubble rising in viscous liquid. J. Comput. Phys. 2007, 222, 769–795. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Cao, Z.; Guo, L. Investigations on bubble growth mechanism during photoelectrochemical and electrochemical conversions. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 505, 86–92. [Google Scholar] [CrossRef]
- Prosperetti, A. Vapor Bubbles. Annu. Rev. Fluid Mech. 2017, 49, 221–248. [Google Scholar] [CrossRef]
- Reddy, C.M.; Arey, J.S.; Seewald, J.S.; Sylva, S.P.; Lemkau, K.L.; Nelson, R.K.; Carmichael, C.A.; McIntyre, C.P.; Fenwick, J.; Venura, G.T.; et al. Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. PNAS 2012, 109, 20229–20234. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taqieddin, A.; Liu, Y.; Alshawabkeh, A.N.; Allshouse, M.R. Computational Modeling of Bubbles Growth Using the Coupled Level Set—Volume of Fluid Method. Fluids 2020, 5, 120. https://doi.org/10.3390/fluids5030120
Taqieddin A, Liu Y, Alshawabkeh AN, Allshouse MR. Computational Modeling of Bubbles Growth Using the Coupled Level Set—Volume of Fluid Method. Fluids. 2020; 5(3):120. https://doi.org/10.3390/fluids5030120
Chicago/Turabian StyleTaqieddin, Amir, Yuxuan Liu, Akram N. Alshawabkeh, and Michael R. Allshouse. 2020. "Computational Modeling of Bubbles Growth Using the Coupled Level Set—Volume of Fluid Method" Fluids 5, no. 3: 120. https://doi.org/10.3390/fluids5030120