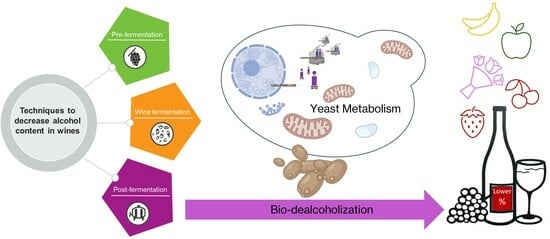
Bio-Dealcoholization of Wines: Can Yeast Make Lighter Wines?
Abstract
:1. Climate Change and Wine Quality
1.1. Impact of Climate Changes on Wine Sensory Perception
1.1.1. Influence of the Higher Alcohol Content
1.1.2. Imbalance Acidity and Perceived Sourness
1.1.3. Phenolic Compounds and Health-Promoting Compounds Deficiency
2. Techniques to Decrease Alcohol Content in Wines
2.1. Non-Microbial Alcohol Reduction in Wines
2.1.1. Reducing Fermentable Sugars in the Grapes
Juice Dilution
Juice Filtration with Membranes
Use of Enzymes
Viticultural Practices
2.1.2. Reduce or Limit Ethanol after Winemaking
Alcohol Removal via Extraction Methods
Alcohol Removal through Membrane-Based Processes
Alcohol Removal: Thermal Processes in Winemaking
Multi-Stage Membrane-Based Systems
2.2. Microbial Strategies for Producing Low-Alcohol Wines
2.2.1. GMO Microorganisms
2.2.2. Yeast Selection for Low Alcohol Production
2.2.3. Co-Inoculations and Sequential Inoculations (Non-Saccharomyces and S. cerevisiae)
2.2.4. Abiotic Factors Control during Fermentation
2.2.5. Wines Biological Dealcoholisation
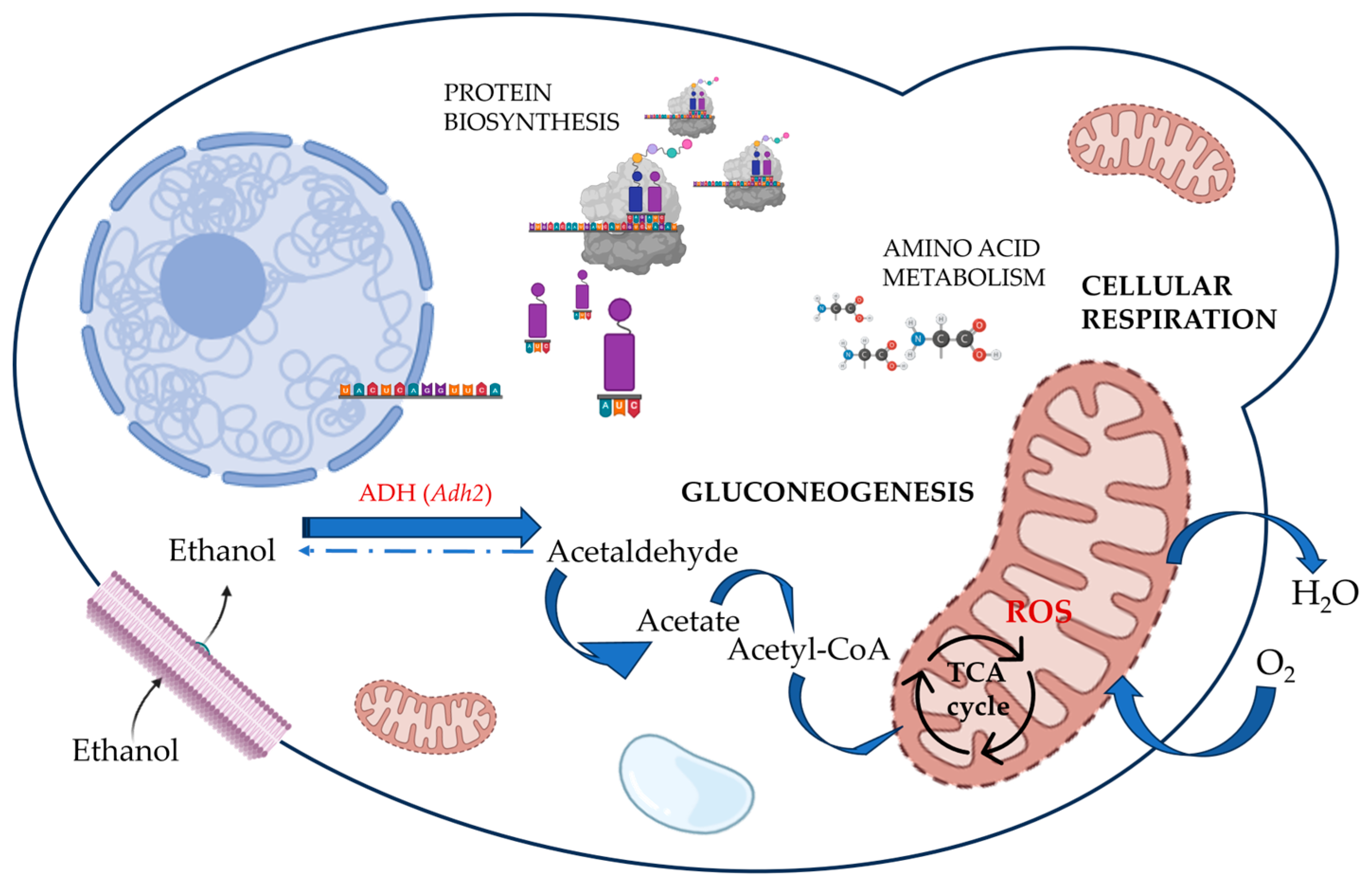
3. Consumers Perception and Behavior Related to Low-Alcohol Wine
4. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Climate Change 2021—The Physical Science Basis: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; pp. 3–32. [Google Scholar]
- Dinis, L.T.; Bernardo, S.; Yang, C.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Mediterranean viticulture in the context of climate change. Ciência Téc. Vitiv. 2022, 37, 139–158. [Google Scholar] [CrossRef]
- Adão, F.; Campos, J.C.; Santos, J.A.; Malheiro, A.C.; Fraga, H. Relocation of bioclimatic suitability of Portuguese grapevine varieties under climate change scenarios. Front. Plant Sci. 2023, 14, 974020. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Fraga, H.; Santos, J.A. Exposure of Portuguese viticulture to weather extremes under climate change. Clim. Serv. 2023, 30, 100357. [Google Scholar] [CrossRef]
- IPCC. Working Group II Contribution to the IPCC Sixth Assessment Report. In Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parlker, A.; de Reséguier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Arfini, F. Mediterranean agriculture facing climate change: Challenges and policies. Bio-Based Appl. Econ. 2021, 10, 87–88. [Google Scholar] [CrossRef]
- Lamonaca, E.; Santeramo, F.G.; Seccia, A. Climate changes and new productive dynamics in the global wine sector. Bio-Based Appl. Econ. 2021, 10, 123–135. [Google Scholar] [CrossRef]
- Deschênes, O.; Greenstone, M. The economic impacts of climate change: Evidence from agricultural output and random fluctuations in weather. Am. Econ. Rev. 2007, 97, 354–385. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Fraga, H. Viticulture and winemaking under climate change. Agronomy 2019, 9, 783. [Google Scholar] [CrossRef]
- State of the World Vine and Wine Sector in 2022. Available online: https://www.oiv.int/sites/default/files/documents/OIV_State_of_the_world_Vine_and_Wine_sector_in_2022_2 (accessed on 1 November 2023).
- Macedo, A.; Gouveia, S.; Rebelo, J.; Santos, J.; Fraga, H. International trade, non-tariff measures and climate change: Insights from Port wine exports. J. Econ. Stud. 2021, 48, 1228–1243. [Google Scholar] [CrossRef]
- Bai, H.; Gambetta, G.A.; Wang, Y.; Kong, J.; Long, Q.; Fan, P.; Duan, W.; Liang, Z.; Dai, Z. Historical long-term cultivar× climate suitability data to inform viticultural adaptation to climate change. Sci. Data 2022, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.K.; de Cortázar-Atauri, I.G.; Gény, L.; Spring, J.L.; Destrac, A.; Schultz, H.; Van Leeuwen, C. Temperature-based grapevine sugar ripeness modelling for a wide range of Vitis vinifera L. cultivars. Agric. For. Meteorol. 2020, 285, 107902. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Friant, P.; Chone, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar] [CrossRef]
- Jones, G.V.; Davis, R.E. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar] [CrossRef]
- De Orduna, R.M. Climate change associated effects on grape and wine quality and production. Int. Food Res. J. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Clemente, N.; Santos, J.A.; Fontes, N.; Graça, A.; Gonçalves, I.; Fraga, H. Grapevine Sugar Concentration Model (GSCM): A decision support tool for the douro superior winemaking region. Agronomy 2022, 12, 1404. [Google Scholar] [CrossRef]
- Gutierrez-Gamboa, G.; Perez-Alvarez, E.P.; Rubio-Breton, P.; Garde-Cerdan, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.) grapevines. Sci. Hortic. Amst. 2019, 244, 257–262. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Schultz, H.R. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Saliba, A.J.; Ovington, L.A.; Moran, C.C. Consumer demand for low-alcohol wine in an Australian sample. Int. J. Wine Res. 2013, 5, 1–8. [Google Scholar] [CrossRef]
- Jordão, A.M.; Vilela, A.; Cosme, F. From sugar of grape to alcohol of wine: Sensorial impact of alcohol in wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Changes in volatile composition and sensory attributes of wines during alcohol content reduction. J. Sci. Food Agric. 2017, 97, 8–16. [Google Scholar] [CrossRef]
- Teslić, N.; Zinzani, G.; Parpinello, G.P.; Versari, A. Climate change trends, grape production, and potential alcohol concentration in wine from the “Romagna Sangiovese” appellation area (Italy). Theor. Appl. Climatol. 2018, 131, 793–803. [Google Scholar] [CrossRef]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- King, E.S.; Dunn, R.L.; Heymann, H. The influence of alcohol on the sensory perception of red wines. Food Qual. Prefer. 2013, 28, 235–243. [Google Scholar] [CrossRef]
- Eliodório, K.P.; Cunha, G.C.D.G.; Müller, C.; Lucaroni, A.C.; Giudici, R.; Walker, G.M.; Alves, S.L., Jr.; Basso, T.O. Chapter Three—Advances in yeast alcoholic fermentations for the production of bioethanol, beer and wine. In Advances in Applied Microbiology; Geoffrey, M.G., Sima, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 109, pp. 61–119. ISBN 9780128176221. ISSN 0065-2164. [Google Scholar] [CrossRef]
- Angus, C.; Holmes, J.; Meier, P.S. Comparing alcohol taxation throughout the European Union. Addiction 2019, 114, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Neufeld, M.; Room, R.; Sornpaisarn, B.; Štelemėkas, M.; Swahn, M.H.; Lachenmeier, D.W. The impact of alcohol taxation changes on unrecorded alcohol consumption: A review and recommendations. Int. J. Drug Policy 2022, 99, 103420. [Google Scholar] [CrossRef]
- IWSR 2023. Key Statistics: The No-Alcohol and Low-Alcohol Market. Available online: https://www.theiwsr.com/key-statistics-the-no-alcohol-and-low-alcohol-market/ (accessed on 1 November 2023).
