The Hard Reality of Biogas Production through the Anaerobic Digestion of Algae Grown in Dairy Farm Effluents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rotating Algal Biofilm Reactor
2.2. Algae Selection
2.3. Theoretical Bioremediation of Algae
2.4. Economic Analysis
3. Results and Discussion
3.1. Biogas Potential According to Different Species
3.2. Theoretical Bioremediation of Algae
3.3. Technoeconomic Assessment
3.3.1. Capital Costs
3.3.2. Operating Costs
3.3.3. Profitability Analysis
3.3.4. Economy of Scale
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, Y.K.; Jang, H.M.; Kan, E. Microalgal Biomass and Lipid Production on Dairy Effluent Using a Novel Microalga, Chlorella sp. Isolated from Dairy Wastewater. Biotechnol. Bioprocess Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Pizarro, C.; Kebede-Westhead, E. Treatment of dairy manure effluent using freshwater algae: Algal productivity and recovery of manure nutrients using pilot-scale algal turf scrubbers. Bioresour. Technol. 2008, 99, 8137–8142. [Google Scholar] [CrossRef]
- Passero, M.; Cragin, B.; Coats, E.R.; McDonald, A.G.; Feris, K. Dairy Wastewaters for Algae Cultivation, Polyhydroxyalkanote Reactor Effluent Versus Anaerobic Digester Effluent. Bioenergy Res. 2015, 8, 1647–1660. [Google Scholar] [CrossRef]
- Pizarro, C.; Mulbry, W.; Blersch, D.; Kangas, P. An economic assessment of algal turf scrubber technology for treatment of dairy manure effluent. Ecol. Eng. 2006, 26, 321–327. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Le, V.-A.; Do, Q.-H.; Le, T.-N.-C.; Vo, T.-D.-H. Emerging revolving algae biofilm system for algal biomass production and nutrient recovery from wastewater. Sci. Total Environ. 2024, 912, 168911. [Google Scholar] [CrossRef]
- Craggs, R.J.; Tanner, C.C.; Sukias, J.P.S.; Davies-Colley, R.J. Dairy farm wastewater treatment by an advanced pond system. Water Sci. Technol. 2003, 48, 291–297. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. 15 years of research on wastewater treatment high rate algal ponds in New Zealand: Discoveries and future directions. N. Z. J. Bot. 2020, 58, 334–357. [Google Scholar] [CrossRef]
- Christenson, L.B.; Sims, R.C. Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol. Bioeng. 2012, 109, 1674–1684. [Google Scholar] [CrossRef]
- Christenson, L. Algal Biofilm Production and Harvesting System for Wastewater Treatment with Biofuels By-Products; Utah State University: Logan, UT, USA, 2011; p. 103. [Google Scholar]
- Woolsey, P.A. Rotating Algal Biofilm Reactors: Mathematical Modeling and Lipid Production; Utah State University: Logan, UT, USA, 2011; p. 108. [Google Scholar]
- Fica, Z.T.; Sims, R.C. Algae-based biofilm productivity utilizing dairy wastewater: Effects of temperature and organic carbon concentration. J. Biol. Eng. 2016, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Pavan, P.; Mata-Alvarez, J. Anaerobic co-digestion of sewage sludge: Application to the macroalgae from the Venice lagoon. Resour. Conserv. Recycl. 1996, 17, 57–66. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Rokicka, M.; Kupczyk, K. The possibility of using macroalgae biomass from natural reservoirs as a substrate in the methane fermentation process. Int. J. Green Energy 2015, 12, 970–977. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, A.; Posten, C. Fate of H2S during the cultivation of Chlorella sp. deployed for biogas upgrading. J. Environ. Manag. 2017, 191, 252–257. [Google Scholar] [CrossRef]
