Valorisation Process Using Lactic Acid Bacteria Fermentation Induces Significant Changes in the Physical and Functional Properties of Brewers Spent Yeast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. α-,β-, and Total Glucans
2.3. Fatty Acid Composition
2.4. FODMAPs and Trehalose
2.5. Resistant, Digestible, and Total Starch
2.6. pH and Total Titratable Acidity (TTA)
2.7. Foaming Capacity and Stability
2.8. Water- and Oil-Holding Capacities
2.9. Surface Hydrophobicity
2.10. Isoelectric Point Determination
2.11. Protein Solubility
2.12. Particle Size
2.13. Emulsion Properties
2.14. Minimum Gelation Concentration
2.15. Rheological Analysis
2.16. Statistical Analysis
3. Results
3.1. α-, β-, and Total Glucans
3.2. Lipid and Fatty Acid Composition
3.3. Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols
3.4. Resistant and Digestible Carbohydrates
3.5. pH and Total Titratable Acidity
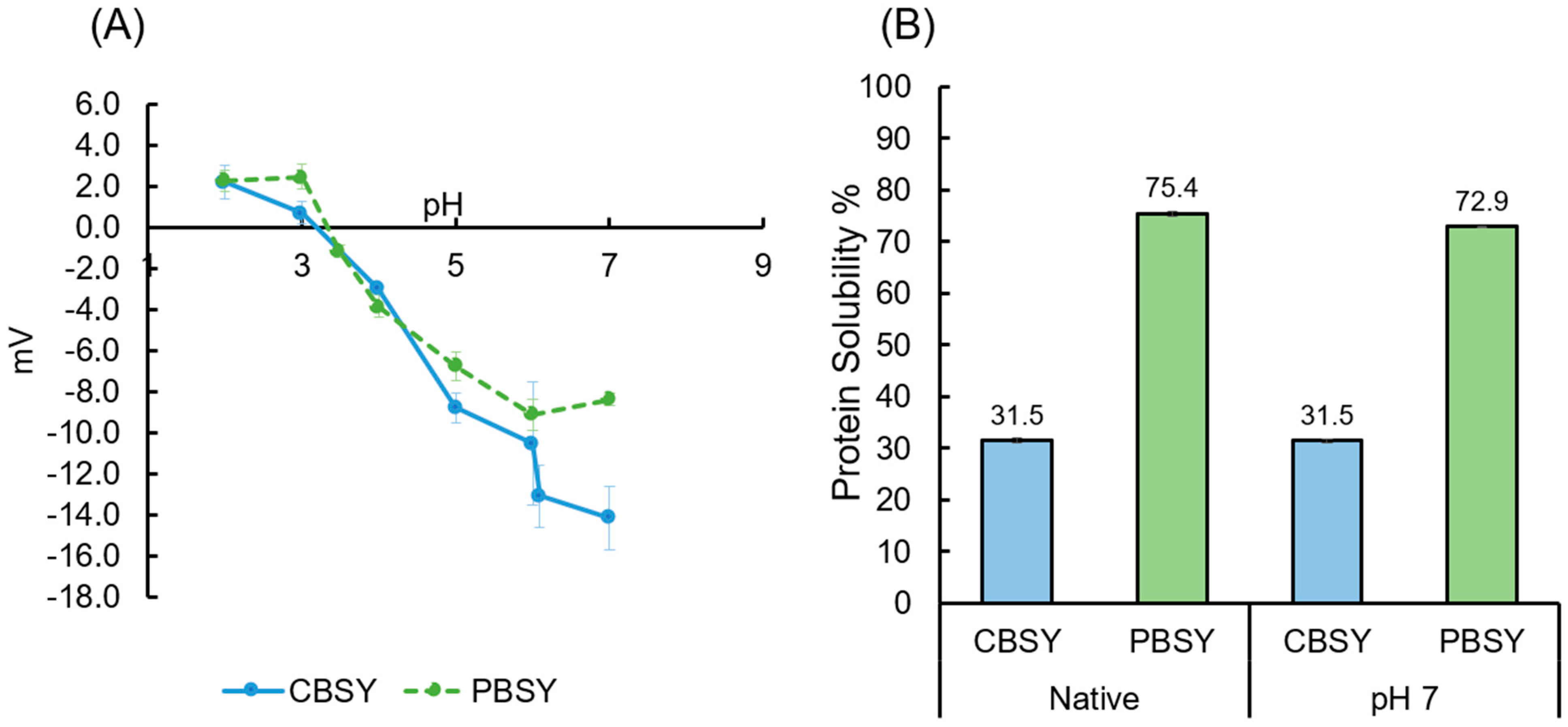
3.6. Surface Hydrophobicity, Zeta Potential, and Protein Solubility
3.7. Oil- and Water-Holding Capacity