- Agra CEAS Consulting, S.A.; Areté, s.r.l. Directorate-General for Agriculture and Rural Development (European Commission). In Study on Low/No Alcohol Beverages; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Fraga, H.; Molitor, D.; Leolini, L.; Santos, J.A. What is the impact of heatwaves on European viticulture? A modelling assessment. Appl. Sci. 2020, 10, 3030. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Castellarin, S.D. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario–A review. Front. Plant Sci. 2021, 12, 262. [Google Scholar] [CrossRef]
- Brillante, L.; Martínez-Luscher, J.; Yu, R.; Plank, C.M.; Sanchez, L.; Bates, T.L.; Brenneman, C.; Oberholster, A.; Kurtural, S.K.K. Assessing spatial variability of grape skin flavonoids at the vineyard scale based on plant water status mapping. J. Agric. Food Chem. 2017, 65, 5255–5265. [Google Scholar] [CrossRef]
- Petoumenou, D.G.; Biniari, K.; Xyrafis, E.; Mavronasios, D.; Daskalakis, I.; Palliotti, A. Effects of natural hail on the growth, physiological characteristics, yield, and quality of Vitis vinifera L. cv. Thompson Seedless under Mediterranean growing conditions. Agronomy 2019, 9, 197. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Mitigating heat wave and exposure damage to “Cabernet-Sauvignon” wine grape with partial shading under two irrigation mounts. Front. Plant Sci. 2020, 11, 579192. [Google Scholar] [CrossRef] [PubMed]
- Bigard, A.; Berhe, D.T.; Maoddi, E.; Sire, Y.; Boursiquot, J.M.; Ojeda, H.; Péros, J.P.; Doligez, A.; Romieu, C.; Torregrosa, L. Vitis vinifera L. fruit diversity to breed varieties anticipating climate changes. Front. Plant Sci. 2018, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Ausseil, A.G.E.; Law, R.M.; Parker, A.K.; Teixeira, E.I.; Sood, A. Projected wine grape cultivar shifts due to climate change in New Zealand. Front. Plant Sci. 2021, 12, 618039. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate changes and food quality: The potential of microbial activities as mitigating strategies in the wine sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Future scenarios for viticultural zoning in Europe: Ensemble projections and uncertainties. Int. J. Biometeorol. 2013, 57, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Fujishima, H.; Chijiwa, H. Evaluation of table grape genetic resources for sugar, organic acid, and amino acid composition of berries. Euphytica 2010, 174, 1–13. [Google Scholar] [CrossRef]
- Dai, Z.W.; Ollat, N.; Gomès, E.; Decroocq, S.; Tandonnet, J.-P.; Bordenave, L.; Pieri, P.; Hilbert, G.; Kappel, C.; van Leeuwen, C.; et al. Ecophysiological, genetic, and molecular causes of variation in grape berry weight and composition: A review. Am. J. Enol. Vitic. 2011, 62, 413–425. [Google Scholar] [CrossRef]
- Bock, A.; Sparks, T.H.; Estrella, N.; Menzel, A. Climate-induced changes in grapevine yield and must sugar content in Franconia (Germany) between 1805 and 2010. PLoS ONE 2013, 8, e69015. [Google Scholar] [CrossRef]
- Alston, J.M.; Fuller, K.B.; Lapsley, J.T.; Soleas, G. Too much of a good thing? Causes and consequences of increases in sugar content of California wine grapes. J. Wine Econ. 2011, 6, 135–159. [Google Scholar] [CrossRef]
- Navrátilová, M.; Beranová, M.; Severová, L.; Šrédl, K.; Svoboda, R.; Abrhám, J. The impact of climate change on the sugar content of grapes and the sustainability of their production in the Czech Republic. Sustainability 2020, 13, 222. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Kurtural, S.K. Global warming and wine quality: Are we close to the tipping point? Oeno One 2021, 55, 353–361. [Google Scholar] [CrossRef]
- Duchêne, E.; Schneider, C. Grapevine, and climatic changes: A glance at the situation in Alsace. Agron. Sustain. Dev. 2005, 24, 93–99. [Google Scholar] [CrossRef]
- Godden, P.; Wilkes, E.; Johnson, D. Trends in the composition of Australian wine 1984–2014: Composition of Australian wine 1984–2014. Aust. J. Grape Wine Res. 2015, 21, 741–753. [Google Scholar] [CrossRef]
- Suter, B.; Destrac Irvine, A.; Gowdy, M.; Dai, Z.; van Leeuwen, C. Adapting wine grape ripening to global change requires a multi-trait approach. Front. Plant Sci. 2021, 12, 624867. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, S.Y.; Greer, D.H.; Liu, Y.; Baby, T.; Xiao, Z. Impact of climate change on grape berry ripening: An assessment of adaptation strategies for the Australian vineyard. Front. Plant Sci. 2022, 13, 1094633. [Google Scholar] [CrossRef] [PubMed]
- Petrie, P.R.; Sadras, V.O. Advancement of grapevine maturity in Australia between 1993 and 2006: Putative causes, magnitude of trends and viticultural consequences. Aust. J. Grape Wine Res. 2008, 14, 33–45. [Google Scholar] [CrossRef]
- Sherman, E.; Greenwood, D.R.; Villas-Boâs, S.G.; Heymann, H.; Harbertson, J.F. Impact of grape maturity and ethanol concentration on sensory properties of Washington State Merlot wines. Am. J. Enol. Vitic. 2017, 68, 344–356. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J. Current research related to wine sensory perception since 2010. Beverages 2020, 6, 47. [Google Scholar] [CrossRef]
- Pham, D.-T.; Stockdale, V.J.; Jeffery, D.W.; Tuke, J.; Wilkinson, K.L. Investigating Alcohol Sweet spot Phenomena in Reduced Alcohol Red Wines. Foods 2019, 8, 491. [Google Scholar] [CrossRef]
- Conibear, H. Rising alcohol levels in wine—Is this a cause for concern. AIM Dig. 2006, 18, 1–3. [Google Scholar]
- Antalick, G.; Suklje, K.; Blackman, J.; Schmidtke, L.; Deloire, A. Performing sequential harvests based on berry sugar accumulation (mg/berry) to obtain specific wine sensory profiles. Oeno One 2021, 55, 131–146. [Google Scholar] [CrossRef]
- Shepherd, H.; Parr, W.V.; Monaco, G.L.; Rodrigues, H. The meaning of the word elegance as a wine descriptor: Effect of expertise and wine type. Food Res. Int. 2023, 164, 112399. [Google Scholar] [CrossRef] [PubMed]
- Sokolowsky, M.; Fischer, U. Evaluation of bitterness in white wine applying descriptive analysis, time-intensity analysis, and temporal dominance of sensations analysis. Anal. Chim. Acta 2012, 732, 46–52. [Google Scholar] [CrossRef]
- King, E.S.; Heymann, H. The effect of reduced alcohol on the sensory profiles and consumer preferences of white wine. J. Sens. Stud. 2014, 29, 33–42. [Google Scholar] [CrossRef]
- Ofoedu, C.E.; Ofoedu, E.O.; Chacha, J.S.; Owuamanam, C.I.; Efekalam, I.S.; Awuchi, C.G. Comparative evaluation of physicochemical, antioxidant, and sensory properties of red wine as markers of its quality and authenticity. Int. J. Food Sci. 2022, 2022, 8368992. [Google Scholar] [CrossRef]
- Goldner, M.C.; Zamora, M.C.; di Leo Lira, P.; Gianninoto, H.; Bandoni, A. Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J. Sens. Stud. 2009, 24, 243–257. [Google Scholar] [CrossRef]
- Scinska, A.; Koros, E.; Habrat, B.; Kukwa, A.; Kostowski, W.; Bienkowski, P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol. Depend. 2000, 60, 199–206. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odour families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Meillon, S.; Dugas, V.; Urbano, C.; Schlich, P. Preference and acceptability of partially dealcoholized white and red wines by consumers and professionals. Am. J. Enol. Vitic. 2010, 61, 42–52. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between polyphenols and volatile compounds in wine: A literature review on physicochemical and sensory insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Leolini, L.; Moriondo, M.; Romboli, Y.; Gardiman, M.; Costafreda-Aumedes, S.; de Cortazar-Atauri, I.G.; Bindi, M.; Granchi, L.; Brilli, L. Modelling sugar and acid content in Sangiovese grapes under future climates: An Italian case study. Clim. Res. 2019, 78, 211–224. [Google Scholar] [CrossRef]
- Vicente, J.; Baran, Y.; Navascués, E.; Santos, A.; Calderón, F.; Marquina, D.; Rauhut, D.; Benito, S. Biological management of acidity in wine industry: A review. Int. J. Food Microbiol. 2022, 375, 109726. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A. Use of nonconventional yeasts for modulating wine acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef]
- Villette, J.; Cuéllar, T.; Verdeil, J.L.; Delrot, S.; Gaillard, I. Grapevine potassium nutrition and fruit quality in the context of climate change. Front. Plant Sci. 2020, 11, 123. [Google Scholar] [CrossRef]
- Famiani, F.; Farinelli, D.; Frioni, T.; Palliotti, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Malate as substrate for catabolism and gluconeogenesis during ripening in the pericarp of different grape cultivars. Biol. Plant 2016, 60, 155–162. [Google Scholar] [CrossRef]
- Ganichot, B. Evolution of harvesting dates in meridional Rhône. In Proceedings of 6th Rencontres Rhodaiennes; Institut Rhodanien: Orange, France, 2002; pp. 38–41. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, E.R. Principles and Practices of Winemaking; Chapman & Hall: New York, NY, USA, 1996; pp. 146–150. [Google Scholar] [CrossRef]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Santos-Sánchez, F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic acid pathway in biosynthesis of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., Ed.; Intechopen: London, UK, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- An, X.; Liang, Y.; Zhu, S.; Mu, L.; Yin, H. Changes of health-promoting bioactive compounds and related enzymes of ‘Hutai No. 8’grape (Vitis vinifera L.) in response to deficit irrigation. J. Hortic. Sci. Biotechnol. 2021, 96, 494–507. [Google Scholar] [CrossRef]
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between wine composition and temperature: Impact on Bordeaux wine typicity in the context of global warming. Crit. Rev. Food Sci. Nutr. 2019, 59, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.-T. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]
- Fournand, D.; Vicens, A.; Sidhoum, L.; Souquet, J.M.; Moutounet, M.; Cheynier, V. Accumulation and extractability of grape skin tannins and anthocyanins at different advanced physiological stages. J. Agric. Food Chem. 2006, 54, 7331–7338. [Google Scholar] [CrossRef] [PubMed]
- Keller, M. Managing grapevines to optimise fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Bonada, M.; Jeffery, D.W.; Petrie, P.R.; Moran, M.A.; Sadras, V.O. Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines. Aust. J. Grape Wine Res. 2015, 21, 240–253. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Pieri, P.; Charon, J.; Pillet, J.; Hilbert, G.; Renaud, C.; Gomè, E.; Delrot, S.; Lecourieux, D. Dissecting the biochemical and transcriptomic effects of a locally applied heat treatment on developing cabernet sauvignon grape berries. Front. Plant Sci. 2017, 8, 53. [Google Scholar] [CrossRef]
- Arias, L.A.; Berli, F.; Fontana, A.; Bottini, R.; Piccoli, P. Climate change effects on grapevine physiology and biochemistry: Benefits and challenges of high altitude as an adaptation strategy. Front. Plant Sci. 2022, 13, 835425. [Google Scholar] [CrossRef]
- Jeffery, D.W. Spotlight on varietal thiols and precursors in grapes and wines. Aust. J. Chem. 2016, 69, 1323–1330. [Google Scholar] [CrossRef]
- Kwasniewski, M.T.; Vanden Heuvel, J.E.; Pan, B.S.; Sacks, G.L. Timing of cluster light environment manipulation during grape development affects C13 norisoprenoid and carotenoid concentrations in Riesling. Trends Food Sci. 2010, 58, 6841–6849. [Google Scholar] [CrossRef]
- Artero, A.; Artero, A.; Tarín, J.J.; Cano, A. The impact of moderate wine consumption on health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Conesa, M.R.; Falagán, N.; de la Rosa, J.M.; Aguayo, E.; Domingo, R.; Pastor, A.P. Post-veraison deficit irrigation regimes enhance berry coloration and health-promoting bioactive compounds in ‘Crimson Seedless’ table grapes. Agric. Water Managt. 2016, 163, 9–18. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Petsini, F.; Choleva, M.; Detopoulou, M.S.; Arvaniti, O.; Kallinikou, E.; Sakantani, E.; Tsolou, A.; Nomikos, T.; Samaras, Y. Evaluation of anti-inflammatory, anti-platelet and anti-oxidant activity of wine extracts prepared from ten different grape varieties. Molecules 2020, 25, 5054. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Guo, H.; Fang, Y.; Zhou, G. The mechanisms of wine phenolic compounds for preclinical anticancer therapeutics. Food Nutr. Res. 2021, 65, 6507. [Google Scholar] [CrossRef] [PubMed]