- Hinks, J.; Edwards, S.; Sallis, P.J.; Caldwell, G.S. The steady state anaerobic digestion of Laminaria hyperborea--effect of hydraulic residence on biogas production and bacterial community composition. Bioresour. Technol. 2013, 143, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Karray, R.; Hamza, M.; Sayadi, S. Production and characterization of enzymatic cocktail produced by Aspergillus niger using green macroalgae as nitrogen source and its application in the pre-treatment for biogas production from Ulva rigida. Bioresour. Technol. 2016, 216, 622–628. [Google Scholar] [CrossRef]
- Karray, R.; Karray, F.; Loukil, S.; Mhiri, N.; Sayadi, S. Anaerobic co-digestion of Tunisian green macroalgae Ulva rigida with sugar industry wastewater for biogas and methane production enhancement. Waste Manag. 2017, 61, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kocer, A.T.; Ozcimen, D. Investigation of the biogas production potential from algal wastes. Waste Manag. Res. 2018, 36, 1100–1105. [Google Scholar] [CrossRef]
- Meier, L.; Stará, D.; Bartacek, J.; Jeison, D. Removal of H2S by a continuous microalgae-based photosynthetic biogas upgrading process. Process Saf. Environ. Prot. 2018, 119, 65–68. [Google Scholar] [CrossRef]
- Wirth, R.; Lakatos, G.; Bojti, T.; Maroti, G.; Bagi, Z.; Kis, M.; Kovacs, A.; Acs, N.; Rakhely, G.; Kovacs, K.L. Metagenome changes in the mesophilic biogas-producing community during fermentation of the green alga Scenedesmus obliquus. J. Biotechnol. 2015, 215, 52–61. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Murphy, J.D. Innovation in biological production and upgrading of methane and hydrogen for use as gaseous transport biofuel. Biotechnol. Adv. 2016, 34, 451–472. [Google Scholar] [CrossRef]
- Hull-Cantillo, M.; Lay, M.; Rosentrater, K. Agriculture waste bioremediation with algae and potential for methane production. In An Integration of Phycoremediation Processes in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 419–450. [Google Scholar]
- Allen, E.; Wall, D.M.; Herrmann, C.; Murphy, J.D. Investigation of the optimal percentage of green seaweed that may be co-digested with dairy slurry to produce gaseous biofuel. Bioresour. Technol. 2014, 170, 436–444. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Choudhary, P.; Malik, A.; Vijay, V.K. Algae mediated treatment and bioenergy generation process for handling liquid and solid waste from dairy cattle farm. Bioresour. Technol. 2014, 167, 260–268. [Google Scholar] [CrossRef]
- Briand, X.; Morand, P. Anaerobic digestion of Ulva sp. 1. Relationship between Ulva composition and methanisation. J. Appl. Phycol. 1997, 9, 511–524. [Google Scholar]
- Cabrita, T.M.; Santos, M.T. Biochemical Methane Potential Assays for Organic Wastes as an Anaerobic Digestion Feedstock. Sustainability 2023, 15, 11573. [Google Scholar] [CrossRef]