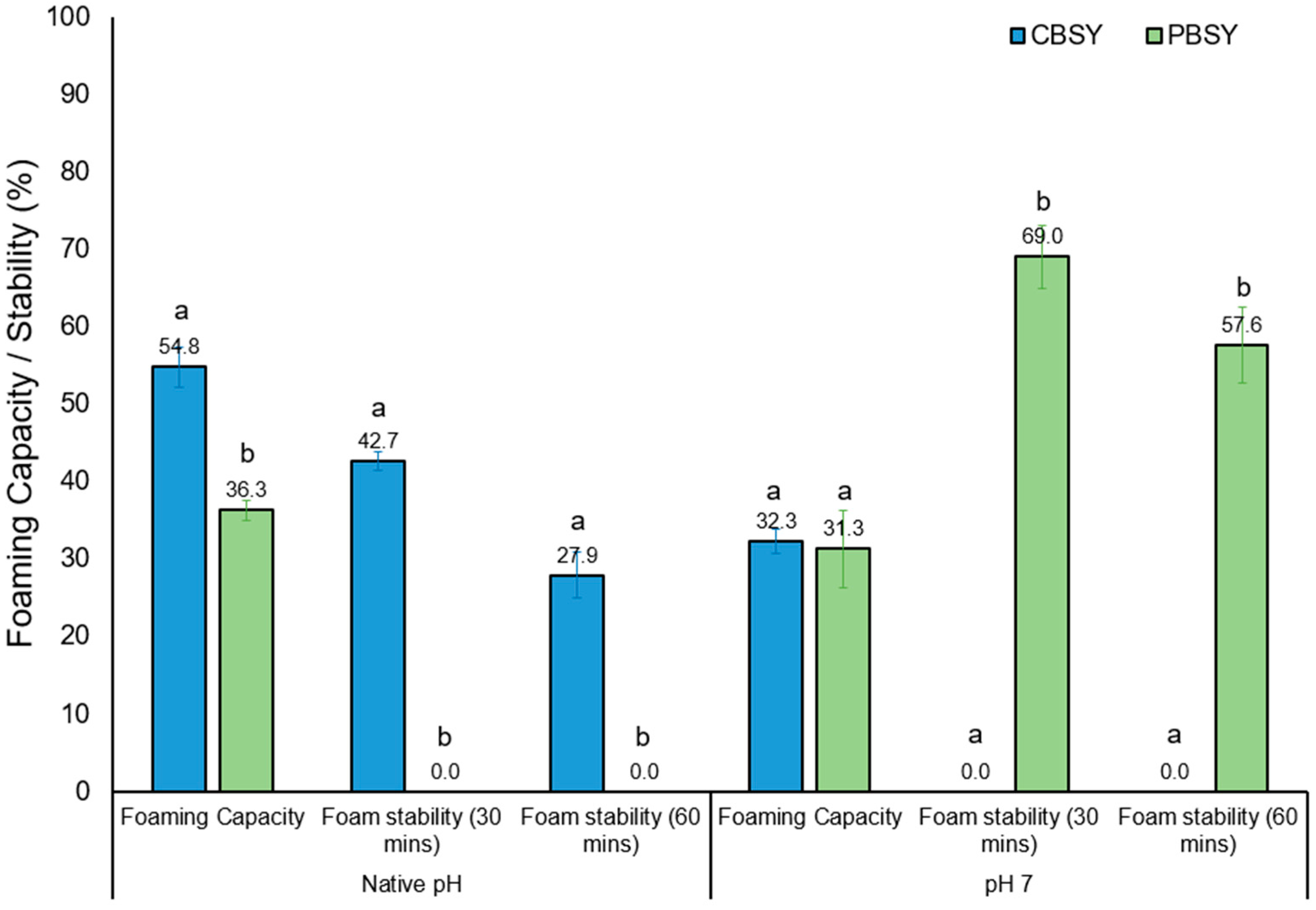
3.8. Foaming Capacity and Stability
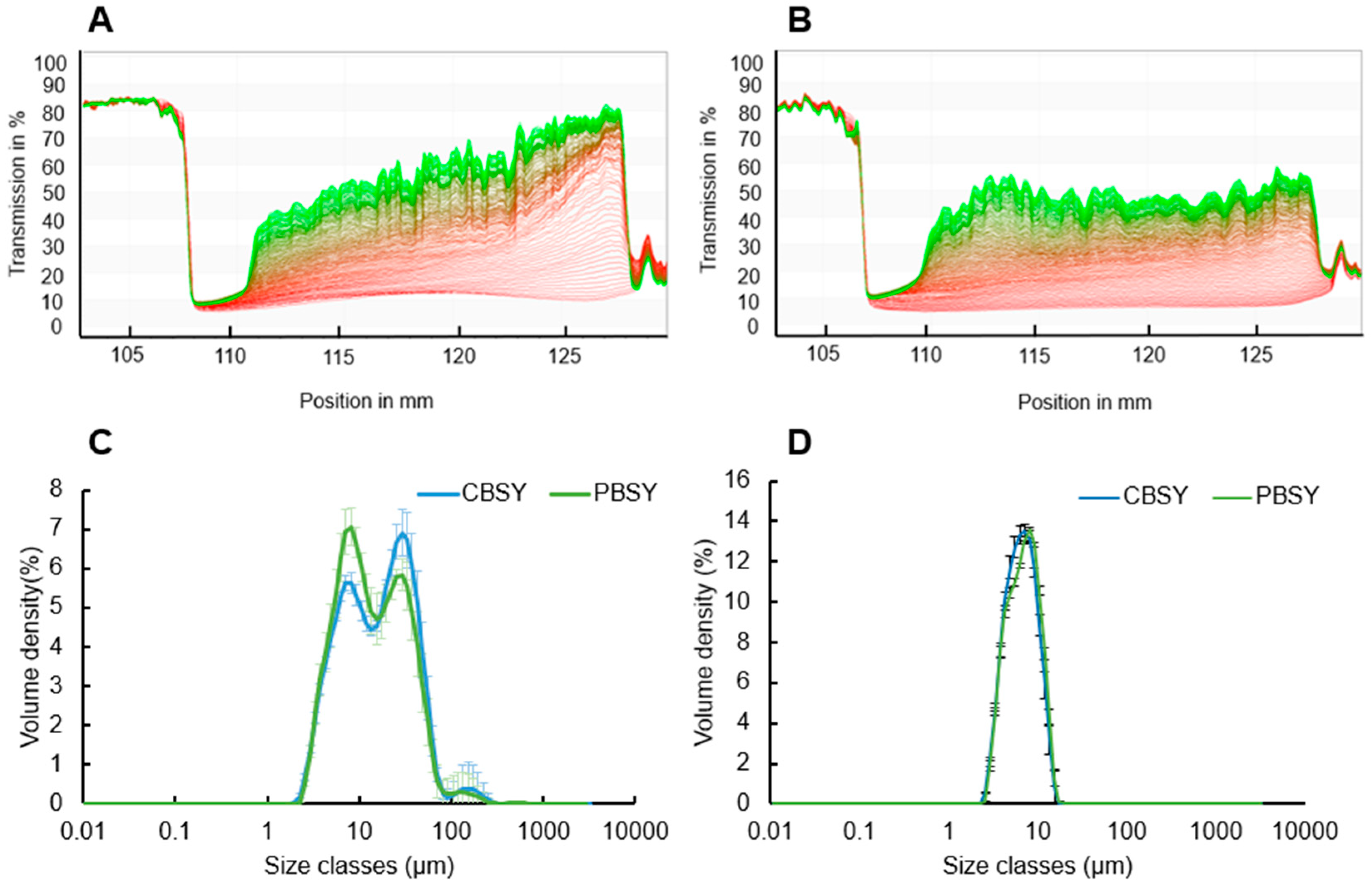
3.9. Particle Size Distribution
3.10. Stabilisation Behaviour and Emulsion Particle Size Distribution
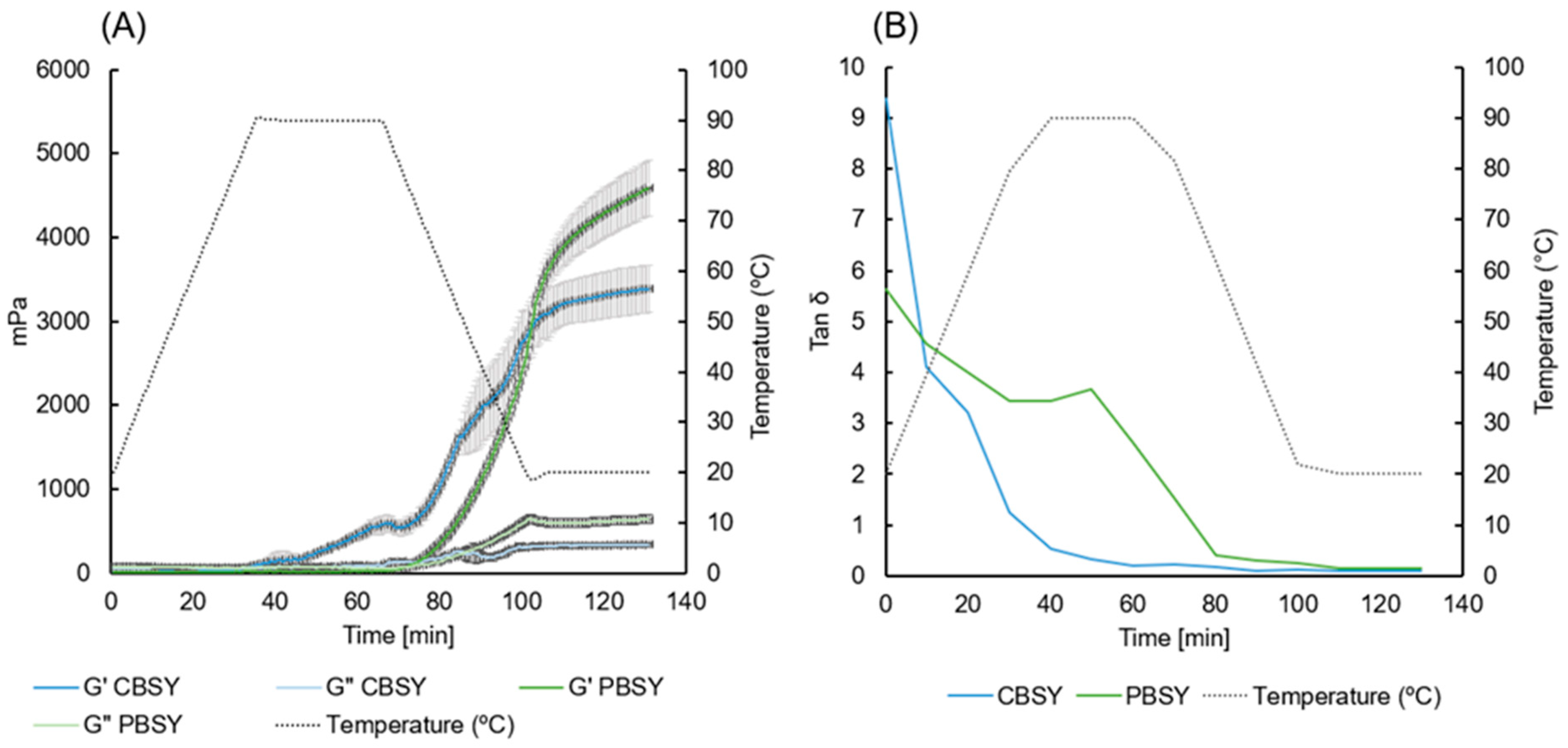
3.11. Rheological Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunze, W. Technology Brewing and Malting, 2nd ed.; VLB: Berlin, Germany, 1999. [Google Scholar]
- Jan Conway Global Beer Production 1998–2022. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 30 January 2024).
