Biodiversity and Safety: Cohabitation Experimentation in Undefined Starter Cultures for Traditional Dairy Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Plan
2.2. Microbial Counts and Isolation
2.3. Microbial Identification and Biodiversity Evaluation
2.3.1. Species Identification
2.3.2. Molecular Biotyping
2.4. Safety Assessment
2.4.1. Antibiotic Resistance and Virulence Gene Detection
2.4.2. Antibiotic Susceptibility
2.5. Statistical Analysis
3. Results
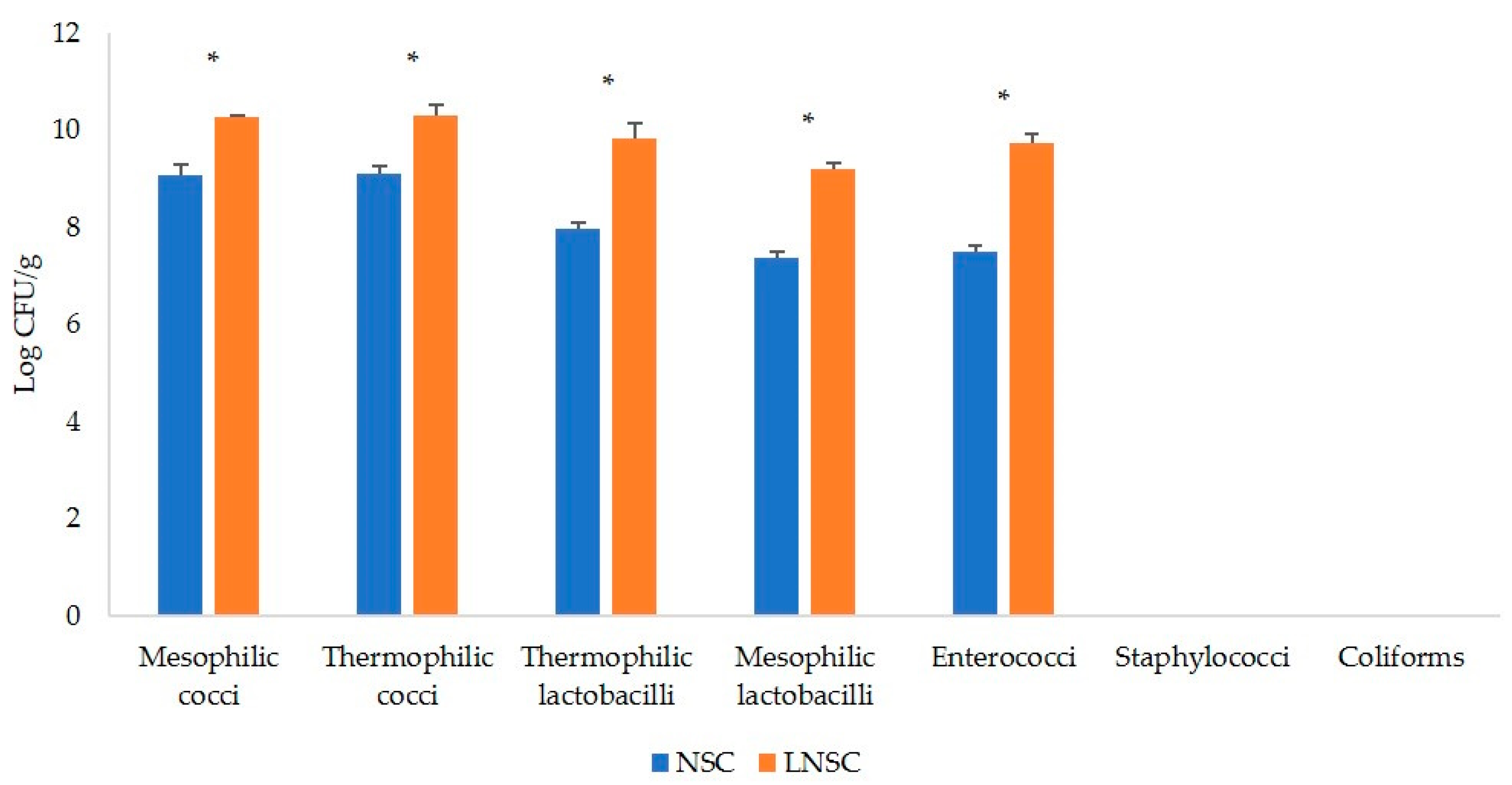
3.1. Microbial Counts of the Natural Starter Cultures
3.2. Biodiversity Evaluation
3.2.1. Biodiversity at Species Level
3.2.2. Biodiversity at Strain Level
3.3. Safety Assessment
3.3.1. Antibiotic Resistance and Virulence Genes Investigation
3.3.2. Minimum Inhibitory Concentration Determination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassi, D.; Puglisi, E.; Cocconcelli, P.S. Comparing natural and selected starter cultures in meat and cheese fermentations. Curr. Opin. Food Sci. 2015, 2, 118–122. [Google Scholar] [CrossRef]
- Powell, I.B.; Broome, M.C.; Limsowtin, G.K.Y. Cheese|Starter Cultures: General Aspects. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 552–558. [Google Scholar] [CrossRef]
- Durso, L.; Hutkins, R. Starter cultures. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5583–5593. [Google Scholar] [CrossRef]
- Chessa, L.; Paba, A.; Daga, E.; Dupré, I.; Piga, C.; Di Salvo, R.; Mura, M.; Addis, M.; Comunian, R. Autochthonous Natural Starter Cultures: A Chance to Preserve Biodiversity and Quality of Pecorino Romano PDO Cheese. Sustainability 2021, 13, 8214. [Google Scholar] [CrossRef]
- Parente, E.; Cogan, T.M.; Powell, I. Starter Cultures: General Aspects; Academic Press: Cambridge, MA, USA, 2017; pp. 201–226. [Google Scholar] [CrossRef]
- Abarquero, D.; Bodelón, R.; Manso, C.; Rivero, P.; Fresno, J.M.; Tornadijo, M.E. Effect of autochthonous starter and non-starter cultures on the physicochemical, microbiological and sensorial characteristics of Castellano cheese. Int. J. Dairy Technol. 2023. [Google Scholar] [CrossRef]
- European Food Safety Authority. The EFSA’s 2nd Scientific Colloquium Report—Qps. EFSA Support. Publ. 2005, 2, 109E. [Google Scholar] [CrossRef]
- EFSA Panel on Additives Products or Substances used in Animal Feed. Update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA J. 2023, 21, e07747. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 15: Suitability of taxonomic units notified to EFSA until September 2021. EFSA J. 2022, 20, 7045. [Google Scholar] [CrossRef]
- EFSA Panel on Additives Products or Substances used in Animal Feed. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [CrossRef]
- Chessa, L.; Paba, A.; Dupré, I.; Daga, E.; Fozzi, M.C.; Comunian, R. A Strategy for the Recovery of Raw Ewe’s Milk Microbiodiversity to Develop Natural Starter Cultures for Traditional Foods. Microorganisms 2023, 11, 823. [Google Scholar] [CrossRef]