- Hull-Cantillo, M.; Lay, M.; Kovalsky, P. Anaerobic Digestion of Dairy Effluent in New Zealand, Time to Revisit the Idea? Energies 2023, 16, 2859. [Google Scholar] [CrossRef]
- New Zealand Dairy Statistics; Dairy NZ: Hamilton, New Zealand; LIC: Mumbai, India, 2022–2023.
- Park, J.B.; Craggs, R.J. Biogas production from anaerobic waste stabilisation ponds treating dairy and piggery wastewater in New Zealand. Water Sci. Technol. 2007, 55, 257–264. [Google Scholar] [CrossRef]
- Hariz, H.B.; Lawton, R.J.; Craggs, R.J. Effects of seeding method and single versus mixed species assemblages on the performance of Filamentous Algae Nutrient Scrubbers (FANS) for the treatment of agricultural drainage. Agric. Water Manag. 2023, 280, 108238. [Google Scholar] [CrossRef]
- Weiland, P. Fundamentals of methane fermentation-biology and substrates; Grundlagen der Methangaerung-Biologie und Substrate. Biogas Als Regen. Energ.—Stand Und Perspekt. VDI-Ber. 2001, 1620, 19–32. [Google Scholar]
- Baserga, U. Land wirtschaftliche Co-Vergärungs-Biogasanlagen. FAT-Berichte Nr. 512, Eidg. Forschungsanstalt für Agrarwirtschaft und Landtechnik, Tänikon, Schweiz. Cited by Gerber, M., Span, R., 2008. In Proceedings of the An Analysis of Available Mathematical Models for Anaerobic Digestion of Organic Substances for Production of Biogas”, International Gas Union Research Conference, Paris, France, 8–10 October 2008. [Google Scholar]
- Ingenieure, V.D. VDI 4630: Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Beuth Verlag: Berlin, Germany, 2016. [Google Scholar]
- Weissbach, F. Evaluation of the renewable primary products for biogas production. Part I: Gas production potential of the fermentable nutrients. Pflanzenbauwissenschaften 2009, 13, 72–85. [Google Scholar]
- Neveux, N.; Yuen, A.K.L.; Jazrawi, C.; He, Y.; Magnusson, M.; Haynes, B.S.; Masters, A.F.; Montoya, A.; Paul, N.A.; Maschmeyer, T.; et al. Pre- and post-harvest treatment of macroalgae to improve the quality of feedstock for hydrothermal liquefaction. Algal Res. 2014, 6, 22–31. [Google Scholar] [CrossRef]
- Ge, S.; Madill, M.; Champagne, P. Use of freshwater macroalgae Spirogyra sp. for the treatment of municipal wastewaters and biomass production for biofuel applications. Biomass Bioenergy 2018, 111, 213–223. [Google Scholar] [CrossRef]
- Moore, J.W. Seasonal changes in the proximate and fatty acid composition of some naturally grown freshwater chlorophytes. J. Phycol. 1975, 11, 205–211. [Google Scholar] [CrossRef]
- Renaud, S.M.; Parry, D.L.; Thinh, L.-V. Microalgae for use in tropical aquaculture I: Gross chemical and fatty acid composition of twelve species of microalgae from the Northern Territory, Australia. J. Appl. Phycol. 1994, 6, 337–345. [Google Scholar] [CrossRef]
- Nordin, N.; Yusof, N.; Samsudin, S. Biomass Production of Chlorella sp., Scenedesmus sp., and Oscillatoria sp. in Nitrified Landfill Leachate. Waste Biomass Valorization 2017, 8, 2301–2311. [Google Scholar] [CrossRef]