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Burki, T. Food Security and Nutrition in the World. Lancet Diabetes Endocrinol. 2022, 10, 622. [Google Scholar] [CrossRef]
- Shotipruk, A.; Kittianong, P.; Suphantharika, M.; Muangnapoh, C. Application of Rotary Microfiltration in Debittering Process of Spent Brewer’s Yeast. Bioresour. Technol. 2005, 96, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of Protein-Rich Extracts from Spent Brewer’s Yeast (Saccharomyces cerevisiae): An Overview. Biomass Convers. Biorefinery 2022, 11, 4002. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Odila Pereira, J.; Ferreira, C.; Faustino, M.; Durão, J.; Pereira, A.M.; Oliveira, C.M.; Pintado, M.E.; Carvalho, A.P. Spent Yeast Valorization for Food Applications: Effect of Different Extraction Methodologies. Foods 2022, 11, 4002. [Google Scholar] [CrossRef]
- Olivares-Galván, S.; Marina, M.L.; García, M.C. Extraction of Valuable Compounds from Brewing Residues: Malt Rootlets, Spent Hops, and Spent Yeast. Trends Food Sci. Technol. 2022, 127, 181–197. [Google Scholar] [CrossRef]
- Ma, C.; Xia, S.; Song, J.; Hou, Y.; Hao, T.; Shen, S.; Li, K.; Xue, C.; Jiang, X. Yeast Protein as a Novel Dietary Protein Source: Comparison with Four Common Plant Proteins in Physicochemical Properties. Curr. Res. Food Sci. 2023, 7, 100555. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein-State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations 2022, 9, 178. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Jaeger, A.; Nyhan, L.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Lactic Acid Fermentation as a Valorising Agent for Brewer’s Spent Yeast—Improving the Sensory Quality and Nutritional Potential. Fermentation 2024, 10, 54. [Google Scholar] [CrossRef]
- ISO 16958:2015|IDF 231:2015; International Organization for Standardization Determination of Fatty Acids Composition—Milk, Milk Products, Infant Formula and Adult Nutritionals. Capillary Gas Chromatographic Method. International Organization for Standardization: Geneva, Switzerland, 2015.
- Ispiryan, L.; Heitmann, M.; Hoehnel, A.; Zannini, E.; Arendt, E.K. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. J. Agric. Food Chem. 2019, 67, 4384–4392. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; Jeske, S.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; Wriessnegger, C.L.; O’Mahony, J.A.; Zannini, E.; Arendt, E.K. Membrane Filtration and Isoelectric Precipitation Technological Approaches for the Preparation of Novel, Functional and Sustainable Protein Isolate from Lentils. Eur. Food Res. Technol. 2019, 245, 1855–1869. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the Functional Properties of Pea, Chickpea and Lentil Protein Concentrates Processed Using Ultrafiltration and Isoelectric Precipitation Techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Hayakawa, S.; Nakai, S. Relationships of Hydrophobicity and Net Charge to the Solubility of Milk and Soy Proteins. J. Food Sci. 1985, 50, 486–491. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Chickpea, Faba Bean, Lentil and Pea Proteins Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- AACC. Method 46-12–Crude Protein—Kjeldahl Method, Boric Acid Modification 2011. In International Approved Methods of Analysis, 11th ed.; AACC: Arnold, MD, USA, 2011. [Google Scholar]
- Jaeger, A.; Sahin, A.W.; Nyhan, L.; Zannini, E.; Arendt, E.K. Functional Properties of Brewer’s Spent Grain Protein Isolate: The Missing Piece in the Plant Protein Portfolio. Foods 2023, 12, 798. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal View: Food for Thought-Western Lifestyle and Susceptibility to Crohn’s Disease. The FODMAP Hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, J.; Hu, J.; Li, Z.; Liu, R.; Liu, Y.; Cao, Y.; Ge, Q.; Yu, H. Effect of Typical Starch on the Rheological Properties and NMR Characterization of Myofibrillar Protein Gel. J. Sci. Food Agric. 2020, 100, 258–267. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Ramachandra Swamy, S.R.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Kumar, S.; Tharanathan, R.N. Grinding Characteristics and Hydration Properties of Coconut Residue: A Source of Dietary Fiber. J. Food Eng. 2006, 72, 281–286. [Google Scholar] [CrossRef]
- Escamilla-Silva, E.M.; Guzmán-Maldonado, S.H.; Cano-Medinal, A.; González-Alatorre, G. Simplified Process for the Production of Sesame Protein Concentrate. Differential Scanning Calorimetry and Nutritional, Physicochemical and Functional Properties. J. Sci. Food Agric. 2003, 83, 972–979. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of Brewers Spent Yeast Polysaccharides: A Structural Characterization Approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef]