- Balanov, P.E.; Smotraeva, I.V.; Abdullaeva, M.S.; Volkova, D.A.; Ivanchenko, O.B. Study on resveratrol content in grapes and wine products. In Proceedings of the E3S Web of Conferences, Kryvyi Rih, Ukraine, 19–21 May 2021; EDP Sciences: Lejulis, France, 2021; Volume 247, p. 01063. [Google Scholar]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L.) byproducts. J. Food Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Xynas, B.; Barnes, C. Yeast or water: Producing wine with lower alcohol levels in a warming climate: A review. J. Sci. Food Agric. 2023, 103, 3249–3260. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Dry, P.R.; Kutyna, D.R.; Francis, I.L.; Henschke, P.A.; Curtin, C.D.; Chambers, P.J. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 2015, 21, 670–679. [Google Scholar] [CrossRef]
- Varela, J.; Varela, C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019, 56, 88–96. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.Z.; Salifu, R.; Wang, J.; Jiang, Y.M.; Zhang, B.; Han, S.Y. Techniques for dealcoholization of wines: Their impact on wine phenolic composition, volatile composition, and sensory characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef]
- Ma, T.Z.; Sam, F.E.; Zhang, B. Low-Alcohol and nonalcoholic wines: Production methods, compositional changes, and aroma improvement. In Recent Advances in Grapes and Wine Production-New Perspectives for Quality Improvement; IntechOpen: London, UK, 2022. [Google Scholar]
- Schelezki, O.J.; Deloire, A.; Jeffery, D.W. Substitution or dilution? Assessing Pre-fermentative water implementation to produce lower alcohol shiraz wines. Molecules 2020, 25, 2245. [Google Scholar] [CrossRef]
- Filimon, V.R.; Filimon, R.; Nechita, A.; Bora, F.D.; Cotea, V.V. Compositional characteristics of low-alcohol wines obtained by staggered grape harvesting technology. Lucr. Ştiinţifice 2021, 64, 69–74. [Google Scholar]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.-M.; Zamora, F. Use of Unripe Grapes Harvested during Cluster Thinning as a Method for Reducing Alcohol Content and pH of Wine. Aust. J. Grape Wine Res. 2011, 17, 230–238. [Google Scholar] [CrossRef]
- Salgado, C.M.; Fernández-Fernández, E.; Palacio, L.; Hernández, A.; Prádanos, P. Alcohol reduction in red and white wines by nanofiltration of musts before fermentation. Food Bioprocess Proc. 2015, 96, 285–295. [Google Scholar] [CrossRef]
- Mira, H.; De Pinho, M.N.; Guiomar, A.; Geraldes, V. Membrane processing of grape must for control of the alcohol content in fermented beverages. J. Membr. Sci. Res. 2017, 3, 308–312. [Google Scholar] [CrossRef]
- Mangas, R.; González, M.R.; Martín, P.; Rodríguez-Nogales, J.M. Impact of glucose oxidase treatment in high sugar and pH musts on volatile composition of white wines. LWT 2023, 184, 114975. [Google Scholar] [CrossRef]
- de Toda, F.M.; Balda, P. Decreasing the alcohol level in quality red wines by the “double harvest” technique. In Proceedings of the 17th International Symposium Giesco, Asti, Italy, 29 August–2 September 2011. [Google Scholar]
- Piccardo, D.; Favre, G.; Pascual, O.; Canals, J.; Zamora, F.; González-Neves, G. Influence of the use of unripe grapes to reduce ethanol content and pH on the color, polyphenol and polysaccharide composition of conventional and hot macerated Pinot Noir and Tannat wines. Eur. Food Res. Technol. 2019, 245, 1321–1335. [Google Scholar] [CrossRef]
- Böttcher, C.; Harvey, K.; Forde, C.G.; Boss, P.K.; Davies, C. Auxin treatment of pre-veraison grape (Vitis Vinifera L.) berries both delays ripening and increases the synchronicity of sugar accumulation. Aust. J. Grape Wine Res. 2011, 17, 1–8. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, X.; Needs, S.; Liu, D.; Fuentes, S.; Howell, K. The influence of apical and basal defoliation on the canopy structure and biochemical composition of Vitis vinifera cv. Shiraz grapes and wine. Front. Chem. 2017, 5, 48. [Google Scholar] [CrossRef]
- Caccavello, G.; Giaccone, M.; Scognamiglio, P.; Forlani, M.; Basile, B. Influence of intensity of post-veraison defoliation or shoot trimming on vine physiology, yield components, berry and wine composition in Aglianico grapevines. Aust. J. Grape Wine Res. 2016, 22, 226–239. [Google Scholar] [CrossRef]
- Teng, B.; Petrie, P.R.; Smith, P.A.; Bindon, K.A. Comparison of water addition and early-harvest strategies to decrease alcohol concentration in Vitis vinifera cv. Shiraz wine: Impact on wine phenolics, tannin composition and colour properties. Aust. J. Grape Wine Res. 2020, 26, 158–171. [Google Scholar] [CrossRef]
- Cassano, A.; Mecchia, A.; Drioli, E. Analyses of hydrodynamic resistances and operating parameters in the ultrafiltration of grape must. J. Food Eng. 2008, 89, 171–177. [Google Scholar] [CrossRef]
- Filimon, R.V.; Nechita, B.; Bora, F.D.; Nechita, A.; Filimon, R. Analysis of volatile compounds in low alcoholic wines obtained by reverse osmosis of grape must. Adv. Agric. Bot. 2021, 13, 1–7. [Google Scholar]
- Ivić, I.; Kopjar, M.; Jukić, V.; Bošnjak, M.; Maglica, M.; Mesić, J.; Pichler, A. Aroma profile and chemical composition of reverse osmosis and nanofiltration concentrates of red wine Cabernet Sauvignon. Molecules 2021, 26, 874. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.H.; Vakili, O.; Ahmadi, N.; Soltani Fard, E.; Mousavi, P.; Khalvati, B.; Maleksabet, A.; Savardashtaki, A.; Taheri-Anganeh, M.; Movahedpour, A. Glucose oxidase: Applications, sources, and recombinant production. Biotechnol. Appl. Biochem. 2022, 69, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The use of glucose oxidase and catalase for the enzymatic reduction of the potential ethanol content in wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G. The Production of Reduced-Alcohol Wine Using Glucose Oxidase. Ph.D. Thesis, Lincoln University, Lincoln, UK, 1997. [Google Scholar]
- Petkova, V.; Mladenoska, I.; Dimitrovski, D.; Stafilov, T.; Stefova, M. Pre-fermentative treatment of grape juice and must from vranec variety with a glucose oxidase from Aspergillus niger. In Experimental and Computational Investigations in Engineering: Proceedings of the International Conference of Experimental and Numerical Investigations and New Technologies, CNNTech 2020; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 83–90. [Google Scholar]
- Asproudi, A.; Ferrandino, A.; Bonello, F.; Vaudano, E.; Pollon, M.; Petrozziello, M. Key norisoprenoid compounds in wines from early-harvested grapes in view of climate change. Food Chem. 2018, 268, 143–152. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Harvesting and blending options for lower alcohol wines: A sensory and chemical investigation. J. Sci. Food Agric. 2018, 98, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Volatile and sensory profiling of Shiraz wine in response to alcohol management: Comparison of harvest timing versus technological approaches. Food Res. Int. 2018, 109, 561–571. [Google Scholar] [CrossRef]
- Palliotti, A.; Poni, S.; Berrios, J.G.; Bernizzoni, F. Vine Performance and Grape Composition as Affected by Early-Season Source Limitation Induced with Anti-Transpirants in Two Red Vitis vinifera L. Cultivars. Aust. J. Grape Wine Res. 2010, 16, 426–433. [Google Scholar] [CrossRef]
- Filimon, V.R.; Tudose Sandu Ville, Ș.; Bora, F.D.; Tudor, G.; Filimon, R.; Nechita, A.; Damian, D. Methods for Producing Low-Alcohol Wine I. Viticultural and Pre-Fermentation Strategies. 2020. Available online: https://www.uaiasi.ro/revista_horti/files/Nr1_2020/vol%2063_1_2020%20(15).pdf (accessed on 1 November 2023).
- Tyagi, K.; Maoz, I.; Lapidot, O.; Kochanek, B.; Butnaro, Y.; Shlisel, M.; Lerno, L.; Ebeler, S.; Lichter, A. Effects of gibberellin and cytokinin on phenolic and volatile composition of Sangiovese grapes. Sci. Hortic. 2022, 295, 110860. [Google Scholar] [CrossRef]
- Ozturk, B.; Anli, E. Different techniques for reducing alcohol levels in wine: A review. In Proceedings of the BIO Web of Conferences, Mendoza, Argentina, 9–14 November 2014; EDP Sciences: Lejulis, France, 2014; Volume 3, p. 02012. [Google Scholar]
- Poni, S.; Gatti, M.; Bernizzoni, F.; Civardi, S.; Bobeica, N.; Magnanini, E.; Palliotti, A. Late leaf removal aimed at delaying ripening in cv. S angiovese: Physiological assessment and vine performance. Aust. J. Grape Wine Res. 2013, 19, 378–387. [Google Scholar] [CrossRef]
- Palliotti, A.; Cini, R.; Silvestroni, O.; Leoni, F.; Poni, S. Effects of late mechanized leaf removal above the clusters zone to delay grape ripening in ‘Sangiovese’ vines. In Proceedings of the I International Workshop on Vineyard Mechanization and Grape and Wine Quality 978, Piacenza, Italy, 27–29 June 2012; pp. 301–307. [Google Scholar]
- Parker, A.K.; Hofmann, R.W.; Van Leeuwen, C.; McLachlan, A.R.; Trought, M.C. Manipulating the leaf area to fruit mass ratio alters the synchrony of total soluble solids accumulation and titratable acidity of grape berries. Aust. J. Grape Wine Res. 2015, 21, 266–276. [Google Scholar] [CrossRef]
- Cincotta, F.; Verzera, A.; Prestia, O.; Tripodi, G.; Lechhab, W.; Sparacio, A.; Condurso, C. Influence of leaf removal on grape, wine and aroma compounds of Vitis vinifera L. cv. Merlot under Mediterranean climate. Eur. Food Res. Technol. 2022, 248, 403–413. [Google Scholar] [CrossRef]
- Sun, Q.; Sacks, G.L.; Lerch, S.D.; Heuvel, J.E.V. Impact of shoot and cluster thinning on yield, fruit composition, and wine quality of Corot noir. Am. J. Enol. Vitic. 2012, 63, 49–56. [Google Scholar] [CrossRef]
- Torres, N.; Martínez-Lüscher, J.; Porte, E.; Yu, R.; Kurtural, S.K. Impacts of leaf removal and shoot thinning on cumulative daily light intensity and thermal time and their cascading effects of grapevine (Vitis vinifera L.) berry and wine chemistry in warm climates. Food Chem. 2021, 343, 128447. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sacks, G.; Lerch, S.; Vanden-Heuvel, J.E. Impact of shoot thinning and harvest date on yield components, fruit composition, and wine quality of Marechal Foch. Am. J. Enol. Vitic. 2011, 62, 32–41. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, A.; Fornari, T.; Jaime, L.; Vázquez, E.; Amador, B.; Nieto, J.A.; Reglero, G. Supercritical CO2 extraction applied toward the production of a functional beverage from wine. J. Supercrit. Fluids 2012, 61, 92–100. [Google Scholar] [CrossRef]
- Catarino, M.; Mendes, A. Dealcoholizing wine by membrane separation processes. Innov. Food Sci. Emerg. Technol. 2011, 12, 330–337. [Google Scholar] [CrossRef]
- Meillon, S.; Viala, D.; Medel, M.; Urbano, C.; Guillot, G.; Schlich, P. Impact of partial alcohol reduction in Syrah wine on perceived complexity and temporality of sensations and link with preference. Food Qual. Pref. 2010, 21, 732–740. [Google Scholar] [CrossRef]
- Gonçalves, F.; Ribeiro, R.; Neves, L.; Lemperle, T.; Lança, M.; Ricardo da Silva, J.; Laureano, O. Alcohol reduction in wine by nanofiltration. Some comparisons with reverse osmosis technique. In Proceedings of the 1st Oenoviti International Symposium—Alcohol Level Reduction in Wine, Bordeaux, France, 6 September 2013; VIGNE et vin Publications Internationales: Bordeaux, France; pp. 64–67. [Google Scholar]
- Corona, O.; Liguori, L.; Albanese, D.; Di Matteo, M.; Cinquanta, L.; Russo, P. Quality and volatile compounds in red wine at different degrees of dealcoholization by membrane process. Eur. Food Res. Technol. 2019, 245, 2601–2611. [Google Scholar] [CrossRef]
- Liguori, L.; Russo, P.; Albanese, D.; Di Matteo, M. Evolution of quality parameters during red wine dealcoholization by osmotic distillation. Food Chem. 2013, 140, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Liguori, L.; Albanese, D.; Crescitelli, A.; Di Matteo, M.; Russo, P. Impact of dealcoholization on quality properties in white wine at various alcohol content levels. J. Food Sci. Technol. 2019, 56, 3707–3720. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dang, G.; Ding, X.; Shen, C.; Liu, G.; Zuo, C.; Chen, X.; Xing, W.; Jin, W. Production of alcohol-free wine and grape spirit by pervaporation membrane technology. Food Bioprod. Process. 2020, 123, 262–273. [Google Scholar] [CrossRef]
- Salgado, C.M.; Fernández-Fernández, E.; Palacio, L.; Carmona, F.J.; Hernández, A.; Prádanos, P. Application of pervaporation and nanofiltration membrane processes for the elaboration of full flavored low alcohol white wines. Food Bioprod. Process. 2017, 101, 11–21. [Google Scholar] [CrossRef]
- Pham, D.T.; Ristic, R.; Stockdale, V.J.; Jeffery, D.W.; Tuke, J.; Wilkinson, K. Influence of partial dealcoholization on the composition and sensory properties of Cabernet Sauvignon wines. Food Chem. 2020, 325, 126869. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Petrozziello, M.; Ciambotti, A.; Panero, L.; Solomita, M.; Bosso, A. Comparison of the physicochemical and volatile composition of wine fractions obtained by two different dealcoholization techniques. Food Chem. 2017, 221, 1–10. [Google Scholar] [CrossRef]