- Di Cello, F.; Pepi, M.; Baldi, F.; Fani, R. Molecular characterization of an n-alkane-degrading bacterial community and identification of a new species, Acinetobacter venetianus. Res. Microbiol. 1997, 148, 237–249. [Google Scholar] [CrossRef]
- Poyart, C. Taxonomic dissection of the Streptococcus bovis group by analysis of manganese-dependent superoxide dismutase gene (sodA) sequences: Reclassification of ‘Streptococcus infantarius subsp. coli’ as Streptococcus lutetiensis sp. nov. and of Streptococcus bovis biotype II.2 as Streptococcus pasteurianus sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1247–1255. [Google Scholar] [CrossRef]
- Odamaki, T.; Yonezawa, S.; Kitahara, M.; Sugahara, Y.; Xiao, J.-Z.; Yaeshima, T.; Iwatsuki, K.; Ohkuma, M. Novel multiplex polymerase chain reaction primer set for identification of Lactococcus species. Lett. Appl. Microbiol. 2011, 52, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, P.; Vanoni, L.; Morandi, S.; Silvetti, T.; Castiglioni, B.; Brasca, M. Development of a pentaplex PCR assay for the simultaneous detection of Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, L. delbrueckii subsp. lactis, L. helveticus, L. fermentum in whey starter for Grana Padano cheese. Int. J. Food Microbiol. 2011, 146, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Chagnaud, P.; Machinis, K.; Coutte, L.c.A.; Marecat, A.; Mercenier, A. Rapid PCR-based procedure to identify lactic acid bacteria: Application to six common Lactobacillus species. J. Microbiol. Methods 2001, 44, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.J.; Timmins, M.J. Differentiation of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus by polymerase chain reaction. Lett. Appl. Microbiol. 1999, 29, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Picard, F.J.; Martineau, F.; Menard, C.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Development of a PCR assay for rapid detection of enterococci. J. Clin. Microbiol. 1999, 37, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B. Use of a Genus- and Species-Specific Multiplex PCR for Identification of Enterococci. J. Clin. Microbiol. 2004, 42, 3558–3565. [Google Scholar] [CrossRef]
- Chessa, L.; Paba, A.; Daga, E.; Comunian, R. Effect of growth media on natural starter culture composition and performance evaluated with a polyphasic approach. Int. J. Dairy Technol. 2019, 72, 152–158. [Google Scholar] [CrossRef]
- Graves, L.M.; Swaminathan, B. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int. J. Food Microbiol. 2001, 65, 55–62. [Google Scholar] [CrossRef]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef]
- Paba, A.; Chessa, L.; Cabizza, R.; Daga, E.; Urgeghe, P.P.; Testa, M.C.; Comunian, R. Zoom on starter lactic acid bacteria development into oxytetracycline spiked ovine milk during the early acidification phase. Int. Dairy J. 2019, 96, 15–20. [Google Scholar] [CrossRef]