- Alexandre, H.; Guilloux-Benatier, M. Yeast Autolysis in Sparkling Wine—A Review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Balmaseda, A.; Aniballi, L.; Rozès, N.; Bordons, A.; Reguant, C. Use of Yeast Mannoproteins by Oenococcus Oeni during Malolactic Fermentation under Different Oenological Conditions. Foods 2021, 10, 1540. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Bao, J.; Lidén, G. Optically Pure Lactic Acid Production from Softwood-Derived Mannose by Pediococcus Acidilactici. J. Biotechnol. 2021, 335, 1–8. [Google Scholar] [CrossRef]
- Van Steenwijk, H.P.; Bast, A.; De Boer, A. Immunomodulating Effects of Fungal Beta-Glucans. Nutrients 2021, 13, 1333. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal β-Glucans and Mammalian Immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vashishta, A.; Saraswat-Ohri, S.; Vetvickova, J. Immunological Effects of Yeast- and Mushroom-Derived β-Glucans. J. Med. Food 2008, 11, 615–622. [Google Scholar] [CrossRef]
- Dharsono, T.; Rudnicka, K.; Wilhelm, M.; Schoen, C. Effects of Yeast (1,3)-(1,6)-Beta-Glucan on Severity of Upper Respiratory Tract Infections: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Subjects. J. Am. Coll. Nutr. 2019, 38, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Liu, Z.; Lu, Y.; Xu, X. Effects of Yeast β-Glucans for the Prevention and Treatment of Upper Respiratory Tract Infection in Healthy Subjects: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2021, 60, 4175–4187. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.M.; Talbott, J.A. Baker’s Yeast Beta-Glucan Supplement Reduces Upper Respiratory Symptoms and Improves Mood State in Stressed Women. J. Am. Coll. Nutr. 2012, 31, 295–300. [Google Scholar] [CrossRef]
- François, J.; Parrou, J.L. Reserve Carbohydrates Metabolism in the Yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001, 25, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Peyer, L.C.; Zarnkow, M.; Jacob, F.; De Schutter, D.P.; Arendt, E.K. Sour Brewing: Impact of Lactobacillus Amylovorus FST2.11 on Technological and Quality Attributes of Acid Beers. J. Am. Soc. Brew. Chem. 2017, 75, 207–216. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Follador, R. Metabolism of Oligosaccharides and Starch in Lactobacilli: A Review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef] [PubMed]
- Hernawan, T.; Fleet, G. Chemical and Cytological Changes during the Autolysis of Yeasts. J. Ind. Microbiol. 1995, 14, 440–450. [Google Scholar] [CrossRef]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant Starch in Food: A Review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef]
- Aklujkar, P.P.; Sankh, S.N.; Arvindekar, A.U. A Simplified Method for the Isolation and Estimation of Cell Wall Bound Glycogen in Saccharomyces cerevisiae. J. Inst. Brew. 2008, 114, 205–208. [Google Scholar] [CrossRef]
- Guyot, S.; Ferret, E.; Gervais, P. Responses of Saccharomyces cerevisiae to Thermal Stress. Biotechnol. Bioeng. 2005, 92, 403–409. [Google Scholar] [CrossRef]
- Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. Trehalose Promotes the Survival of Saccharomyces cerevisiae during Lethal Ethanol Stress, but Does Not Influence Growth under Sublethal Ethanol Stress. FEMS Yeast Res. 2009, 9, 1208–1216. [Google Scholar] [CrossRef]
- Eleutherio, E.; Panek, A.; Freire, J.; Eduardo, D.M. Revisiting Yeast Trehalose Metabolism. Curr. Genet. 2015, 61, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Trevisol, E.T.V.; Panek, A.D.; Mannarino, S.C.; Eleutherio, E.C.A. The Effect of Trehalose on the Fermentation Performance of Aged Cells of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2011, 90, 697–704. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E.; Diowksz, A. Physiological State of Reused Brewing Yeast. Czech J. Food Sci. 2013, 31, 264–269. [Google Scholar] [CrossRef]
- Du Toit, M.; Dicks, L.M.T.; Holzapfel, W.H. Taxonomy of Obligately Homofermentative and Facultatively Heterofermentative Lactobacilli in Pig Faeces. Lett. Appl. Microbiol. 2001, 32, 199–204. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.J. Yeast Extract Production Using Spent Yeast from Beer Manufacture: Influence of Industrially Applicable Disruption Methods on Selected Substance Groups with Biotechnological Relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182. [Google Scholar] [CrossRef]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food Composition, Defining Cutoff Values and International Application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef]