- Puglisi, C.; Ristic, R.; Saint, J.; Wilkinson, K. Evaluation of Spinning Cone Column Distillation as a Strategy for Remediation of Smoke Taint in Juice and Wine. Molecules 2022, 27, 8096. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical carbon dioxide applications in food processing. Food Eng. Rev. 2021, 13, 570–591. [Google Scholar] [CrossRef]
- Raventós, M.; Duarte, S.; Alarcón, R. Application and possibilities of supercritical CO2 extraction in food processing industry: An overview. Food Sci. Technol. Int. 2002, 8, 269–284. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Winemaking using immobilized kefir cells on natural zeolites. LWT 2020, 133, 110043. [Google Scholar] [CrossRef]
- Akyereko, Y.G.; Wireko-Manu, F.D.; Alemawor, F.; Adzanyo, M. Effects of production methods on flavour characteristics of nonalcoholic wine. J. Food Qual. 2021, 2021, 3014793. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. A comprehensive review of membrane distillation and osmotic distillation in agro-food applications. J. Membr. Sci. Res. 2020, 6, 304–318. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.; Liang, Y.; Qiang, W.; Atuna, R.A.; Amagloh, F.K.; Han, S. Comparison between membrane and thermal dealcoholization methods: Their impact on the chemical parameters, volatile composition, and sensory characteristics of wines. Membranes 2021, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Banvolgyi, S.; Savaş Bahçeci, K.; Vatai, G.; Bekassy, S.; Bekassy-Molnar, E. Partial dealcoholization of red wine by nanofiltration and its effect on anthocyanin and resveratrol levels. Food Sci. Technol. Int. 2016, 22, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Castro-Muñoz, R.; Cassano, A. Nanofiltration in beverage industry. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–548. [Google Scholar]
- Mangindaan, D.; Khoiruddin, K.; Wenten, I.G. Beverage dealcoholization processes: Past, present, and future. Trends Food Sci. Technol. 2018, 71, 36–45. [Google Scholar] [CrossRef]
- Labanda, J.; Vichi, S.; Llorens, J.; López-Tamames, E. Membrane separation technology for the reduction of alcoholic degree of a white model wine. LWT 2009, 42, 1390–1395. [Google Scholar] [CrossRef]
- Massot, A.; Mietton-Peuchot, M.; Peuchot, C.; Milisic, V. Nanofiltration and reverse osmosis in winemaking. Desalination 2008, 231, 283–289. [Google Scholar] [CrossRef]
- Ambrosi, A.; Motke, M.B.; Souza-Silva, É.A.; Zini, C.A.; McCutcheon, J.R.; Cardozo, N.S.M.; Tessaro, I.C. Beer dealcoholization by forward osmosis diafiltration. Innov. Food Sci. Emerg. Technol. 2020, 63, 102371. [Google Scholar] [CrossRef]
- Lisanti, M.T.; Gambuti, A.; Genovese, A.; Piombino, P.; Moio, L. Partial dealcoholization of red wines by membrane contactor technique: Effect on sensory characteristics and volatile composition. Food Bioproc. Technol. 2013, 6, 2289–2305. [Google Scholar] [CrossRef]
- Fedrizzi, B.; Nicolis, E.; Camin, F.; Bocca, E.; Carbognin, C.; Scholz, M.; Barbieri, P.; Finato, F.; Ferrarini, R. Stable isotope ratios and aroma profile changes induced due to innovative wine dealcoholisation approaches. Food Bioproc. Technol. 2014, 7, 62–70. [Google Scholar] [CrossRef]
- Crespo, J.G.; Brazinha, C. Fundamentals of pervaporation. In Pervaporation, Vapour Permeation and Membrane Distillation; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–17. [Google Scholar]
- Figoli, A.; Santoro, S.; Galiano, F.; Basile, A. Pervaporation membranes: Preparation, characterization, and application. In Pervaporation, Vapour Permeation and Membrane Distillation; Woodhead Publishing: Cambridge, UK, 2015; pp. 19–63. [Google Scholar] [CrossRef]
- Castro-Muñoz, R. Pervaporation-based membrane processes for the production of non-alcoholic beverages. J. Food Sci. Technol. 2019, 56, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Plaza, E.; López-Nicolás, J.M.; López-Roca, J.M.; Martínez-Cutillas, A. Dealcoholization of wine. Behaviour of the aroma components during the process. LWT Food Sci. Technol. 1999, 32, 384–386. [Google Scholar] [CrossRef]
- Belisario-Sánchez, Y.Y.; Taboada-Rodriguez, A.; Marin-Iniesta, F.; Lopez-Gomez, A. Dealcoholized wines by spinning cone column distillation: Phenolic compounds and antioxidant activity measured by the 1,1-diphenyl-2-picrylhydrazyl method. J. Agric. Food Chem. 2009, 57, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Belisario-Sánchez, Y.Y.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Iguaz-Gainza, A.; López-Gómez, A. Aroma recovery in wine dealcoholization by SCC distillation. Food Bioproc. Technol. 2012, 5, 2529–2539. [Google Scholar] [CrossRef]
- Di Giacomo, G.; Romano, P. Advanced fractionation process for wine-based products diversification. J. Food Sci. Technol. 2021, 58, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Paredes, D.A.F.; Sánchez, R.J.; Morero, B.; Fernández, M.B.; Espinosa, J. Enriching the conceptual modelling approach with environmental considerations: Application to the partial dealcoholization of wines. Sep. Purif. Technol. 2023, 308, 122950. [Google Scholar] [CrossRef]
- Pham, D.T.; Stockdale, V.J.; Wollan, D.; Jeffery, D.W.; Wilkinson, K.L. Compositional consequences of partial dealcoholization of red wine by reverse osmosis-evaporative perstraction. Molecules 2019, 24, 1404. [Google Scholar] [CrossRef] [PubMed]
- Esteras-Saz, J.; de la Iglesia, Ó.; Kumakiri, I.; Peña, C.; Escudero, A.; Téllez, C.; Coronas, J. Pervaporation of the low ethanol content extracting stream generated from the dealcoholization of red wine by membrane osmotic distillation. J. Ind. Eng. Chem. 2023, 122, 231–240. [Google Scholar] [CrossRef]
- Russo, P.; Liguori, L.; Corona, O.; Albanese, D.; Matteo, M.D.; Cinquanta, L. Combined membrane process for dealcoholization of wines. Osmotic distillation and reverse osmosis. Chem. Eng. Trans. 2019, 75, 7–12. [Google Scholar] [CrossRef]
- Liguori, L.; Russo, P.; Albanese, D.; Di Matteo, M. Production of low-alcohol beverages: Current status and perspectives. In Food Processing for Increased Quality and Consumption; Academic Press: Cambridge, MA, USA, 2018; pp. 347–382. [Google Scholar]
- Nevoigt, E.; Stahl, U. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 1996, 12, 1331–1337. [Google Scholar] [CrossRef]
- Remize, F.; Barnavon, L.; Dequin, S. Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatase, are rate limiting for glycerol production in Saccharomyces cerevisiae. Metab. Eng. 2001, 3, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Cambon, B.; Monteil, V.; Remize, F.; Camarasa, C.; Dequin, S. Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl. Environ. Microbiol. 2006, 72, 4688–4694. [Google Scholar] [CrossRef] [PubMed]
- Cuello, R.A.; Flores Montero, K.J.; Mercado, L.A.; Combina, M.; Ciklic, I.F. Construction of low-ethanol–wine yeasts through partial deletion of the Saccharomyces cerevisiae PDC2 gene. AMB Express 2017, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Drewke, C.; Thielen, J.; Ciriacy, M. Ethanol formation in adh0 mutants reveals the existence of a novel acetaldehyde-reducing activity in Saccharomyces cerevisiae. J. Bacteriol. 1990, 172, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Compagno, C.; Boschi, F.; Ranzi, B.M. Glycerol production in a triose phosphate isomerase deficient mutant of Saccharomyces cerevisiae. Biotechnol. Prog. 1996, 12, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Heux, S.; Sablayrolles, J.M.; Cachon, R.; Dequin, S. Engineering a Saccharomyces cerevisiae wine yeast that exhibits reduced ethanol production during fermentation under controlled microoxygenation conditions. Appl. Environ. Microbiol. 2006, 72, 5822–5828. [Google Scholar] [CrossRef]
- Malherbe, D.F.; Du Toit, M.; Cordero Otero, R.R.; Van Rensburg, P.; Pretorius, I.S. Expression of the Aspergillus niger glucose oxidase gene in Saccharomyces cerevisiae and its potential applications in wine production. Appl. Microbiol. Biotechnol. 2003, 61, 502–511. [Google Scholar] [CrossRef]
- Henricsson, C.; de Jesus Ferreira, M.C.; Hedfalk, K.; Elbing, K.; Larsson, C.; Bill, R.M.; Gustafsson, L. Engineering of a novel Saccharomyces cerevisiae wine strain with a respiratory phenotype at high external glucose concentrations. Appl. Environ. Microbiol. 2005, 71, 6185–6192. [Google Scholar] [CrossRef]
- Magyar, I.; Toth, T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100. [Google Scholar] [CrossRef]
- Gobbi, M.; De Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Puškaš, V.S.; Miljić, U.D.; Djuran, J.J.; Vučurović, V.M. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Nutr. Food Sci. 2020, 8, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Physiological features of Schizosaccharomyces pombe of interest in making of white wines. Eur. Food Res. Technol. 2013, 236, 29–36. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, D.; Bauer, F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The oenological potential of Hanseniaspora uvarum in simultaneous and sequential co-fermentation with Saccharomyces cerevisiae for industrial wine production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and phenotypic characterization of Metschnikowia pulcherrima strains from Douro wine region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef]
- Contreras, A.; Curtin, C.; Varela, C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Sáenz-Navajas, M.P.; Morales, P.; De-La-Fuente-Blanco, A.; Fernández-Zurbano, P.; Ferreira, V.; Gonzalez, R. Aroma profiling of an aerated fermentation of natural grape must with selected yeast strains at pilot scale. Food Microbiol. 2018, 70, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality, and composition of Airén wines fermented by sequential inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135. [Google Scholar] [CrossRef] [PubMed]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; Chamoro, C.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017, 243, 2175–2185. [Google Scholar] [CrossRef]
- Izquierdo Canas, P.M.; García Romero, E.; Huertas Nebreda, B.; Gómez Alonso, S.; Gómez-Alonso, S.; Collins, V.J.; Corona, G. Enhancement of flavour properties in wines using sequential inoculations of non. VITIS-J. Grapevine Res. 2011, 50, 177–182. [Google Scholar]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Puertas, B.; Jiménez, M.J.; Cantos-Villar, E.; Cantoral, J.M.; Rodríguez, M. Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in semi-industrial sequential inoculation to improve quality of palomino and chardonnay wines in warm climates. J. Appl. Microbiol. 2017, 122, 733–746. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef]
- Soden, A.; Francis, I.L.; Oakey, H.; Henschke, P.A. Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust. J. Grape Wine Res. 2000, 6, 21–30. [Google Scholar] [CrossRef]
- Ferraro, L.; Fatichenti, F.; Ciani, M. Pilot scale vinification process using immobilized Candida stellata cells and Saccharomyces cerevisiae. Process Biochem. 2000, 35, 1125–1129. [Google Scholar] [CrossRef]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Röcker, J.; Strub, S.; Ebert, K.; Grossmann, M. Usage of different aerobic non-Saccharomyces yeasts and experimental conditions as a tool for reducing the potential ethanol content in wines. Eur. Food Res. Technol. 2016, 242, 2051–2070. [Google Scholar] [CrossRef]
- Tronchoni, J.; Gonzalez, R.; Guindal, A.M.; Calleja, E.; Morales, P. Exploring the suitability of Saccharomyces cerevisiae strains for winemaking under aerobic conditions. Food Microbiol. 2022, 101, 103893. [Google Scholar] [CrossRef] [PubMed]
- Torija, M.J.; Rozes, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003, 80, 47–53. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Llauradó, J.M.; Rozès, N.; Constantí, M.; Mas, A. Study of some Saccharomyces cerevisiae strains for winemaking after preadaptation at low temperatures. J. Agric. Food Chem. 2005, 53, 1003–1011. [Google Scholar] [CrossRef]
- Edwards, C.G.; Aplin, J.J. Application of cool fermentation temperatures to encourage non-Saccharomyces yeasts to yield lower ethanol concentrations in wines. Fermentation 2022, 8, 421. [Google Scholar] [CrossRef]
- Tanguler, H. Influence of temperatures and fermentation behaviour of mixed cultures of Williopsis saturnus var. saturnus and Saccharomyces cerevisiae associated with winemaking. Food Sci. Technol. Res. 2013, 19, 781–793. [Google Scholar] [CrossRef]