- 20776-1:2019; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices. In Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- Clinical and Laboratory Standards Institute (CLSI). M100—Performance Standards for Antimicrobial Susceptibility Testing, Clinical and Laboratory Standards Institute, 30th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.0. 2023. Available online: http://www.eucast.org (accessed on 15 October 2023).
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.P.; Lau, A.F. An Update on the Streptococcus bovis Group: Classification, Identification, and Disease Associations. J. Clin. Microbiol. 2016, 54, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Hinse, D.; Vollmer, T.; Erhard, M.; Welker, M.; Moore, E.R.; Kleesiek, K.; Dreier, J. Differentiation of species of the Streptococcus bovis/equinus-complex by MALDI-TOF Mass Spectrometry in comparison to sodA sequence analyses. Syst. Appl. Microbiol. 2011, 34, 52–57. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives Products or Substances used in Animal Feed. Guidance on the safety assessment of Enterococcus faecium in animal nutrition. EFSA J. 2012, 10, 2682. [Google Scholar] [CrossRef]
- Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Jans, C.; Meile, L.; Kaindi, D.W.M.; Kogi-Makau, W.; Lamuka, P.; Renault, P.; Kreikemeyer, B.; Lacroix, C.; Hattendorf, J.; Zinsstag, J.; et al. African fermented dairy products—Overview of predominant technologically important microorganisms focusing on African Streptococcus infantarius variants and potential future applications for enhanced food safety and security. Int. J. Food Microbiol. 2017, 250, 27–36. [Google Scholar] [CrossRef]
- Ogier, J.-C.; Serror, P. Safety assessment of dairy microorganisms: The Enterococcus genus. Int. J. Food Microbiol. 2008, 126, 291–301. [Google Scholar] [CrossRef]
- Hazards, E. Panel o.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Update of the list of QPS-recommended microbiological agents intentionally added to food or feed as notified to EFSA 16: Suitability of taxonomic units notified to EFSA until March 2022. EFSA J. 2022, 20, e07408. [Google Scholar] [CrossRef]
- Chaffanel, F.; Charron-Bourgoin, F.; Libante, V.; Leblond-Bourget, N.; Payot, S. Resistance Genes and Genetic Elements Associated with Antibiotic Resistance in Clinical and Commensal Isolates of Streptococcus salivarius. Appl. Environ. Microbiol. 2015, 81, 4155–4163. [Google Scholar] [CrossRef]
- Joyce, L.R.; Youngblom, M.A.; Cormaty, H.; Gartstein, E.; Barber, K.E.; Akins, R.L.; Pepperell, C.S.; Palmer, K.L. Comparative Genomics of Streptococcus oralis Identifies Large Scale Homologous Recombination and a Genetic Variant Associated with Infection. mSphere 2022, 7, e00509-22. [Google Scholar] [CrossRef] [PubMed]