- Lomakin, I.B.; Xiong, Y.; Steitz, T.A. The Crystal Structure of Yeast Fatty Acid Synthase, a Cellular Machine with Eight Active Sites Working Together. Cell 2007, 129, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Speers, A.; Forbes, J. Yeast: An overview. In Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 3–9. ISBN 9781782423492. [Google Scholar]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The Chemical Profiling of Fatty Acids during the Brewing Process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty Acid Synthesis and Elongation in Yeast. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 255–270. [Google Scholar] [CrossRef]
- Technology, F.; Blagovi, B. Lipid Composition of Brewer’ s Yeast. Food Technol. Biotechnol. 2015, 39, 175–181. [Google Scholar]
- Mennah-Govela, Y.A.; Singh, R.P.; Bornhorst, G.M. Buffering Capacity of Protein-Based Model Food Systems in the Context of Gastric Digestion. Food Funct. 2019, 10, 6074–6087. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering Capacity of Dairy Products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Thomas, K.C.; Hynes, S.H.; Ingledew, W.M. Influence of Medium Buffering Capacity on Inhibition of Saccharomyces cerevisiae Growth by Acetic and Lactic Acids. Appl. Environ. Microbiol. 2002, 68, 1616–1623. [Google Scholar] [CrossRef]
- Lavaisse, L.M.; Hollmann, A.; Nazareno, M.A.; Disalvo, E.A. Zeta Potential Changes of Saccharomyces cerevisiae during Fermentative and Respiratory Cycles. Colloids Surf. B Biointerfaces 2019, 174, 63–69. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Złoch, M.; Railean-Plugaru, V.; Król, A.; Rafińska, K.; Szultka-Młyńska, M.; Buszewski, B. The Influence of Different PH on the Electrophoretic Behaviour of Saccharomyces cerevisiae Modified by Calcium Ions. Sci. Rep. 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, Plasmolysis and Enzymatic Hydrolysis of Baker’s Yeast (Saccharomyces Cerevisiae): A Comparative Study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Lagos, L.; Lei, P.; Reveco-Urzua, F.E.; Morales-Lange, B.; Hansen, L.D.; Schiavone, M.; Mydland, L.T.; Arntzen, M.Ø.; Mercado, L.; et al. Down-Stream Processing of Baker’s Yeast (Saccharomyces cerevisiae)—Effect on Nutrient Digestibility and Immune Response in Atlantic Salmon (Salmo salar). Aquaculture 2021, 530, 735707. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Application of Fermentation Strategy in Aquafeed for Sustainable Aquaculture. Rev. Aquac. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- Mercier-Bonin, M.; Ouazzani, K.; Schmitz, P.; Lorthois, S. Study of Bioadhesion on a Flat Plate with a Yeast/Glass Model System. J. Colloid Interface Sci. 2004, 271, 342–350. [Google Scholar] [CrossRef]
- Marson, G.V.; Pereira, D.T.V.; da Costa Machado, M.T.; Di Luccio, M.; Martínez, J.; Belleville, M.P.; Hubinger, M.D. Ultrafiltration Performance of Spent Brewer’s Yeast Protein Hydrolysate: Impact of PH and Membrane Material on Fouling. J. Food Eng. 2021, 302, 110569. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Shimoi, H.; Sakamoto, K.; Okuda, M.; Atthi, R.; Iwashita, K.; Ito, K. The AWA1 Gene Is Required for the Foam-Forming Phenotype and Cell Surface Hydrophobicity of Sake Yeast. Appl. Environ. Microbiol. 2002, 68, 2018–2025. [Google Scholar] [CrossRef]
- Vidgren, V.; Londesborough, J. 125th Anniversary Review: Yeast Flocculation and Sedimentation in Brewing. J. Inst. Brew. 2011, 117, 475–487. [Google Scholar] [CrossRef]
- Mahmoud, M.I.; Malone, W.T.; Cordle, C.T. Enzymatic Hydrolysis of Casein: Effect of Degree of Hydrolysis on Antigenicity and Physical Properties. J. Food Sci. 1992, 57, 1223–1229. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, X.; Huang, L.; Chen, Z.; Cheng, J. Influence of Hydrolysis Behaviour and Microfluidisation on the Functionality and Structural Properties of Collagen Hydrolysates. Food Chem. 2017, 227, 211–218. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Jin, Y.L.; Speers, R.A. Effect of Enviromnental Conditions on the Flocculation of Saccharomyces cerevisiae. J. Am. Soc. Brew. Chem. 2000, 58, 108–116. [Google Scholar] [CrossRef]
- Murray, B.S.; Ettelaie, R. Foam Stability: Proteins and Nanoparticles. Curr. Opin. Colloid Interface Sci. 2004, 9, 314–320. [Google Scholar] [CrossRef]