- Carroll, D.E. Effects of carbonic maceration on chemical, physical and sensory characteristics of Muscadine wines. J. Food Sci. 1986, 51, 1195–1196. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Du, G.; Gao, Y.T.; Wang, L.W.; Meng, D.; Li, B.J.; Guan, W.Q. The effect of carbonic maceration during winemaking on the color, aroma and sensory properties of ‘Muscat Hamburg’ wine. Molecules 2019, 24, 3120. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.R.; Santamaría, P.; Olarte, C.; López-Alfaro, I.; Garijo, P.; González-Arenzana, L.; Sanz, S. Influence of microbial population on the characteristics of carbonic maceration wines. LWT 2022, 166, 113783. [Google Scholar] [CrossRef]
- Gutiérrez, A.R.; Portu, J.; López, R.; Garijo, P.; González-Arenzana, L.; Santamaría, P. Carbonic maceration vinification: A tool for wine alcohol reduction. Food Chem. 2023, 426, 136558. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, D.; Heyns, E.H.; Setati, M.E.; Bosch, S.; Bauer, F.F. Adjustment of trehalose metabolism in wine Saccharomyces cerevisiae strains to modify ethanol yields. Appl. Environ. Microbiol. 2013, 79, 5197–5207. [Google Scholar] [CrossRef] [PubMed]
- Tilloy, V.; Cadière, A.; Ehsani, M.; Dequin, S. Reducing alcohol levels in wines through rational and evolutionary engineering of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2015, 213, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Kutyna, D.R.; Solomon, M.R.; Black, C.A.; Borneman, A.; Henschke, P.A.; Pretorius, I.S.; Chambers, P.J. Evaluation of gene modification strategies for the development of low-alcohol-wine yeasts. Appl. Environ. Microbiol. 2012, 78, 6068–6077. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Guindal, A.M.; Tronchoni, J.; Morales, P. Biotechnological approaches to lowering the ethanol yield during wine fermentation. Biomolecules 2021, 11, 1569. [Google Scholar] [CrossRef]
- Kutyna, D.R.; Varela, C.; Henschke, P.A.; Chambers, P.J.; Stanley, G.A. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci. Technol. 2010, 21, 293–302. [Google Scholar] [CrossRef]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s balancing act between ethanol and glycerol production in low-alcohol wines. J. Microbiol. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.B.; Lonvaud, A.A.; Darriet, P.; Towey, J. The Microbiology of Wine and Vinifications. In Handbook of Enology; Wiley: Hoboken, NJ, USA, 2006; pp. 208–209. [Google Scholar]
- Scanes, K.T.; Hohmann, S.; Prior, B.A. Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: A review. S. Afr. J. Enol. Vitic. 1998, 19, 17–24. [Google Scholar] [CrossRef]
- Lopes, M.; De, B.; Ur-Rehman, A.; Gockowiak, H.; Heinrich, A.J.; Langridge, P.; Henschke, P.A. Fermentation properties of a wine yeast over-expressing the Saccharomyces cerevisiae Glycerol 3-Phosphate Dehydrogenase Gene (GPD2). Aust. J. Grape Wine Res. 2000, 6, 208–215. [Google Scholar] [CrossRef]
- Eglinton, J.M.; Heinrich, A.J.; Pollnitz, A.P.; Langridge, P.; Henschke, P.A.; de Barros Lopes, M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast 2002, 19, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Boidron, J.N.; Chatonnet, P.; Pons, M. Influence du bois sur certaines substances odorantesdes vin. OENO One 1988, 22, 275–294. [Google Scholar] [CrossRef]
- Saint-Prix, F.; Bonquist, L.; Dequin, S. Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: The NADP+-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiology 2004, 150, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, M.; Fernández, M.R.; Biosca, J.A.; Julien, A.; Dequin, S. Engineering of 2,3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Luyten, K.; Albertyn, J.; Skibbe, W.F.; Prior, B.A.; Ramos, J.; Thevelein, J.M.; Hohmann, S. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 1995, 14, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Tamás, M.J.; Luyten, K.; Sutherland, F.C.W.; Hernandez, A.; Albertyn, J.; Valadi, H.; Hohmann, S. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 1999, 31, 1087–1104. [Google Scholar] [CrossRef]
- Tamás, M.J.; Rep, M.; Thevelein, J.M.; Hohmann, S. Stimulation of the yeast high osmolarity glycerol (HOG) pathway: Evidence for a signal generated by a change in turgor rather than by water stress. FEBS Lett. 2000, 472, 159–165. [Google Scholar] [CrossRef]
- Flikweert, M.T.; de Swaaf, M.; van Dijken, J.P.; Pronk, J.T. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1999, 174, 73–79. [Google Scholar] [CrossRef]
- Compagno, C.; Brambilla, L.; Capitanio, D.; Boschi, F.; Maria Ranzi, B.; Porro, D. Alterations of the glucose metabolism in a triose phosphate isomerase-negative Saccharomyces cerevisiae mutant. Yeast 2001, 18, 663–670. [Google Scholar] [CrossRef]
- Overkamp, K.M.; Bakker, B.M.; Kötter, P.; Luttik, M.A.; Van Dijken, J.P.; Pronk, J.T. Metabolic engineering of glycerol production in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2002, 68, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Fraenkel, D.G. Glucose metabolism in gcr mutants of Saccharomyces cerevisiae. J. Bacteriol. 1999, 181, 4719–4723. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.W.; Baker, H.V. Concerted action of the transcriptional activators REB1, RAP1, and GCR1 in the high-level expression of the glycolytic gene TPI. Mol. Cell Biol. 1993, 13, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Kim, J.H. Glucose as a hormone: Receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem. Soc. Trans. 2005, 33, 247–252. [Google Scholar] [CrossRef]
- Fazio, N.A.; Russo, N.; Foti, P.; Pino, A.; Caggia, C.; Randazzo, C.L. Inside current winemaking challenges: Exploiting the potential of conventional and unconventional yeasts. Microorganisms 2023, 11, 1338. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Blackman, J.W.; Agboola, S.O. Production technologies for reduced alcoholic wines. J. Food Sci. 2012, 77, R25–R41. [Google Scholar] [CrossRef]
- Piškur, J.; Rozpędowska, E.; Polakova, S.; Merico, A.; Compagno, C. How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006, 22, 183–186. [Google Scholar] [CrossRef]
- Varela, C.; Kutyna, D.; Henschke, P.A.; Chambers, P.J.; Herderich, M.J.; Pretorius, I.S. Taking control of alcohol. Aust. N. Z. Wine Ind. J. 2008, 23, 41–43. [Google Scholar]
- Palacios, A.; Raginel, F.; Ortiz-Julien, A. Can the selection of Saccharomyces cerevisiae yeast lead to variations in the final alcohol degree of wines? Aust. N. Z. Grapegrow. Winemak. 2007, 527, 71–75. [Google Scholar]
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of non-Saccharomyces and Saccharomyces non-Cerevisiae yeasts on ethanol and glycerol levels in wine. Fermentation 2020, 6, 77. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces yeasts to the aroma complexity of wine: A Review. Foods 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Quiros, M.; Morales, P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Technol. 2013, 29, 55–61. [Google Scholar] [CrossRef]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muratore, G.; Asmundo, C.N.; Lanza, C.M.; Caggia, C.; Licciardello, F.; Restuccia, C. Influence of Saccharomyces uvarum on volatile acidity, aromatic and sensory profile of Malvasia delle Lipari wine. Food Technol. Biotechnol. 2007, 45, 101–106. [Google Scholar]
- Arroyo-López, F.N.; Pérez-Torrado, R.; Querol, A.; Barrio, E. Modulation of the glycerol and ethanol syntheses in the yeast Saccharomyces kudriavzevii differs from that exhibited by Saccharomyces cerevisiae and their hybrid. Food Microbiol. 2010, 27, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Rocker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Vilela, A. Lachancea thermotolerans, the non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef]
- Canonico, L.; Galli, E.; Ciani, E.; Comitini, F.; Ciani, M. Exploitation of three non-conventional yeast species in the brewing process. Microorganisms 2019, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Belda, I.; Santos, A.; Navascués, E.; Marquina, D. Advances in the control of the spoilage caused by Zygosaccharomyces species on sweet wines and concentrated grape musts. Food Control 2015, 51, 129–134. [Google Scholar] [CrossRef]
- Mehlomakulu, N.N.; Setati, M.E.; Divol, B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int. J. Food Microbiol. 2014, 188, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; San Mauro, M.; Bravo, E.; Marquina, D. PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis. Microbiology 2009, 155, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Ciani, M.; Bizzaro, D.; Comitini, F. Evaluation of damage induced by Kwkt and Pikt zymocins against Brettanomyces/Dekkera spoilage yeast, as compared to sulphur dioxide. J. Appl. Microbiol. 2016, 121, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef]
- Comitini, F.; Canonico, L.; Agarbati, A.; Ciani, M. Biocontrol and probiotic function of non-Saccharomyces yeasts: New insights in agri-food industry. Microorganisms 2023, 11, 1450. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R. The yeast Torulaspora delbrueckii: An interesting but difficult-to-use tool for winemaking. Fermentation 2018, 4, 94. [Google Scholar] [CrossRef]
- Čuš, F.; Jenko, M. The influence of yeast strains on the composition and sensory quality of Gewürztraminer wine. Food Technol. Biotechnol. 2013, 51, 547–553. [Google Scholar]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Redón, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effect of lipid supplementation upon Saccharomyces cerevisiae lipid composition and fermentation performance at low temperature. Eur. Food Res. Technol. 2009, 228, 833–840. [Google Scholar] [CrossRef]
- Du, Q.; Ye, D.; Zang, X.; Nan, H.; Liu, Y. Effect of low temperature on the shaping of yeast-derived metabolite compositions during wine fermentation. Food Res. Int. 2022, 162, 112016. [Google Scholar] [CrossRef] [PubMed]
- Zakhartsev, M.; Yang, X.; Reuss, M.; Pörtner, H.O. Metabolic efficiency in yeast Saccharomyces cerevisiae in relation to temperature dependent growth and biomass yield. J. Therm. Biol. 2015, 52, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.J.; Raimbourg, T.; Gonzalez, R.; Morales, P. Environmental factors influencing the efficacy of different yeast strains for alcohol level reduction in wine by respiration. LWT 2016, 65, 1038–1043. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Gao, C.; Fleet, G.H. The effects of temperature and pH on the ethanol tolerance of the wine yeasts, Saccharomyces cerevisiae, Candida stellata and Kloeckera apiculata. J. Appl. Bacteriol. 1988, 65, 405–409. [Google Scholar] [CrossRef]
- Heard, G.M.; Fleet, G.H. The effects of temperature and pH on the growth of yeast species during the fermentation of grape juice. J. Appl. Bacteriol. 1988, 65, 23–28. [Google Scholar] [CrossRef]
- Abeln, F.; Chuck, C.J. The role of temperature, pH and nutrition in process development of the unique oleaginous yeast Metschnikowia pulcherrima. J. Chem. Technol. Biotechnol. 2020, 95, 1163–1172. [Google Scholar] [CrossRef]
- Pickering, G.J. Low-and reduced-alcohol wine: A review. J. Wine Res. 2000, 11, 129–144. [Google Scholar] [CrossRef]
- Piornos, J.A.; Koussissi, E.; Balagiannis, D.P.; Brouwer, E.; Parker, J.K. Alcohol-free and low-alcohol beers: Aroma chemistry and sensory characteristics. Compr. Rev. Food Sci. Food Saf. 2023, 22, 233–259. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.; Stewart, G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma Precursors in Grapes and Wine: Flavor Release during Wine Production and Consumption. J. Agric. Food Chem. 2018, 66, 2281–2286. [Google Scholar] [CrossRef]
- Capece, A.; Romano, P. Yeasts and Their Metabolic Impact on Wine Flavour. In Yeasts in the Production Wine; Romano, P., Ciani, M., Fleet, G.H., Eds.; Springer: New York, NY, USA, 2019; pp. 43–80. [Google Scholar]
- Nikolaou, A.; Kourkoutas, Y. High-temperature semi-dry and sweet low alcohol winemaking using immobilized kefir culture. Fermentation 2021, 7, 45. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Tesniere, C.; Flanzy, C. Carbonic maceration wines: Characteristics and winemaking process. Adv. Food Nutr. Res. 2011, 63, 1–15. [Google Scholar] [CrossRef]
- Carnacini, A.; Del Pozzo, A. Characteristics of wines obtained by carbonic maceration. L’Enotecnico 1985, 21, 1115–1119. [Google Scholar]
- Navarro, G.; Zuñel, C.; Méndez, C.; Navarro, S. Vinificaciones Comparadas, en Tinto Tradicional y por Maceración Carbónica, de Vitis vinifera Variedad Monastrell. I. Aspectos Generales y Evolución de los Parámetros de Control de la Fermentación. 1988. Anales de Bromatología XL-1, 163–174. Available online: https://www.semanticscholar.org/paper/Vinificaciones-comparadas%2C-en-tinto-tradicional-y-y-Navarro-Zu%C3%B1el/b990e849eeeb1c9078dbd3f641422bdf438501db#citing-papers (accessed on 1 November 2023).