- Malhotra-Kumar, S.; Lammens, C.; Martel, A.; Mallentjer, C.; Chapelle, S.; Verhoeven, J.; Wijdooghe, M.; Haesebrouck, F.; Goossens, H. Oropharyngeal carriage of macrolide-resistant viridans group streptococci: A prevalence study among healthy adults in Belgium. J. Antimicrob. Chemother. 2004, 53, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Di Bonaventura, G.; Gherardi, G. An Overview on Streptococcus bovis/Streptococcus equinus Complex Isolates: Identification to the Species/Subspecies Level and Antibiotic Resistance. Int. J. Mol. Sci. 2019, 20, 480. [Google Scholar] [CrossRef] [PubMed]
- Blaiotta, G.; Sorrentino, A.; Ottombrino, A.; Aponte, M. Short communication: Technological and genotypic comparison between Streptococcus macedonicus and Streptococcus thermophilus strains coming from the same dairy environment. J. Dairy Sci. 2011, 94, 5871–5877. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Calasso, M. STREPTOCOCCUS|Introduction. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 535–553. [Google Scholar] [CrossRef]
- Chen, P.; Qiu, Y.; Liu, G.; Li, X.; Cheng, J.; Liu, K.; Qu, W.; Zhu, C.; Kastelic, J.P.; Han, B.; et al. Characterization of Streptococcus lutetiensis isolated from clinical mastitis of dairy cows. J. Dairy Sci. 2021, 104, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.T.; Shapiro, K.; Beneri, C.A.; Wilks-Gallo, L.S. Streptococcus lutetiensis neonatal meningitis with empyema. Access Microbiol. 2021, 3, 000264. [Google Scholar] [CrossRef]
- Fugl, A.; Berhe, T.; Kiran, A.; Hussain, S.; Laursen, M.F.; Bahl, M.I.; Hailu, Y.; Sørensen, K.I.; Guya, M.E.; Ipsen, R.; et al. Characterisation of lactic acid bacteria in spontaneously fermented camel milk and selection of strains for fermentation of camel milk. Int. Dairy J. 2017, 73, 19–24. [Google Scholar] [CrossRef]
- Baldeh, E.; Bah, S.A.; Camara, S.; Fye, B.L.; Nakamura, T. Bacterial diversity of Gambian traditional fermented milk, “Kosam”. Anim. Sci. J. 2022, 93, e13699. [Google Scholar] [CrossRef]
- Alexandraki, V.; Kazou, M.; Angelopoulou, A.; Arena, M.P.; Capozzi, V.; Russo, P.; Fiocco, D.; Spano, G.; Papadimitriou, K.; Tsakalidou, E. Chapter 6—The Microbiota of Non-cow Milk and Products. In Non-Bovine Milk and Milk Products; Tsakalidou, E., Papadimitriou, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 117–159. [Google Scholar] [CrossRef]
- Devirgiliis, C.; Barile, S.; Caravelli, A.; Coppola, D.; Perozzi, G. Identification of tetracycline- and erythromycin-resistant Gram-positive cocci within the fermenting microflora of an Italian dairy food product. J. Appl. Microbiol. 2010, 109, 313–323. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Tarrah, A.; Callegaro, S.; Pakroo, S.; Finocchiaro, R.; Giacomini, A.; Corich, V.; Cassandro, M. New insights into the raw milk microbiota diversity from animals with a different genetic predisposition for feed efficiency and resilience to mastitis. Sci. Rep. 2022, 12, 13498. [Google Scholar] [CrossRef] [PubMed]

| Genus/Species | Primer | Gene Target | Primer Sequence (5′–3′) | Annealing (°C) | Size (bp) | Reference |