- Rez, A.; Mason, T.J.; Lelas, V.; Herceg, Z. Effect of Ultrasound Treatment on Solubility and Foaming Properties of Whey Protein Suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- Kinsella, J.E. Functional Properties of Proteins: Possible Relationships between Structure and Function in Foams. Food Chem. 1981, 7, 273–288. [Google Scholar] [CrossRef]
- Nastaj, M.; Sołowiej, B.G. The Effect of Various PH Values on Foaming Properties of Whey Protein Preparations. Int. J. Dairy Technol. 2020, 73, 683–694. [Google Scholar] [CrossRef]
- Tang, T.; Liu, J.; Tang, S.; Xiao, N.; Jiang, Y.; Tu, Y.; Xu, M. Effects of Soy Peptides and PH on Foaming and Physicochemical Properties of Egg White Powder. LWT 2022, 153, 112503. [Google Scholar] [CrossRef]
- Imura, T.; Nakayama, M.; Taira, T.; Sakai, H.; Abe, M.; Kitamoto, D. Interfacial and Emulsifying Properties of Soybean Peptides with Different Degrees of Hydrolysis. J. Oleo Sci. 2015, 64, 183–189. [Google Scholar] [CrossRef]
- Lekkas, C.; Hill, A.E.; Taidi, B.; Hodgson, J.; Stewart, G.G. The Role of Small Wort Peptides in Brewing Fermentations. J. Inst. Brew. 2009, 115, 134–139. [Google Scholar] [CrossRef]
- Núñez, Y.P.; Carrascosa, A.V.; González, R.; Polo, M.C.; Martínez-Rodríguez, A. Isolation and Characterization of a Thermally Extracted Yeast Cell Wall Fraction Potentially Useful for Improving the Foaming Properties of Sparkling Wines. J. Agric. Food Chem. 2006, 54, 7898–7903. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion Stability. In Food Hydrocolloids: Structures, Properties, and Functions; Springer: Boston, MA, USA, 1994; pp. 387–398. [Google Scholar] [CrossRef]
- Firoozmand, H.; Rousseau, D. Microbial Cells as Colloidal Particles: Pickering Oil-in-Water Emulsions Stabilized by Bacteria and Yeast. Food Res. Int. 2016, 81, 66–73. [Google Scholar] [CrossRef]
- Moreira, T.C.P.; da Silva, V.M.; Gombert, A.K.; da Cunha, R.L. Stabilization Mechanisms of Oil-in-Water Emulsions by Saccharomyces cerevisiae. Colloids Surf. B Biointerfaces 2016, 143, 399–405. [Google Scholar] [CrossRef]
- Siddiqi, S.F.; Bulmer, M.; Ayazi Shamlou, P.; Titchener-Hooker, N.J. The Effects of Fermentation Conditions on Yeast Cell Debris Particle Size Distribution during High Pressure Homogenisation. Bioprocess Eng. 1995, 14, 1–8. [Google Scholar] [CrossRef]
- Milo, R.; Phillips, R. Cell Biology by the Numbers. In Physical Biology; Taylor and Francis Group: Oxford, UK, 2015; pp. 217–246. [Google Scholar]
- Zakhartsev, M.; Reuss, M. Cell Size and Morphological Properties of Yeast Saccharomyces cerevisiae in Relation to Growth Temperature. FEMS Yeast Res. 2018, 18, foy052. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, Y.; Yin, Y.; Zhao, H.; Li, X.; Yi, S.; Li, J. Improvement of the Gel Properties and Flavor Adsorption Capacity of Fish Myosin upon Yeast β-Glucan Incorporation. Food Chem. 2022, 397, 133766. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of Yeast β-Glucan on Gel Properties, Spatial Structure and Sensory Characteristics of Silver Carp Surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Worrasinchai, S.; Suphantharika, M.; Pinjai, S.; Jamnong, P. β-Glucan Prepared from Spent Brewer’s Yeast as a Fat Replacer in Mayonnaise. Food Hydrocoll. 2006, 20, 68–78. [Google Scholar] [CrossRef]
- Puppo, M.C.; Añón, M.C. Soybean Protein Dispersions at Acid PH. Thermal and Rheological Properties. J. Food Sci. 1999, 64, 50–56. [Google Scholar] [CrossRef]
- Khemakhem, M.; Attia, H.; Ayadi, M.A. The Effect of PH, Sucrose, Salt and Hydrocolloid Gums on the Gelling Properties and Water Holding Capacity of Egg White Gel. Food Hydrocoll. 2019, 87, 11–19. [Google Scholar] [CrossRef]
| g/100 g DM | ||
|---|---|---|
| CBSY | PBSY | |
| Proteins | 35.588 ± 1.501 | 38.736 ± 1.638 |
| Total Fats | 1.361 ± 0.089 | 1.393 ± 0.001 |
| Carbohydrates | ||
| Insoluble Dietary Fibre | 44.367 ± 1.972 | 36.723 ± 1.767 |
| High MW Soluble | 3.130 ± 0.482 | 2.095 ± 0.386 |
| Low MW Soluble | 2.412 ± 0.064 | 0.925 ± 0.140 |
| Total Dietary Fibre | 49.909 ± 2.122 | 39.731 ± 1.603 |
| Total Sugars | 0.301 ± 0.019 | 10.048 ± 0.644 |
| Ash | 5.628 ± 0.322 | 3.429 ± 0.211 |
| Moisture | 6.710 ± 0.380 | 14.530 ± 0.380 |
| Energy value (kcal) | 314 ± 10 | 294 ± 10 |
| Energy value (kJ) | 1322 ± 45 | 1237 ± 47 |
| mg/kg | ||
| Calcium | 2320 ± 190 | 2700 ± 220 |
| Iron | 65 ± 13 | 68 ± 14 |
| Phosphorus | 9860 ± 790 | 6600 ± 530 |
| Magnesium | 2060 ± 230 | 1310 ± 140 |
| Manganese | 5.7 ± 1.3 | 5.5 ± 1.2 |
