Impact of Glucose Concentration and NaCl Osmotic Stress on Yeast Cell Wall β-d-Glucan Formation during Anaerobic Fermentation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Cells and Culture Growth Conditions
2.2. Yeast Cell Preconditioning and Inoculum Preparation
2.3. Preparation of Yeast Fermentation Medium
2.4. Cell Wall Treatment with Lytic Enzyme and Sonication
2.5. Determination of Cell Concentration
2.6. Cell Viability Determination
- a: number of metabolically active cells
- n: total cell number [27].
2.7. Monitoring of Fermentation Kinetics
2.8. Determination of Cell Dry Weight
2.9. Determination of β-Glucan Concentration (%)
2.10. Determination of β-Glucan Yield (%)
2.11. Statistical Analysis
3. Results
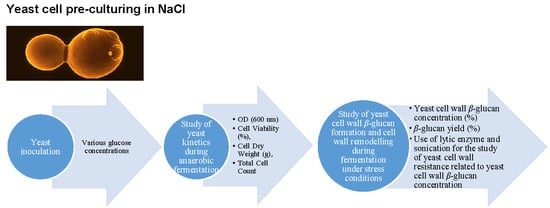
3.1. Experimental Design
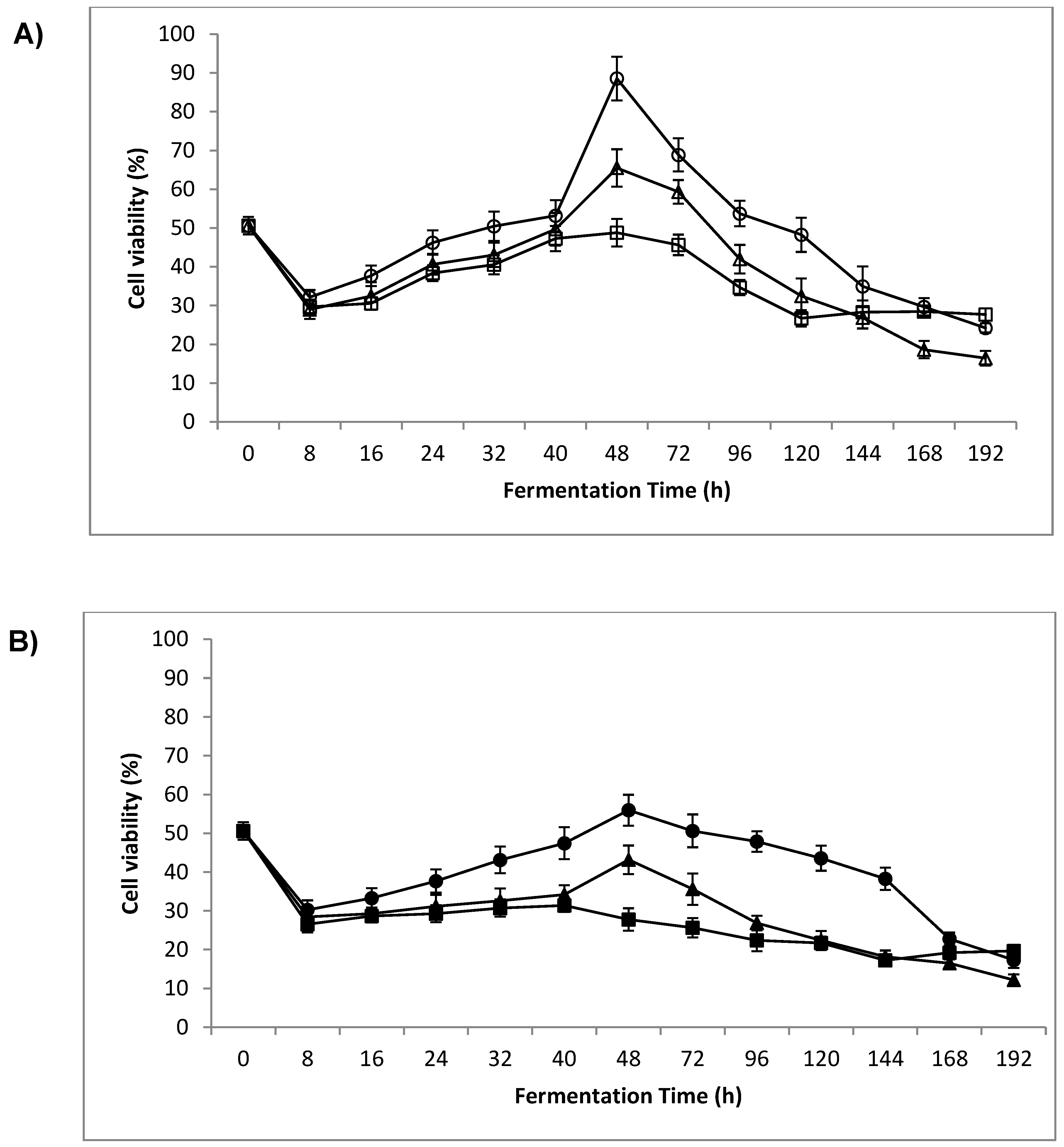