- Etaio, I.; Elortondo, F.P.; Albisu, M.; Gaston, E.; Ojeda, M.; Schlich, P. Effect of winemaking process and addition of white grapes on the sensory and physicochemical characteristics of young red wines. Aust. J. Grape Wine Res. 2008, 14, 211–222. [Google Scholar] [CrossRef]
- Nogueira, A.; Mongruel, C.; Rosana, D.; Simões, S. Effect of Biomass Reduction on the Fermentation of Cider. Braz. Arch. Biol. Technol. 2007, 50, 1083–1092. [Google Scholar] [CrossRef]
- Nogueira, A.; Le Quéré, J.M.; Gestin, P.; Michel, A.; Wosiacki, G.; Drilleau, J.F.; Brew, J.I. Slow Fermentation in French Cider Processing Due to Partial Biomass Reduction. J. Inst. Brew. 2008, 114, 102–110. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M. Yeasts and Wine Off-Flavours: A Technological Perspective. Ann. Microbiol. 2011, 61, 95–102. [Google Scholar] [CrossRef]
- Fan, G.; Shengyun, T.; Rong, W.; Qingbin, L.; Jinsheng, Z.; Xiaodong, Y.; Yang, L. Fermentation process of low-alcohol cider by biomass reduction. China Brew 2012, 31, 186–190. [Google Scholar]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Walker, R.S. Enhancing yeast alcoholic fermentations. Adv. Appl. Microbiol. 2018, 105, 87–129. [Google Scholar] [CrossRef] [PubMed]
- Vamvakas, S.S.; Kapolos, J. Factors affecting yeast ethanol tolerance and fermentation efficiency. World J. Microbiol. Biotechnol. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Maicas, S. Advances in wine fermentation. Fermentation 2021, 7, 187. [Google Scholar] [CrossRef]
- Vilela, A. Modulating wine pleasantness throughout wine-yeast co-inoculation or sequential inoculation. Fermentation 2020, 6, 22. [Google Scholar] [CrossRef]
- Pulcini, L.; Gamalero, E.; Costantini, A.; Vaudano, E.T.; Tsolakis, C.; Garcia-Moruno, E. An overview on Saccharomyces cerevisiae indigenous strains selection methods. In Grapes and Wine; Intechopen: London, UK, 2022; p. 165. [Google Scholar] [CrossRef]
- Alexandre, H. Flor yeasts of Saccharomyces cerevisiae—Their ecology, genetics and metabolism. Int. J. Food Microbiol. 2013, 167, 269–275. [Google Scholar] [CrossRef]
- Ángeles Pozo-Bayón, M.; Victoria Moreno-Arribas, M. Sherry wines. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 63, pp. 17–40. [Google Scholar]
- Avdanina, D.; Zghun, A. Sherry Wines: Worldwide Production, Chemical Composition and Screening Conception for Flor Yeasts. Fermentation 2022, 8, 381. [Google Scholar] [CrossRef]
- De Smidt, O.; du Preez, J.C.; Albertyn, J. Molecular and physiological aspects of alcohol dehydrogenases in the ethanol metabolism of Saccharomyces cerevisiae. FEMS Yeast Res. 2012, 12, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Denis, C.L.; Ferguson, J.; Young, E.T. mRNA levels for the fermentative alcohol dehydrogenase of Saccharomyces cerevisiae decrease upon growth on a nonfermentable carbon source. J. Biol. Chem. 1983, 258, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, I.; Ronzulli, R.; Casatta, N.; Vai, M. Ethanol and acetate acting as carbon/energy sources negatively affect yeast chronological aging. Oxidative Med. Cell. Longev. 2013, 2013, 802870. [Google Scholar] [CrossRef] [PubMed]
- Rahner, A.; Hiesinger, M.; Schüller, H.J. Deregulation of gluconeogenic structural genes by variants of the transcriptional activator Cat8p of the yeast Saccharomyces cerevisiae. Mol. Microbiol. 1999, 34, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Haurie, V.; Perrot, M.; Mini, T.; Jenö, P.; Sagliocco, F.; Boucherie, H. The transcriptional activator Cat8p provides a major contribution to the reprogramming of carbon metabolism during the diauxic shift in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Rahner, A.; Schöler, A.; Martens, E.; Gollwitzer, B.; Schüller, H.J. Dual influence of the yeast Catlp (Snflp) protein kinase on carbon source-dependent transcriptional activation of gluconeogenic genes by the regulatory gene CAT8. Nucleic Acids Res. 1996, 24, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of wines by selected yeast strains. Appl. Microbiol. Biotechnol. 2008, 80, 881–890. [Google Scholar] [CrossRef]
- Vilela, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of acidic wines by immobilized Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2013, 97, 4991–5000. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Falco, V.; Mendes-Faia, A.; Côrte-Real, M. Effect of refermentation conditions and micro-oxygenation on the reduction of volatile acidity by commercial S. cerevisiae strains and their impact on the aromatic profile of wines. Int. J. Food Microbiol. 2010, 141, 165–172. [Google Scholar] [CrossRef]
- Vilela, A. Targeting demalication and deacetification methods: The role of carboxylic acids transporters. Biochem. Physiol. 2017, 6, 224. [Google Scholar] [CrossRef]
- Vilela, A. Biological demalication and deacetification of musts and wines: Can wine yeasts make the wine taste better? Fermentation 2017, 3, 51. [Google Scholar] [CrossRef]
- Moreno-García, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Stress Responsive Proteins of a Flor Yeast Strain during the Early Stages of Biofilm Formation. Process Biochem. 2016, 51, 578–588. [Google Scholar] [CrossRef]
- Anderson, P.; Kokole, D.; Llopis, E.J. Production, consumption, and potential public health impact of low-and no-alcohol products: Results of a scoping review. Nutrients 2021, 13, 3153. [Google Scholar] [CrossRef] [PubMed]
- Bucher, T.; Deroover, K.; Stockley, C. Low-alcohol wine: A narrative review on consumer perception and behaviour. Beverages 2018, 4, 82. [Google Scholar] [CrossRef]
- Bucher, T.; Deroover, K.; Stockley, C. Production and marketing of low-alcohol wine. In Advances in Grape and Wine Biotechnology; Intechopen: London, UK, 2019; pp. 1–15. [Google Scholar]
- Meillon, S.; Urbano, C.; Guillot, G.; Schlich, P. Acceptability of partially dealcoholized wines–Measuring the impact of sensory and information cues on overall liking in real-life settings. Food Qual. Pref. 2010, 21, 763–773. [Google Scholar] [CrossRef]
- World Health Organization. Harmful Use of Alcohol. 2023. Available online: https://www.who.int/health-topics/alcohol#tab=tab_1 (accessed on 1 November 2023).
- Shaw, C.L.; Dolan, R.; Corsi, A.M.; Goodman, S.; Pearson, W. Exploring the barriers and triggers towards the adoption of low-and no-alcohol (NOLO) wines. Food Qual. Prefer. 2023, 110, 104932. [Google Scholar] [CrossRef]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate wine consumption and health: A narrative review. Nutrients 2022, 15, 175. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- Mongan, D.; Long, J. Standard Drink Measures throughout Europe; Peoples’ Understanding of Standard Drinks. RARHA: Joint Actional on Reducing Alcohol Related Harm. 2015. Available online: https://www.rarha.eu/Resources/Deliverables/Lists/Deliverables/Attachments/14/WP5%20Background%20paper%20Standard%20drink%20measures%20HRB.pdf (accessed on 1 November 2023).
- Commonwealth of Australia|Department of Health and Aged Care. Australian Alcohol Guidelines Revised. Available online: https://www.health.gov.au/news/australian-alcohol-guidelines-revised (accessed on 1 December 2023).
- IARD 2023. Drinking Guidelines: General Population (iard.org). Available online: https://www.iard.org/science-resources/detail/Drinking-Guidelines-General-Population (accessed on 1 December 2023).