- Fang, H.; Zhuang, Z.; Huang, L.; Zhao, W.; Niu, J. Dietary Klebsormidium sp. Supplementation Improves Growth Performance, Antioxidant and Anti-Inflammatory Status, Metabolism, and Mid-Intestine Morphology of Litopenaeus Vannamei. Front. Nutr. 2022, 9, 857351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Y.; Cui, B.; Wang, H.; Liu, T. Evaluation of filamentous green algae as feedstocks for biofuel production. Bioresour. Technol. 2016, 220, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemical Engineers, 4th ed.; McGraw-Hill chemical engineering series; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Wang, X.-m.; Chang, S.; Kovalsky, P.; Waite, T.D. Multiphase flow models in quantifying constant pressure dead-end filtration and subsequent cake compression: 1. Dilute slurry filtration. J. Membr. Sci. 2008, 308, 35–43. [Google Scholar] [CrossRef]
- Kovalsky, P.; Gedrat, M.; Bushell, G.; Waite, T.D. Compressible cake characterization from steady-state filtration analysis. AIChE J. 2007, 53, 1483–1495. [Google Scholar] [CrossRef]
- Gagnon, L.; Bélanger, C.; Uchiyama, Y. Life-cycle assessment of electricity generation options: The status of research in year 2001. Energy Policy 2002, 30, 1267–1278. [Google Scholar] [CrossRef]
- Raugei, M.; Fullana-i-Palmer, P.; Fthenakis, V. The energy return on energy investment (EROI) of photovoltaics: Methodology and comparisons with fossil fuel life cycles. Energy Policy 2012, 45, 576–582. [Google Scholar] [CrossRef]
- Ansari, F.A.; Wahal, S.; Gupta, S.K.; Rawat, I.; Bux, F. A comparative study on biochemical methane potential of algal substrates: Implications of biomass pre-treatment and product extraction. Bioresour. Technol. 2017, 234, 320–326. [Google Scholar] [CrossRef]
- Barlow, J.; Sims, R.C.; Quinn, J.C. Techno-economic and life-cycle assessment of an attached growth algal biorefinery. Bioresour. Technol. 2016, 220, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; White, M.A.; Colosi, L.M. Environmental and economic assessment of integrated systems for dairy manure treatment coupled with algae bioenergy production. Bioresour. Technol. 2013, 130, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J. Farm Prices Steady—REINZ. Dairy News, 8 February 2023. [Google Scholar]
- Song, X.; Kong, F.; Liu, B.-F.; Song, Q.; Ren, N.-Q.; Ren, H.-Y. Combined transcriptomic and metabolomic analyses of temperature response of microalgae using waste activated sludge extracts for promising biodiesel production. Water Res. 2024, 251, 121120. [Google Scholar] [CrossRef] [PubMed]

| Species | Protein | Lipid | Carbohydrate | Biogas (m3/year) | Methane (m3/year) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | |||||
| Oedogonium sp. | 38% | 13% | 50% | 11.9 | 63.5 | 6.4 | 39.0 | [35] |
| Spirogyra sp. | 25% | 9% | 66% | 11.9 | 135.5 | 6.3 | 90.6 | [36] |
| Cladophora sp. | 16% | 19% | 65% | 12.6 | 161.2 | 7.0 | 109.6 | [37] |
| Melosira sp. | 48% | 40% | 12% | 13.1 | 139.8 | 8.0 | 101.3 | [38] |
| Oscillatoria sp. | 62% | 8% | 30% | 11.2 | 80.7 | 5.9 | 55.3 | [39] |
| Klebsormidium sp. | 23% | 32% | 29.3% | 11.2 | 140.4 | 6.7 | 99.2 | [40] |
| Ulothrix sp. | 13% | 19% | 68% | 12.6 | 165.4 | 7.0 | 112.2 | [37] |
| Stigeoclonium sp. | 22% | 18.6% | 43.4% | 10.6 | 125.6 | 5.9 | 86.4 | [41] |
| Characteristic | Liquid |
|---|---|
| Volatile solids (VSs) | 1850 g/m3 |
| Total solids (TSs) | 3300 g/m3 |
| Total nitrogen | 290 g/m3 |