| Potassium | 14,900 ± 1500 | 4950 ± 480 |
| Copper | 3.16 ± 0.67 | 3.36 ± 0.71 |
| Sodium | 271 ± 26 | 166 ± 21 |
| Zinc | 72 ± 14 | 78 ± 15 |
| Total | 29,556 | 15,880 |
| g/100g DM | ||
| Glucose | 0.166 ± 0.014 | n.d. |
| Fructose | 0.090 ± 0.009 | 9.924 ± 0.644 |
| Lactose | n.d. | n.d. |
| Sucrose | n.d. | n.d. |
| Maltose | 0.045 ± 0.010 | 0.124 ± 0.020 |
| Total Sugars | 0.301 ± 0.019 | 10.048 ± 0.644 |
| IBU (mg/L) | ||
| Iso-α-acids (IBU) | 129 | 100 |
| g/100 g | |||
|---|---|---|---|
| Carbohydrates | CBSY | PBSY | |
| FODMAPS | |||
| Mono-/disaccharides | Glucose | 0.069 ± 0.003 a | 0.790 ± 0.015 b |
| Fructose | 0.020 ± 0.000 a | 7.994 ± 0.251 b | |
| Excess fructose | - | 7.204 * | |
| Polyols | Xylitol | 0.091 ± 0.001 a | 0.021 ± 0.000 b |
| Sorbitol | 0.035 ± 0.000 a | 0.011 ± 0.000 b | |
| Mannitol | 0.057 ± 0.001 a | 0.043 ± 0.001 b | |
| Total polyols | 0.183 | 0.075 | |
| Oligosaccharides | Raffinose/Stachyose | 0.080 ± 0.001 a | 0.138 ± 0.005 b |
| Verbascose | n.d. | n.d. | |
| Total GOS | 0.08 | 0.138 | |
| Total fructan | n.d. ** | n.d. ** | |
| Other | Trehalose | 5.275 ± 0.056 a | 0.403 ± 0.007 b |
| g/100 g DM | |||
| Glucans | |||
| Total glucan | 39.788 ± 0.443 a | 26.316 ± 0.693 b | |
| α-glucan | 14.822 ± 0.607 a | 11.319 ± 0.356 b | |
| β-glucan | 24.966 ± 0.753 a | 14.997 ± 0.379 b | |
| Resistant starch | 10.550 ± 0.323 a | 5.670 ± 0.271 b | |
| Digestible starch | 17.10 ± 2.06 a | 12.38 ± 0.12 b | |
| Fatty acids | |||
| Caprylic acid (C8:0) | 0.056 ± 0.013 | 0.069 ± 0.015 | |
| Capric acid (C10:0) | 0.135 ± 0.029 | 0.129 ± 0.027 | |
| Lauric acid (C12:0) | 0.030 ± 0.006 | 0.036 ± 0.008 | |
| Myristic acid (C14:0) | 0.004 ± 0.001 | 0.005 ± 0.001 | |
| Palmitic acid (C16:0) | 0.388 ± 0.059 | 0.444 ± 0.066 | |
| Palmitoleic acid (C16:1) | 0.227 ± 0.046 | 0.250 ± 0.050 | |
| Stearic acid (C18:0) | 0.155 ± 0.033 | 0.174 ± 0.036 | |
| Octadecenoic acid (18: 1 total) | 0.101 ± 0.018 | 0.110 ± 0.019 | |
| Octadecenoic acid (18:1 all trans) | 0.013 ± 0.003 | 0.016 ± 0.003 | |
| Cisoctadecenoic acid (18:1 all cis) | 0.088 ± 0.018 | 0.094 ± 0.018 | |
| Linoleic acid (18:2 n6) | 0.028 ± 0.005 | 0.030 ± 0.006 | |
| Octadecatrienoic acid (total 18:3) | 0.005 ± 0.001 | 0.006 ± 0.001 | |
| CBSY | PBSY | ||
|---|---|---|---|
| pH | 6.090 ± 0.000 a | 3.493 ± 0.006 b | |
| TTA (mL 0.1 M NaOH/g) | 2.577 ± 0.040 a | 15.508 ± 0.362 b | |
| Surface hydrophobicity | 2906.933 ± 20.387 a | 189.867 ± 26.942 b | |
| Protein solubility (%) | Native pH | 31.531 ± 0.420 a | 75.426 ± 0.419 b |
| pH 7 | 31.508 ± 0.331 a | 72.916 ± 0.174 b | |
| Zeta potential (mV) | Native pH | −13.1167 ± 1.532 a | −1.179 ± 0.301 b |
| pH 7 | −14.1778 ± 1.525 a | −8.393 ± 0.284 b | |
| Oil-holding capacity (%) | 178.098 ± 1.379 a | 183.310 ± 3.186 a | |
| Water-holding capacity (%) | 52.704 ± 1.587 a | 48.701 ± 0.321 b | |
| Minimum gelling concentration (%w/w) | >20% | >20% | |
| µm | CBSY | PBSY | |
| Particle size | D [3,2] | 6.022 ± 0.114 a | 6.274 ± 0.075 b |
| D [4,3] | 6.976 ± 0.192 a | 7.322 ± 0.103 a | |
| Dv [10] | 3.834 ± 0.032 a | 3.912 ± 0.030 b | |
| Dv [50] | 6.532 ± 0.156 a | 6.941 ± 0.098 b | |
| Dv [90] | 10.844 ± 0.383 a | 11.378 ± 0.222 a | |
| Emulsion particle size | D [3,2] | 10.756 ± 0.204 a | 10.049 ± 0.612 a |
| D [4,3] | 22.967 ± 3.800 a | 20.611 ± 4.170 a | |
| Dv [10] | 4.736 ± 0.103 a | 4.711 ± 0.212 a | |
| Dv [50] | 16.311 ± 0.650 a | 12.967 ± 1.323 b | |
| Dv [90] | 46.267 ± 4.729 a | 43.022 ± 5.094 a | |
| Separation rate | [%/minute] | 3.462 ± 0.067 a | 2.979 ± 0.065 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeger, A.; Nyhan, L.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Valorisation Process Using Lactic Acid Bacteria Fermentation Induces Significant Changes in the Physical and Functional Properties of Brewers Spent Yeast. Fermentation 2024, 10, 136. https://doi.org/10.3390/fermentation10030136
Jaeger A, Nyhan L, Sahin AW, Zannini E, Arendt EK. Valorisation Process Using Lactic Acid Bacteria Fermentation Induces Significant Changes in the Physical and Functional Properties of Brewers Spent Yeast. Fermentation. 2024; 10(3):136. https://doi.org/10.3390/fermentation10030136
Chicago/Turabian StyleJaeger, Alice, Laura Nyhan, Aylin W. Sahin, Emanuele Zannini, and Elke K. Arendt. 2024. "Valorisation Process Using Lactic Acid Bacteria Fermentation Induces Significant Changes in the Physical and Functional Properties of Brewers Spent Yeast" Fermentation 10, no. 3: 136. https://doi.org/10.3390/fermentation10030136