|---|---|---|---|---|---|---|
| Lactococcus lactis | LcLspp-F | 16S rRNA | GTTGTATTAGCTAGTTGGTGAGGTAAA | 55 | 387 | [14] |
| Lc-R | GTTGAGCCACTGCCTTTTAC | |||||
| Lactobacillus delbrueckii lactis | Lac-LACTIS-F733 | dppE | TGCCAAGCTCTACTCCGTTT | 58 | 217 | [15] |
| Lac-LACTIS-R949 | GTCAAGCGGCATAGTGTCAA | |||||
| Lactobacillus delbrueckii bulgaricus | Lac-BULG-F391 | lacZ | GGAAGACTCCGTTTTGGTCA | 58 | 395 | [15] |
| Lac-BULG-R785 | AGTTCAAGTCTGCCCCATTG | |||||
| Lactobacillus helveticus | Lac-HELV-F73 | prtH | GGCGGGGAAAGAGGTAACTA | 58 | 509 | [15] |
| Lac-HELV-R581 | TGACGCAAACTTAATGAACCA | |||||
| Limosilactisbacillus fermentum | Lac-FER-F753 | ArcD | CCAGATCAGCCAACTTCACA | 58 | 310 | [15] |
| Lac-FER-R1062 | GGCAAACTTCAAGAGGACCA | |||||
| Limosilactobacillus reuteri | REUT1 | 16S rRNA | TGAATTGACGATGGATCACCAGTG | 65 | 1000 | [16] |
| LOWLAC | CGACGACCATGAACCACCTGT | |||||
| Lacticaseibacillus paracasei | Y2 | 16S rRNA | CCCACTGCTGCCTCCCGTAGGAGT | 55 | 290 | [17] |
| PARA | CACCGAGATTCAACATGG | |||||
| Lactiplantibacillus plantarum | planF | recA | CCGTTTATGCGGAACACCTA | 56 | 318 | [18] |
| pREV | TCGGGATTACCAAACATCAC | |||||
| Streptococcus thermophilus | Str-THER-F2116 | lacZ | GCTTGTGTTCTGAGGGAAGC | 58 | 577 | [15] |
| Str-THER-R2693 | CTTTCTTCTGCACCGTATCCA | |||||
| Enterococcus | ENT1 | Tuf | TACTGACAAACCATTCATGATG | 59 | 112 | [19] |
| ENT2 | AACTTCGTCACCAACGCGAAC | |||||
| Enterococcus faecium | FM1 | sodA | GAAAAAACAATAGAAGAATTAT | 55 | 215 | [20] |
| FM2 | TGCTTTTTTGAATTCTTCTTTA | |||||
| Enterococcus faecalis | FL1 | sodA | ACTTATGTGACTAACTTAACC | 55 | 360 | [20] |
| FL2 | TAATGGTGAATCTTGGTTTGG | |||||
| Universal | p27f | 16S rRNA | GAGAGTTTGATCCTGGCTCAG | 58 | ≈750 | [12] |
| p765r | CTGTTTGCTCCCCACGCTTTC | |||||
| Degenerate | d1 | sodA | CCITAYICITAYGAYGCIYTIGARCC | 37 | ≈480 | [13] |
| d2 | ARRTARTAIGCARRTARTAIGCRTGYTCCCAIACRTC |
| Species | NSC | LNSC | NSC + LNSC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isolates No. | rep-PCR Profiles No. | PFGE Profiles No. | Isolates No. | rep-PCR Profiles No. | PFGE Profiles No. | Isolates No. | rep-PCR Profiles No. | rep-PCR Profiles in Common | PFGE Profiles in Common | |
| Enterococcus durans | 0 | n.d. | n.d. | 25 | 8 | n.d. | 25 | 8 | n.d. | n.d. |
| Enterococcus faecium | 10 | 6 | n.d. | 45 | 9 | n.d. | 55 | 14 | 1 | n.d. |
| Enterococcus faecalis | 1 | 1 | n.d. | 2 | 1 | n.d. | 3 | 2 | 1 | n.d. |
| Lacticaseibacillus paracasei | 12 | 1 | n.d. | 23 | 5 | n.d. | 35 | 6 | n.d. | n.d. |
| Streptococcus gallolyticus subsp. macedonicus | 13 | 1 | n.d. | 4 | 2 | n.d. | 17 | 2 | 1 | n.d. |
| Streptococcus oralis | 1 | 1 | n.d. | 2 | 1 | n.d. | 3 | 2 | 0 | n.d. |
| Streptococcus salivarius | 1 | 1 | n.d. | 0 | n.d. | n.d. | 1 | 1 | 0 | n.d. |
| Streptococcus lutetiensis | 2 | 1 | 1 | 3 | 1 | 2 | 5 | 1 | 1 | 1 |
| Streptococcus equinus | 13 | 1 | 3 | 0 | n.d. | n.d. | 13 | 1 | 0 | 0 |
| Total isolates | 53 | 104 | 157 | |||||||