| Total ammoniacal-N | 178 g/m3 |
| Nitrate-N + Nitrite-N | <0.10 g/m3 |
| Total Kjeldahl Nitrogen | 290 g/m3 |
| Biochemical oxygen demand a | 720 g O2/m3 |
| Chemical oxygen demand | 3000 g O2/m3 |
| Total Carbon | 1380 g/m3 |
| Oil and grease | 310 g/m3 |
| Tannin | 152 g/m3 |
| Total VFA (as acetic acid) | 320 g/m3 |
| Formic acid | <5 g/m3 |
| Acetic acid | 200 g/m3 |
| Propionic acid | 137 g/m3 |
| Butyric acid | 7 g/m3 |
| NDF | 14.7% DM b |
| ADF | 9.5% DM b |
| Component | Units | Min | Max | Avrg | STDV |
|---|---|---|---|---|---|
| Nitrogen | mg/m2 day | 0.731 | 0.871 | 0.801 | 0.098 |
| Phosphorus | mg/m2 day | 0.099 | 0.119 | 0.109 | 0.014 |
| Total nitrogen | g/year | 2.4 | 2.8 | 2.6 | 0.28 |
| Total phosphorus | g/year | 0.32 | 0.38 | 0.35 | 0.04 |
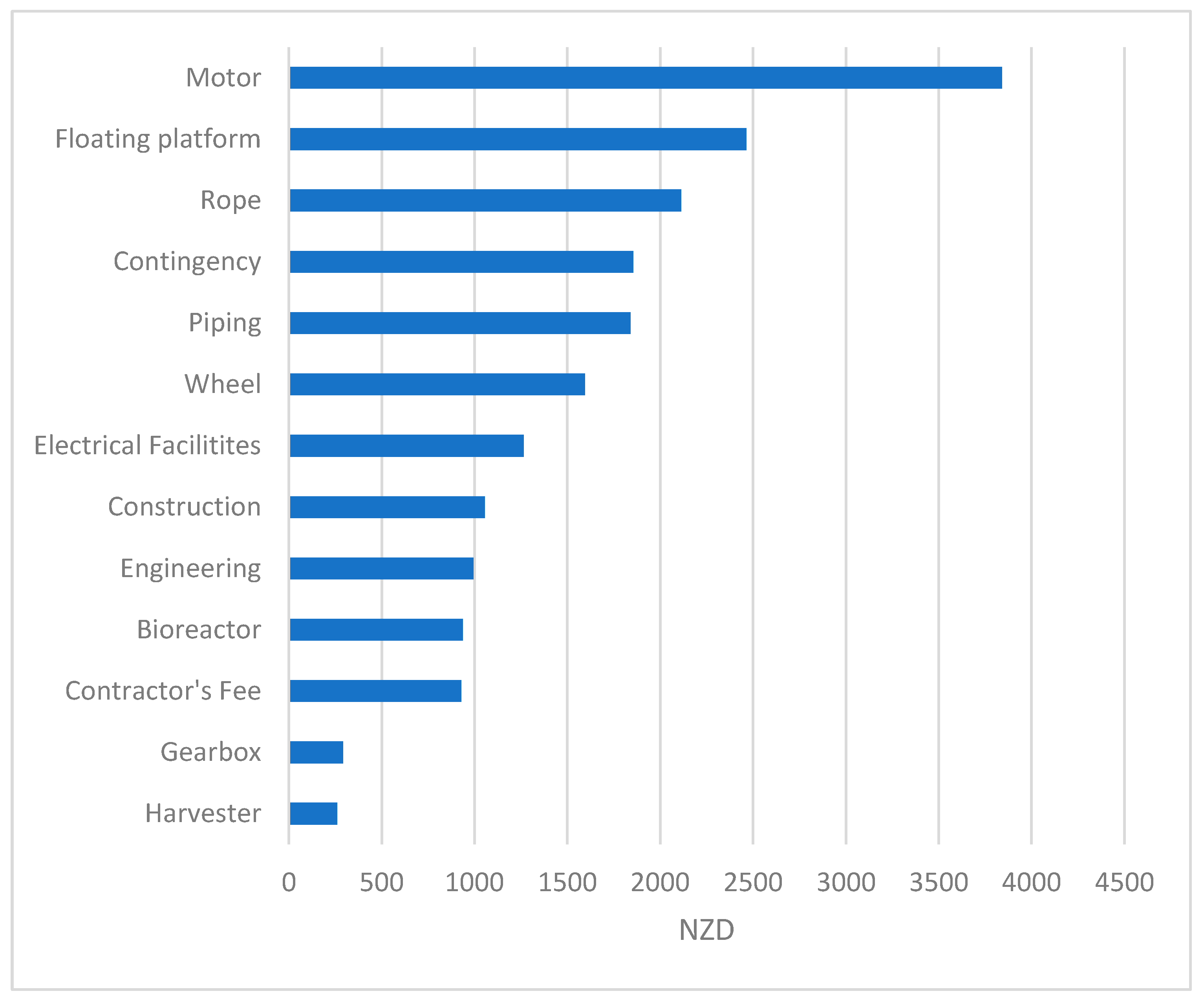
| Component | Price NZD/Unit | QTY | Total Cost (NZD) |
|---|---|---|---|
| Wheel | 1593 | 1 | 1593 |
| Rope | 2112 | 1 | 2112 |
| Floating platform | 2464 | 1 | 2464 |
| Motor | 3840 | 1 | 3840 |
| Harvester | 260 | 1 | 260 |
| Gearbox | 291 | 1 | 291 |
| Bioreactor | 937 | 1 | 937 |
| Total equipment purchase cost (PC) | 11,500 | ||
| Component | Factor | NZD |
|---|---|---|
| Direct cost (DC) | ||
| Piping | 0.16 × PC | 1840 |
| Electrical facilities | 0.11 × PC | 1265 |
| Total direct cost (DC) | 3104 | |
| Indirect cost (IC) | ||
| Construction | 0.34 × DC | 1056 |
| Engineering | 0.32 × DC | 993 |
| Total indirect cost (IC) | 2049 | |
| Other Cost (OC) | ||
| Contractor’s fee | 0.18 × (DC + IC) | 928 |
| Contingency | 0.36 × (DC + IC) | 1855 |
| Total other cost (OC) | 2783 | |
| Direct fixed capital (DFC) | 7936 | |
| Total capital cost | 19,434 | |
| System Operating Costs (NZD/year) | NZD/year |
|---|---|
| Fixed costs | |
| Insurance | 79 |
| Variable costs | |
| Harvesting labor | 1362 |
| Electricity | 192 |
| Maintenance and repair | 476 |
| Total operating costs | 2109 |
| Component | Min | Max |
|---|---|---|
| Biomass production (kg/year) | 17 | 101 |
| Methane production (m3/year) | 6 | 112 |
| Methane yield (m3CH4/kg biomass) | 0.66 | 1.11 |
| Heating value (MJ/m3) | 35.8 | 39.8 |
| Electrical output (kwh/year) | 58 | 1237 |
| Product (NZD/year) | 9 | 187 |
| DFC (NZD) | 19,434 | 19,434 |
| Operating costs (NZD/year) | 2109 | 2109 |
| Gross profit | −2101 | −1922 |
| Net profit | −1453 | −1275 |
| Return on investment | −7.5% | −6.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hull-Cantillo, M.; Lay, M.; Glasgow, G.; Kovalsky, P. The Hard Reality of Biogas Production through the Anaerobic Digestion of Algae Grown in Dairy Farm Effluents. Fermentation 2024, 10, 137. https://doi.org/10.3390/fermentation10030137
Hull-Cantillo M, Lay M, Glasgow G, Kovalsky P. The Hard Reality of Biogas Production through the Anaerobic Digestion of Algae Grown in Dairy Farm Effluents. Fermentation. 2024; 10(3):137. https://doi.org/10.3390/fermentation10030137
Chicago/Turabian StyleHull-Cantillo, Marianne, Mark Lay, Graeme Glasgow, and Peter Kovalsky. 2024. "The Hard Reality of Biogas Production through the Anaerobic Digestion of Algae Grown in Dairy Farm Effluents" Fermentation 10, no. 3: 137. https://doi.org/10.3390/fermentation10030137