- Minzer, S.; Estruch, R.; Casas, R. Wine intake in the framework of a mediterranean diet and chronic non-communicable diseases: A short literature review of the last 5 years. Molecules 2020, 25, 5045. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; Matthews, D.; Duthie, G.G.; Lean, J.; Crozier, A. Extraction of phenolics and changes in antioxidant activity of red wines during vinification. J. Agric. Food Chem. 2001, 49, 5797–5808. [Google Scholar] [CrossRef] [PubMed]
- Bucher, T.; Frey, E.; Wilczynska, M.; Deroover, K.; Dohle, S. Consumer perception and behaviour related to low-alcohol wine: Do people overcompensate? Public Health Nutr. 2020, 23, 1939–1947. [Google Scholar] [CrossRef] [PubMed]

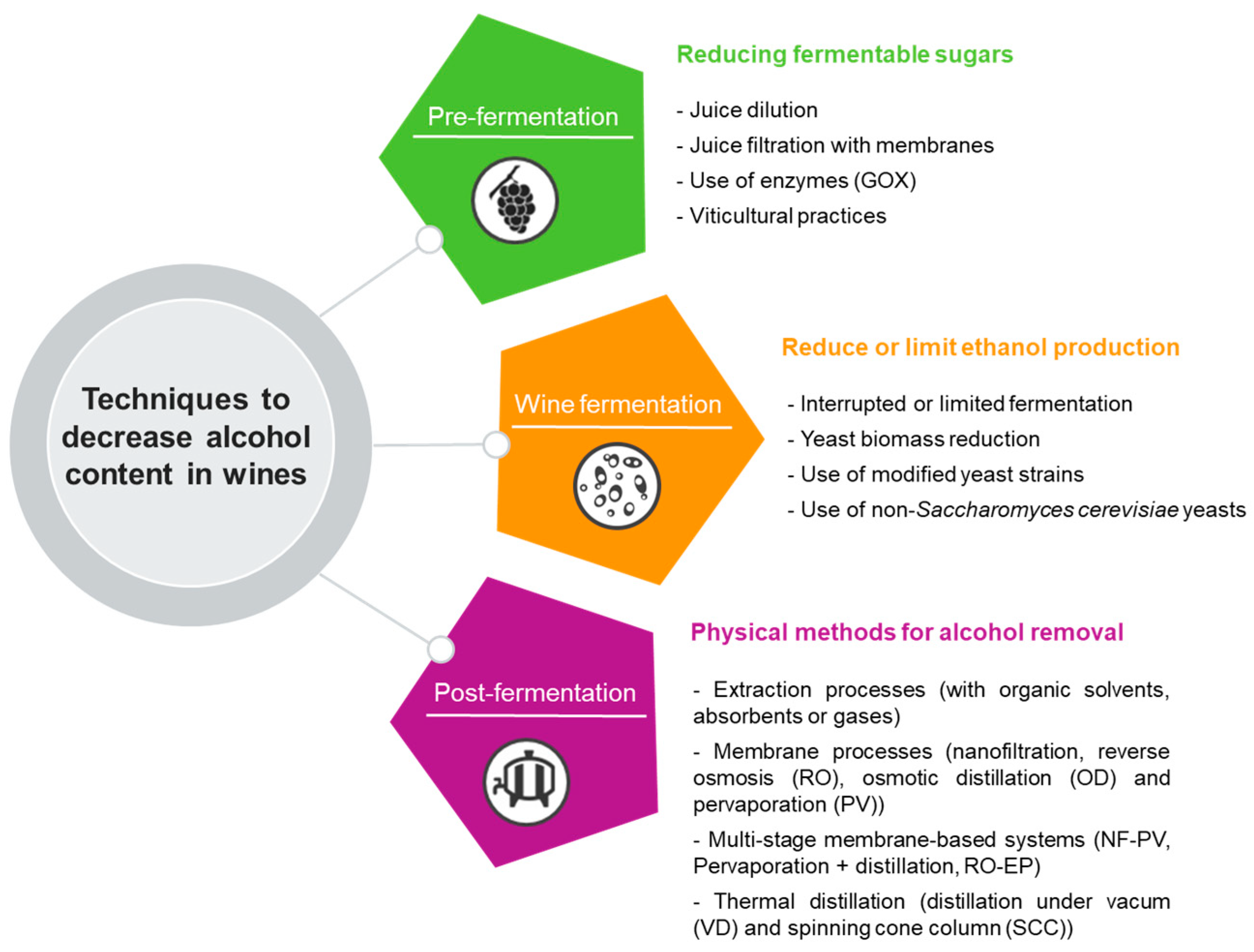


| Country | Wine Production (Mhl) | Rate of Change | % of Global Wine Production |
|---|---|---|---|
| Italy | 49.8 | −1% | 19.3% |
| France | 45.6 | 21% | 17.7% |
| Spain | 35.7 | 1% | 13.8% |
| USA | 22.4 | −7% | 8.7% |
| Australia | 12.7 | −14% | 4.9% |
| Method Used | Type of Wine | Alcohol Reduction | Advantages | Disadvantages | Ref. | ||
|---|---|---|---|---|---|---|---|
| Original Alcohol Content (% v/v) | Final Alcohol Contente (% v/v) | ||||||
| Juice dilution | Shiraz red wine | Fresh Fruits: 13.6 Mature Fruits: 15.5 | 9.6 to 14.5 | Within legal limits, the addition of water may have relatively limited effects on the chemical composition of the wine, preserving many of its characteristics. Wines produced from mature grapes tended to exhibit sensory characteristics closer to the expectations of producers and consumers. | Sensory analyses suggest that dilution can lead to a diminished perception of attributes such as ‘flavor intensity’ and ‘body,’ which could impact the wine’s overall acceptance. | [101] | |
| Juice filtration with membranes | Nanofiltration | Garnacha red wines and Verdejo white wines | Garnacha red wines: 12.40 Verdejo white wines: 13.88 | Garnacha red wines: 11 Verdejo white wines: 11.95 | The two-stage NF process without backflush is the most effective, minimizing sugar content while promoting higher recovery of polyphenolic compounds. Red wines are favorite for odor and color. | Sensory evaluation revealed no consumer preference for wine samples, although white wines present lower persistence, possibly related to their lower alcohol degree. | [104] |
| Reverse Osmosis | The red wine blends Tinta Roriz, Syrah, and Alicante Bouschet. | 15.2 | 5.4 to 13.8 | Production of beverages with lower alcohol content without excessively compromising sensory quality. | Beverages with lower alcohol content were perceived by tasters to have diminished color, reduced aromatic intensity, and increased detection of defects like oxidation and hydrogen sulfite, negatively affecting sensory persistence. | [105] | |
| Use of enzymes (GOX) | Verdejo white wine | 13.8 | 11.1 to 11.7 | Enzymatic treatment led to more balanced wines, reducing alcohol content and pH. The chromatic properties of GOX wines remained unchanged compared to control wines, indicating color stability. GOX wines had lower concentrations of C6-alcohols associated with green-herbaceous notes, contributing to improved aroma. | GOX treatment resulted in lower concentrations of certain wine alcohols with floral notes. The technique did not uniformly affect all volatile compounds, influencing the sensory characteristics differently. | [106] | |
| Viticultural practices | Early grape harvesting | Tempranillo red wine | 14.8 | 10.61 to 10.63 | The early harvesting of grapes, including the use of must or wine obtained from green pruning (green harvest), may lead to producing wines with reduced alcohol content. No significant changes in color intensity and phenolic compounds were observed. Early harvesting can contribute to fresher and less mature wines, although the outcomes may vary depending on the specific conditions of each vintage. | The resulting wines were perceived as more acidic and less full-bodied. | [107] |
| Pinot Noir and Tannat red wines | Pinot Noir: 14.3 Tannat: 14.7 | 11.5 for both Pinot Noir and Tannat | Substituting ripe must with less ripe must result in wines with lower alcohol content (reduction of 14% to 21%) and lower pH. Wines exhibited greater color intensity, concentration of phenolic compounds, total anthocyanins, proanthocyanidins, and polysaccharide concentration. | Possible losses of anthocyanins may occur. Results may vary depending on the specific conditions of each harvest. | [108] | ||
| Growth regulators (Auxin treatment) | Shiraz red wine | 14.3 | 13.9 | No significant differences were observed in the sensory properties of the wine. | Potential alterations in the chemical composition of the wine, particularly in volatile compounds. Understanding the long-term effects of synthetic auxin application is crucial before implementing it on a large scale. The effects of NAA application may vary with climatic conditions and the environment. | [109] | |
| Grapevine canopy management | Shiraz red wine | 2010/2011: 12.40 2015/2016: 13.27 (Apical leaves removal at veraison) | 2010/2011: 11.72 2015/2016: 12.94 | Reduction in alcohol content in wine, especially with apical defoliation. Moderation of wine aromatic properties. Potential mitigation of the effects of global warming on the increase in alcohol content of wine. | Variations in response to the technique among different cultivars and clones. Consider factors such as timing, defoliation method, and location for consistent results. Limited influence of apical defoliation on wine characteristics compared to basal defoliation. | [110] | |
| Aglianico red wine | 2012: 14.1 2013: 13.1 2014: 13.3 (post-veraison pruning techniques, such as leaf removal and shoot trimming) | 2012: 13.2 to 13.8 2013: 11.8 to 12.2 2014: 2.2 to 12.5 | The post-veraison pruning techniques, such as leaf removal and shoot trimming, have shown a significant decrease in alcohol concentration in the wine. Both leaf removal and moderate shoot trimming resulted in improvements in the overall sensory score of the wine. | Results may vary based on climate and vine characteristics. Intensive pruning, whether leaf removal or shoot trimming, may negatively impact wine sensory scores in specific years. The concentration of compounds like anthocyanins and phenolic substances in the berries fluctuated based on pruning intensity and the year. | [111] | ||
| Method Used | Type of Wine | Alcohol Reduction | Advantages | Disadvantages | Ref. | ||
|---|---|---|---|---|---|---|---|
| Original Alcohol Content (%, v/v) | Final Alcohol Contente (%, v/v) | ||||||
| Extraction processes | CO2 | Rose wine | 11.3 | 1.1 | Wine retained several aromatic compounds from the original wine. Slight modification in antioxidant activity, with values similar to the original wine. | Extremely expensive, and their application in the food industry for nonalcoholic wine production is becoming rare. | [134] |
| Membrane processes | Nanofiltration | Cabernet Sauvignon | 13.62 | 7.38 to 11.01 | Retention of desirable compounds, including polyphenols and aromas, preserving wine’s sensorial quality. The technical capability to permeabilize acetic acid can be explored to correct this component in wine, providing sensory improvements. | High energy consumption requirements, especially when dealing with low molecular weight compounds, may lead to increased membrane fouling. Cooling is necessary, adding complexity to the process and potentially increasing operational costs. | [115] |
| Red wine | 12 | 7 to 8 | Nanofiltration produces dealcoholized wine with preserved aromatic compounds, enhancing the gustatory experience. | The membrane selection is crucial, and different membranes exhibit distinct performances in terms of ethanol rejection, aromatic compound rejection, and organoleptic properties. | [135] | ||
| RO | Sauvignon blanc | 13.6 | 10.5 to 12.2 | Reducing alcohol can enhance specific flavors and aromas, providing a unique sensory experience. | Professionals included terms such as “less persistent” and “less balanced”, suggesting a potential loss of desirable characteristics. | [67] | |
| Syrah | 13.4 | 7.9 to 11.4 | The reduction in alcohol-induced an increase in the perception of familiarity, harmony, and balance, reaching an optimum of −4%. The reduction in alcohol is noticeable but not apparent to consumers. | Reverse osmosis can impact the overall sensory experience of wine by decreasing complexity, persistence, and the number of aromas and influencing texture and viscosity. However, it may not be suitable for wines that are sensitive to adjustments in alcohol content. | [136] | ||
| Red wine | 16 | 14.1 | No adverse effects on the treated wines’ color, aroma, and taste were observed, suggesting the preservation of sensory quality. | A reduction in total phenols and anthocyanins was noted, along with a decrease in both total and volatile acidity, concurrent with the reduction in alcohol content. | [137] | ||
| OD | Montepulciano d’Abruzzo red wine | 13.23 | 2.67 to 8.31 | Preservation of satisfactory sensory characteristics. The color and flavor characteristics, assessed by flavonoids and phenolic compounds, remained virtually unchanged in all dealcoholized samples. | The taste of dealcoholized wine is affected by acidity and pH variations. The decline in ethanol concentration affects the taste of red fruits, spices, sweetness, bitterness, and astringency. Samples with less than 5.4% v/v of alcohol are preferred. | [138] | |
| Aglianico red wine | 13 | 0.19 to 6.52 | Essential chemical and physical properties, such as pH and total acidity, remained unchanged from the control wine. | There was a pronounced reduction in volatile acidity. The technique requires constant monitoring throughout the process. | [139] | ||
| Falanghina white wine | 12.5 | 0.3 | No significant changes were observed in the main quality parameters, such as total acidity, pH, organic acids, color, total phenols, and flavonols, during the dealcoholization process. Preservation of antioxidant compounds. | With increasing alcohol removal, the quantity of volatile compounds in the wine decreased significantly. The fully dealcoholized sample saw a total loss of 96% in these compounds. However, the completely dealcoholized sample was perceived as unbalanced in taste and globally unacceptable. | [140] | ||