| Bacteria Tested | Culture | Antibiotics Tested | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Penicillins | Macrolides | Amphenicols | Oxazolidinones | Tetracyclines | Glycycyclines | Glycopeptides | Fluoroquinolones | |||
| AMP | ERY | CHL | LZD | TET | TGC | VAN | TEI | CIP | ||
| E. faecium | NSC | S 1 | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. |
| LNSC | S 1 | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | |
| E. faecalis | NSC | S 1 | I 2 | S 2 | S 1 | R 2 | S 1 | S 1 | S 1 | S 1 |
| LNSC | S 1 | I 2 | S 2 | S 1 | R 2 | S 1 | S 1 | S 1 | S 1 | |
| E. durans | NSC | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. |
| LNSC | S 1 | S 2 | S 2 | S 1 | S 2 | S 1 | S 1 | S 1 | S 1 | |
| Bacteria Tested | Culture | Antibiotics Tested | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillins | Macrolides | Cephalosporins | Amphenicols | Lincosamides | Carbapenems | Fluoroquinolones | Oxazolidinones | Tetracyclines | Glycopeptides | Streptogramins | |||||||
| AMP | PEN | AZI | ERY | FEP | FOT | AXO | CHL | CLI | EPT | MERO | LEVO | LZD | TET | VAN | SYN | ||
| S. gallolyticus macedonicus | NSC | n.t. | S 2 * | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | n.t. |
| LNSC | n.t. | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | n.t. | |
| S. equinus | NSC | n.t. | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | n.t. |
| LNSC | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | |
| S. lutetiensis | NSC | n.t. | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | n.t. |
| LNSC | n.t. | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | n.t. | |
| S. oralis | NSC | S 1 | S 2 | R 2 | R 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | I 2 |
| LNSC | n.t. | S 2 | R 2 | R 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | n.t. | |
| S. salivarius | NSC | S 1 | I 2 | R 2 | R 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 | S 2 |
| LNSC | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | abs. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chessa, L.; Daga, E.; Dupré, I.; Paba, A.; Fozzi, M.C.; Dedola, D.G.; Comunian, R. Biodiversity and Safety: Cohabitation Experimentation in Undefined Starter Cultures for Traditional Dairy Products. Fermentation 2024, 10, 29. https://doi.org/10.3390/fermentation10010029
Chessa L, Daga E, Dupré I, Paba A, Fozzi MC, Dedola DG, Comunian R. Biodiversity and Safety: Cohabitation Experimentation in Undefined Starter Cultures for Traditional Dairy Products. Fermentation. 2024; 10(1):29. https://doi.org/10.3390/fermentation10010029
Chicago/Turabian StyleChessa, Luigi, Elisabetta Daga, Ilaria Dupré, Antonio Paba, Maria C. Fozzi, Davide G. Dedola, and Roberta Comunian. 2024. "Biodiversity and Safety: Cohabitation Experimentation in Undefined Starter Cultures for Traditional Dairy Products" Fermentation 10, no. 1: 29. https://doi.org/10.3390/fermentation10010029