| PV | Cabernet Sauvignon red wine | 12.5 | <0.5 | Enhanced smell and taste. Effective separation of ethanol and aroma substances. Production of high-quality, alcohol-free wine. Stable membrane performance over extended operation. | High investment and operating costs for pilot-scale equipment. Need for membrane cleaning after red wine batches—potential quality issues at higher operating temperatures. | [141] | |
| Multi-stage membrane-based systems | NF–PV | Verdejo white wine | 11.90 | 10.25 | Wines resulting from the NF–PV process exhibited aromatic profiles similar to the original wine. | Nanofiltration and pervaporation equipment can be costly for winemakers, and outcomes may vary based on grape variety and other factors. | [142] |
| RO–EP | 5 Cabernet Sauvignon red wines | Wine A: 17.0 Wine B: 15.5 Wine C: 14.9 Wine D: 14.5 Wine E: 16.0 | Wine A: 14.5 Wine B: 13.3 Wine C: 13.3 Wine D: 13.2 Wine E: 14.2 | Applied in industrial volumes of wine, the technique is suitable for large-scale operations. Preservation of specific volatile and aromatic compounds in the wine contributes to the retention of desirable sensory characteristics, with minor decreases observed in the intensity of specific flavors, such as “dark fruit”, “sweet spice”, and “chocolate”. | Modifications in “body”, “acidity”, “bitterness”, and “astringency” are particularly evident in Wine A following a 2.5% ABV reduction. Installing and maintaining RO–EP equipment may constitute a substantial investment for wine producers. | [143] | |
| Thermal distillation | VD | Langhe Rose Verduno Pelaverga red wine Barbera red wine | Langhe Rose: 13.2 Verduno Pelaverga red wine: 15.2 Barbera red wine: 14.6 | 5 for all wines | Maintained a wine-like physicochemical composition. Richer aromatic profile, particularly in ethyl esters and isoamyl acetate. | Higher losses of alcohol were observed. | [144] |
| SCC | Shiraz Sangiovese red wine Petit Verdot Sangiovese red wine | Shiraz Sangiovese red wine: 15.1 Petit Verdot Sangiovese red wine: 14.2 | 0.3 to 14.5 0.3 to 13.8 | It selectively amplifies key non-volatile elements like organic acids, anthocyanins, and tannins, heightening the sensory intricacy of the wine. | Loss of desirable volatile compounds responsible for fruity and floral aromas compromises the wine’s varietal expression. | [145] | |
| Approaches | Used Approach or Microorganism | Approximate Decrease in Ethanol Content (%) | Inoculation Regime | Scale of Fermentation | Grape Variety or Media | Ref. |
|---|---|---|---|---|---|---|
| GMO-based approaches | Over-expressed GPD1 | 35 | Unspecified | Unspecified | YEPD | [172] |
| 3 | Single | Laboratory | MS | [173] | ||
| 10.5–17.5 | Single | Laboratory | MS | [174] | ||
| Over-expressed GPD2 | 24 | Single | Laboratory | Synthetic Leu-free | [174] | |
| PDC2 deletion mutant | 28–45 | Unspecified | Unspecified | YEPD | [172] | |
| 2 | Single | Laboratory | Diluted white must | [175] | ||
| Alcohol dehydrogenase (ADH) mutants | 63 | Single | Laboratory | YEPD | [176] | |
| Triose phosphate isomerase (TPI) mutants | Undetermined | Single | Laboratory | YEPD | [177] | |
| NADH oxidase (NOX) mutants | 7 | Single | Laboratory | Synthetic MS medium | [178] | |
| Glycerol transporter (FPS) mutants | 10 | Single | Laboratory | Synthetic MS medium | [173] | |
| Glucose oxidase (GOX) mutants | 2 | Single | Laboratory | Chardonnay grape juice | [179] | |
| Hexose transporter (HXT) mutants | Undetermined | Single | Laboratory | 5× defined minimal medium | [180] | |
| non-Saccharomyces (NS) yeasts | C. stellata | 19 | Single | Laboratory | Grape juice | [181] |
| C. zemplinina | 57 | Single | Laboratory | Grape juice | ||
| H. uvarum | 33 | Single | Laboratory | Grape juice | [182] | |
| Z. sapae | 14 | Single | Laboratory | Grape juice | ||
| Z. bailii | 4 | Single | Laboratory | Grape juice | ||
| Z. bisporus | 7 | Single | Laboratory | grape juice | ||
| M. pulcherrima | 0.9 | Sequential fermentations with S. cerevisiae | Laboratory | Chardonnay | [183] | |
| 1.6 | Sequential fermentations with S. cerevisiae | Laboratory | Shiraz | |||
| M. pulcherrima | 0.9 | Sequential fermentations with Saccharomyce bayanus, and S. cerevisiae | Laboratory | Sila | [184] | |
| P. kudriavzevii | 52 | Sequential inoculation | Laboratory | CDGJ medium | [185] | |
| Z. bailii | 16 | Sequential inoculation | Laboratory | CDGJ medium | ||
| S. pombe | 4.9 | Single | Laboratory | Airen | [186] | |
| S. pombe | 3.1 | Sequential fermentations with S. cerevisiae | Laboratory | Airen | [186] | |
| H. uvarum | 1 | Sequential fermentations with S. cerevisiae | Laboratory | Synthetic grape juice and natural grape juice | [187] | |
| H. uvarum | 1.3 | Sequential fermentations with S. cerevisiae | Laboratory | Pinotage | [188] | |
| H. uvarum | 0.8 | Sequential fermentations with S. cerevisiae | Laboratory | Sauvignon Blanc | [188] | |
| H. uvarum | 3.3 | Sequential fermentations with S. cerevisiae | Laboratory | Negroamaro | [189] | |
| H. osmophila | 1.2 | Sequential fermentations with S. cerevisiae | Laboratory | synthetic grape juice and natural grape juice | [187] | |
| H. opuntiae | 2 | Sequential fermentations with S. cerevisiae | Laboratory | Pinotage | [188] | |
| H. opuntiae | 11 | Sequential fermentations with S. cerevisiae | Laboratory | Sauvignon Blanc | [188] | |
| M. pulcherrima | 67 | Single | Laboratory | Tinta Roriz | [190] | |
| M. pulcherrima | 7 | Co-fermentations with S. cerevisiae | Laboratory | Tinta Roriz | [190] | |
| M. pulcherrima | 8 | Co-fermentations with S. cerevisiae | Laboratory | Malvasia and Viura | [191] | |
| M. pulcherrima and S. uvarum mixed inoculum | 1.6 | Sequential fermentations with S. cerevisiae | Laboratory | CDGJ medium | [192] | |
| M. pulcherrima | 1.3 | Sequential fermentations with S. cerevisiae | Laboratory | Synthetic grape juice and natural grape juice | [187] | |
| M. pulcherrima | 7 | Single | pilot-scale | Viura-Malvasía | [193] | |
| T. delbruekii | 4 | Single | pilot-scale | Viura-Malvasía | [193] | |
| L. thermotolerans | 1 | Sequential fermentations with S. cerevisiae | Laboratory | Riesling | [186] | |
| P. kluyveri | 1.8 | Sequential fermentations with S. cerevisiae | Laboratory | Riesling | [186] | |
| M. pulcherrima | 1 | Sequential fermentations with S. cerevisiae | Laboratory | Riesling | [186] | |
| L. thermotolerans | 1 | Co- fermentations with S. cerevisiae | industrial | Sangiovese | [194] | |
| L. thermotolerans | 5 | Sequential fermentations with S. cerevisiae | industrial | Sangiovese | [194] | |
| L. thermotolerans | 3 | Sequential fermentations with S. cerevisiae | Pilot scale | Shiraz | [195] | |
| L. thermotolerans | 0.5 | Co- fermentations with S. cerevisiae | Laboratory | Airen | [196] | |
| L. thermotolerans | 3 | Sequential fermentations with S. cerevisiae | Laboratory | Airen | [196] | |
| L. thermotolerans | 8 | Sequential fermentations with S. cerevisiae | Laboratory | Tempranillo | [197] | |
| T. delbrueckii | 3 | Single | pilot-scale | Viura-Malvasía | [193] | |
| T. delbrueckii | 2 | Sequential fermentations with S. cerevisiae | pilot-scale | Airén | [198] | |
| T. delbrueckii | 0.8 | Co- fermentations with S. cerevisiae | pilot-scale | Amarone | [199] | |
| T. delbrueckii | 3 | Sequential fermentations with S. cerevisiae | pilot-scale | Amarone | [199] | |
| T. delbrueckii | 4 | Sequential fermentations with S. cerevisiae | Semi-industrial scale | Chardonnay | [200] | |
| T. delbrueckii | 1 | Sequential fermentations with S. cerevisiae | Semi-industrial scale | Palomino Fino | [200] | |
| T. delbrueckii | 4 | Sequential fermentations with S. cerevisiae | Laboratory | Verdejo | [201] | |
| C. stellata | 54 | Single | Laboratory | Chardonnay | [202] | |
| C. stellata | 0.8 | Co- fermentations with S. cerevisiae | Laboratory | Chardonnay | [202] | |
| C. stellata | 6 | Sequential fermentations with S. cerevisiae | Laboratory | Chardonnay | [202] | |
| C. stellata | 6 | Immobilized and sequential fermentations with S. cerevisiae | Semi-industrial scale | Grape must | [203] | |
| Abiotic factors control | Controlled factor | |||||
| M. pulcherrima | 42 | Controlled aeration | Laboratory | Synthetic grape must | [204] | |
| M. pulcherrima | 14 | Controlled aeration and Co- fermentations with S. cerevisiae | Laboratory | Natural white grape must | [191] | |
| M. pulcherrima | 4 | Controlled aeration | Laboratory | Riesling must | [205] | |
| S. cerevisiae | 30 | Controlled aeration | Laboratory | Natural white must | [206] | |
| S. cerevisiae | 15 | Controlled temperature | Laboratory | Concentrated white must | [207] | |
| S. cerevisiae | 9 | Controlled temperature | Laboratory | Modified MS300 medium | [208] | |
| S. cerevisiae | 3 | Controlled temperature | Laboratory | Carinyena | [209] | |
| M. pulcherrima | 6 | Controlled temperature and sequential fermentations with S. cerevisiae | Semi-industrial scale | Merlot | [210] | |
| M. guilliermondii | 3 | Controlled temperature and sequential fermentations with S. cerevisiae | Semi-industrial scale | Merlot | [210] | |
| W. saturnus | 3 | Controlled temperature and Co-fermentations with S. cerevisiae | Laboratory | Emir | [211] | |
| Carbonic maceration | 16 | Unspecified | Laboratory | Carlos | [212] | |
| Carbonic maceration | 5 | Unspecified | Laboratory | Noble | [212] | |
| Carbonic maceration | 1.5 | S. cerevisiae | Laboratory | Muscat Hamburg | [213] | |
| Carbonic maceration | 25 | Several species | Semi-industrial scale | Tempranillo | [214] | |
| Carbonic maceration | 24 | Unspecified | Semi-industrial scale | Tempranillo and Graciano | [215] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, S.M.; Inês, A.; Vilela, A. Bio-Dealcoholization of Wines: Can Yeast Make Lighter Wines? Fermentation 2024, 10, 36. https://doi.org/10.3390/fermentation10010036
Afonso SM, Inês A, Vilela A. Bio-Dealcoholization of Wines: Can Yeast Make Lighter Wines? Fermentation. 2024; 10(1):36. https://doi.org/10.3390/fermentation10010036
Chicago/Turabian StyleAfonso, Sílvia Martins, António Inês, and Alice Vilela. 2024. "Bio-Dealcoholization of Wines: Can Yeast Make Lighter Wines?" Fermentation 10, no. 1: 36. https://doi.org/10.3390/fermentation10010036