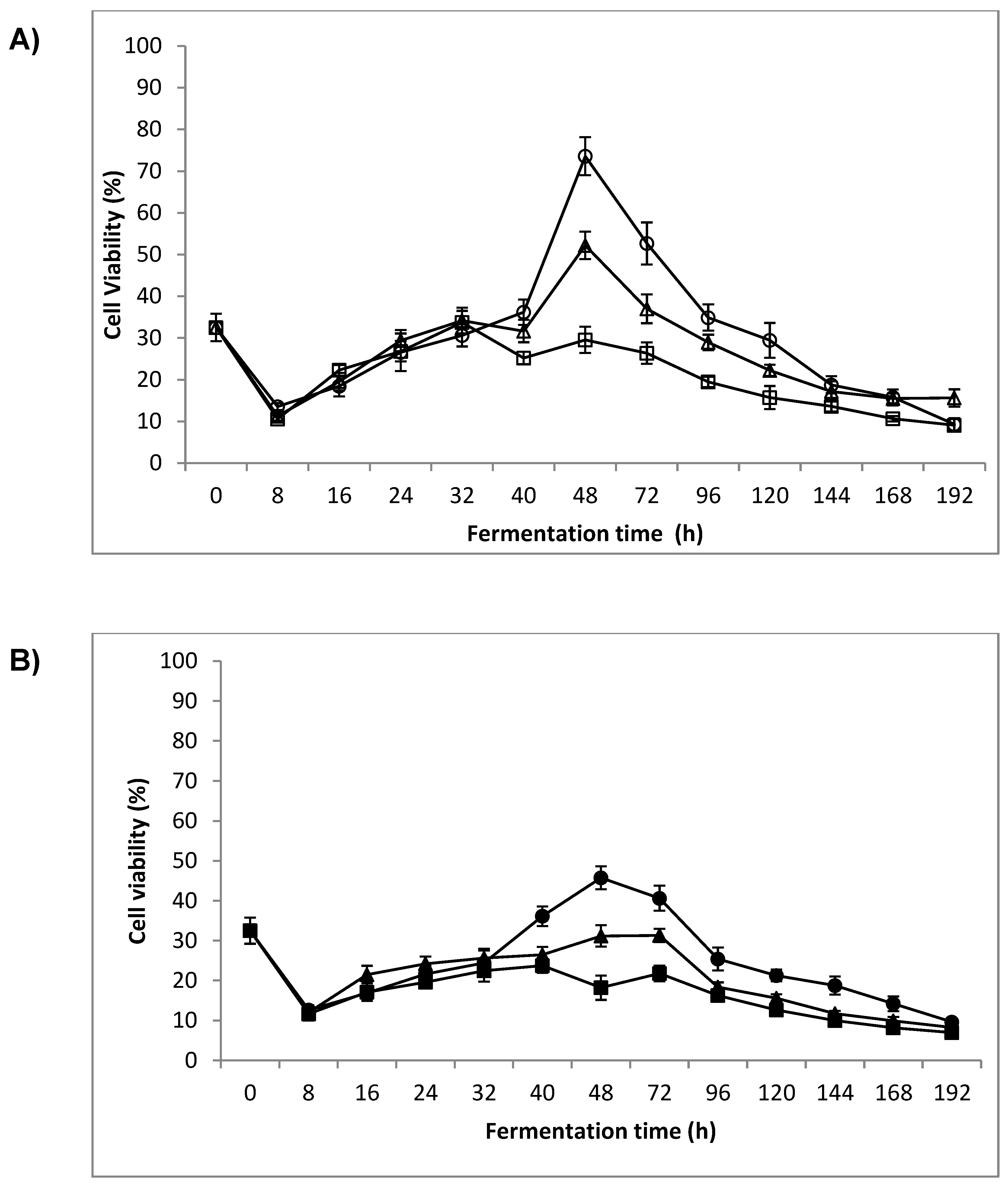
3.2. Cell Viability
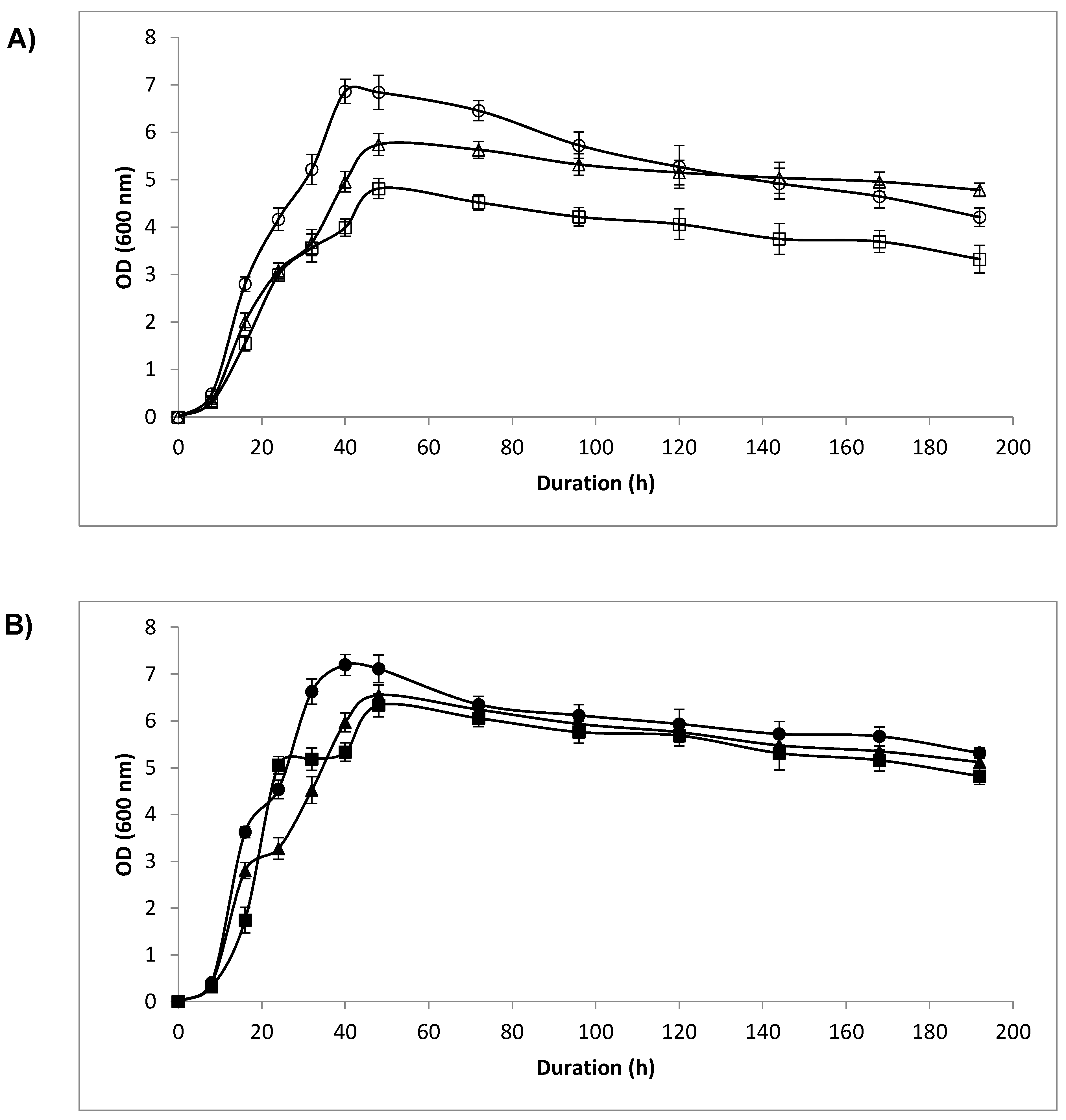
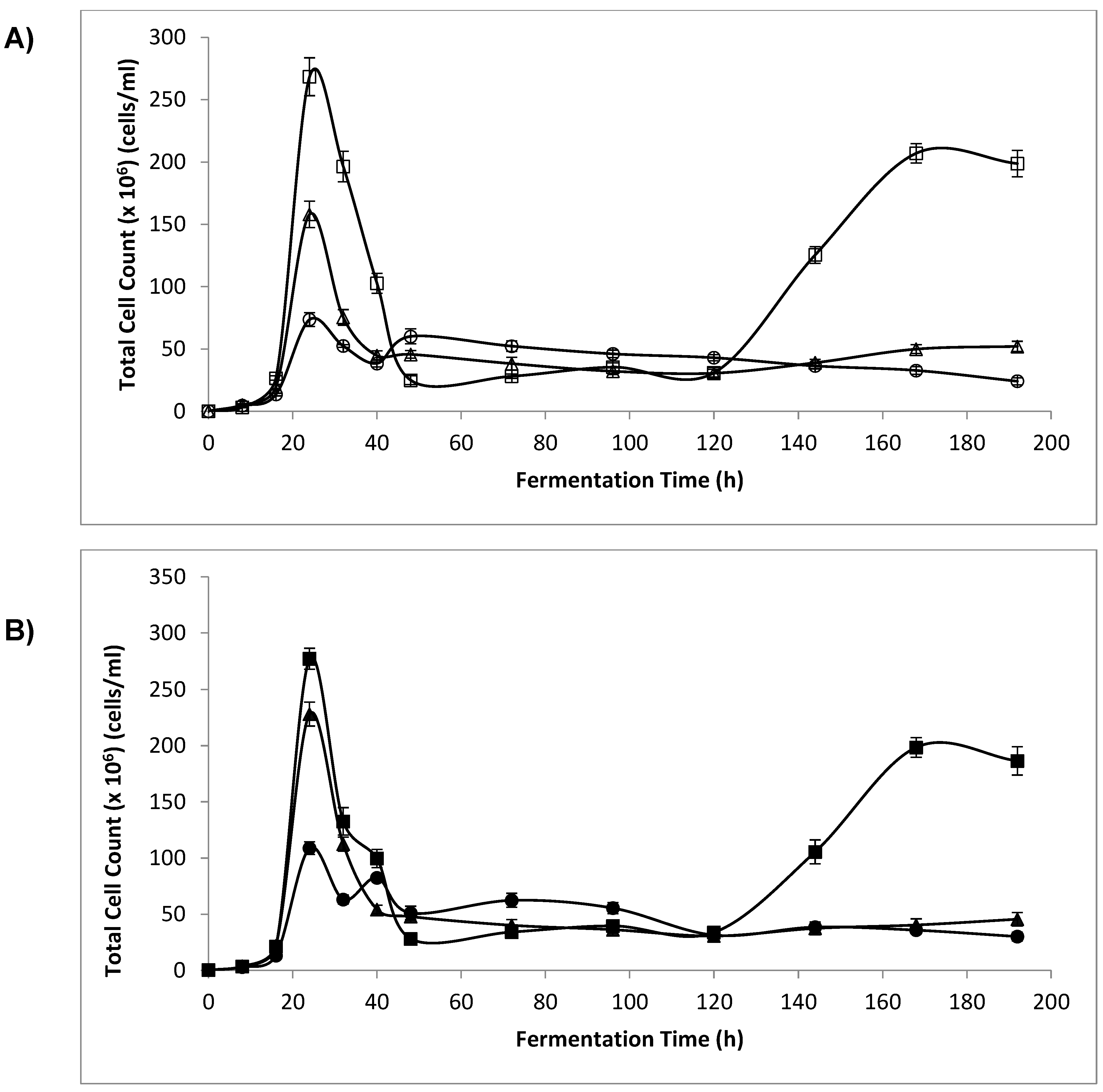
3.3. Optical Density (OD) and Cell Count
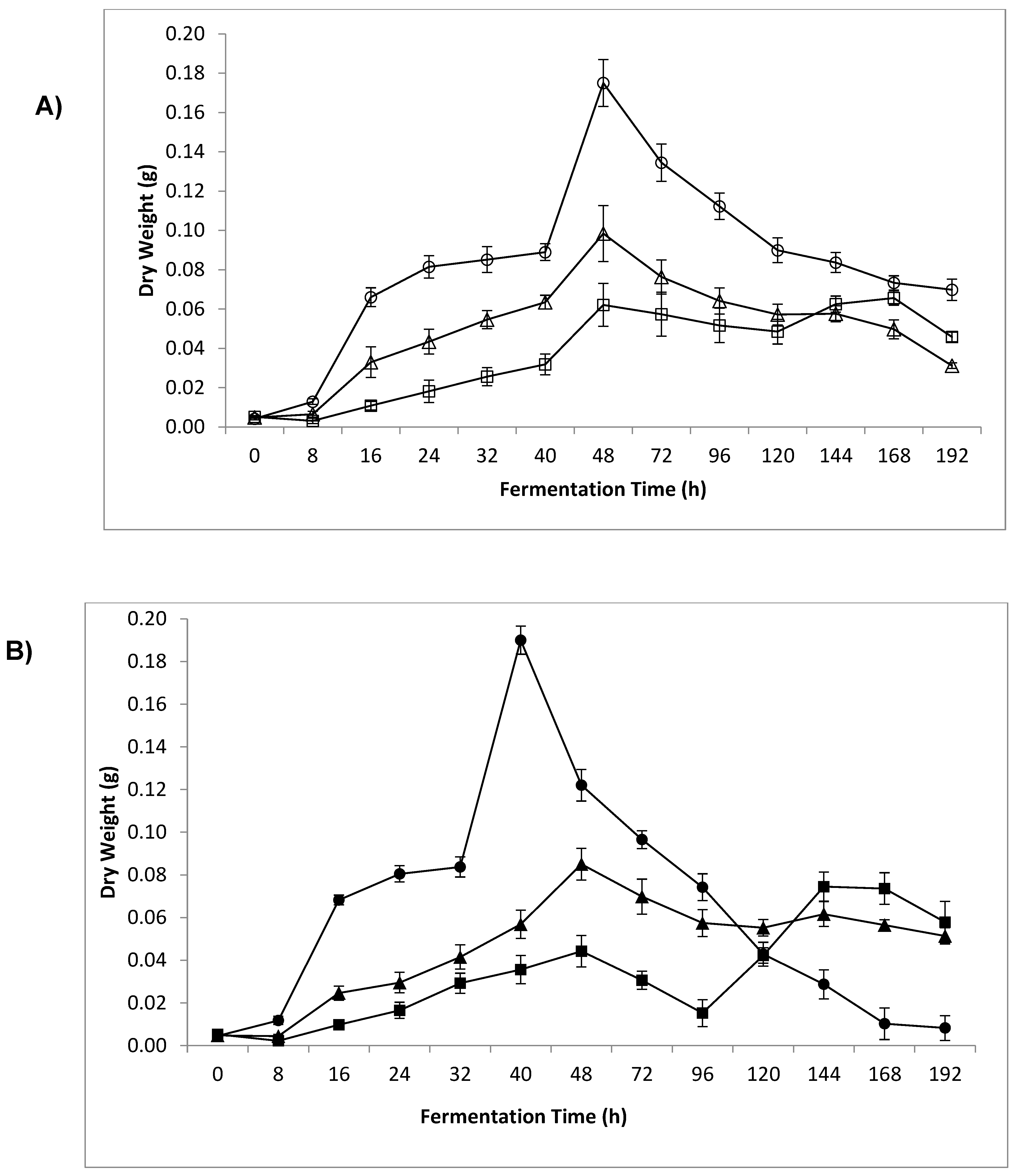
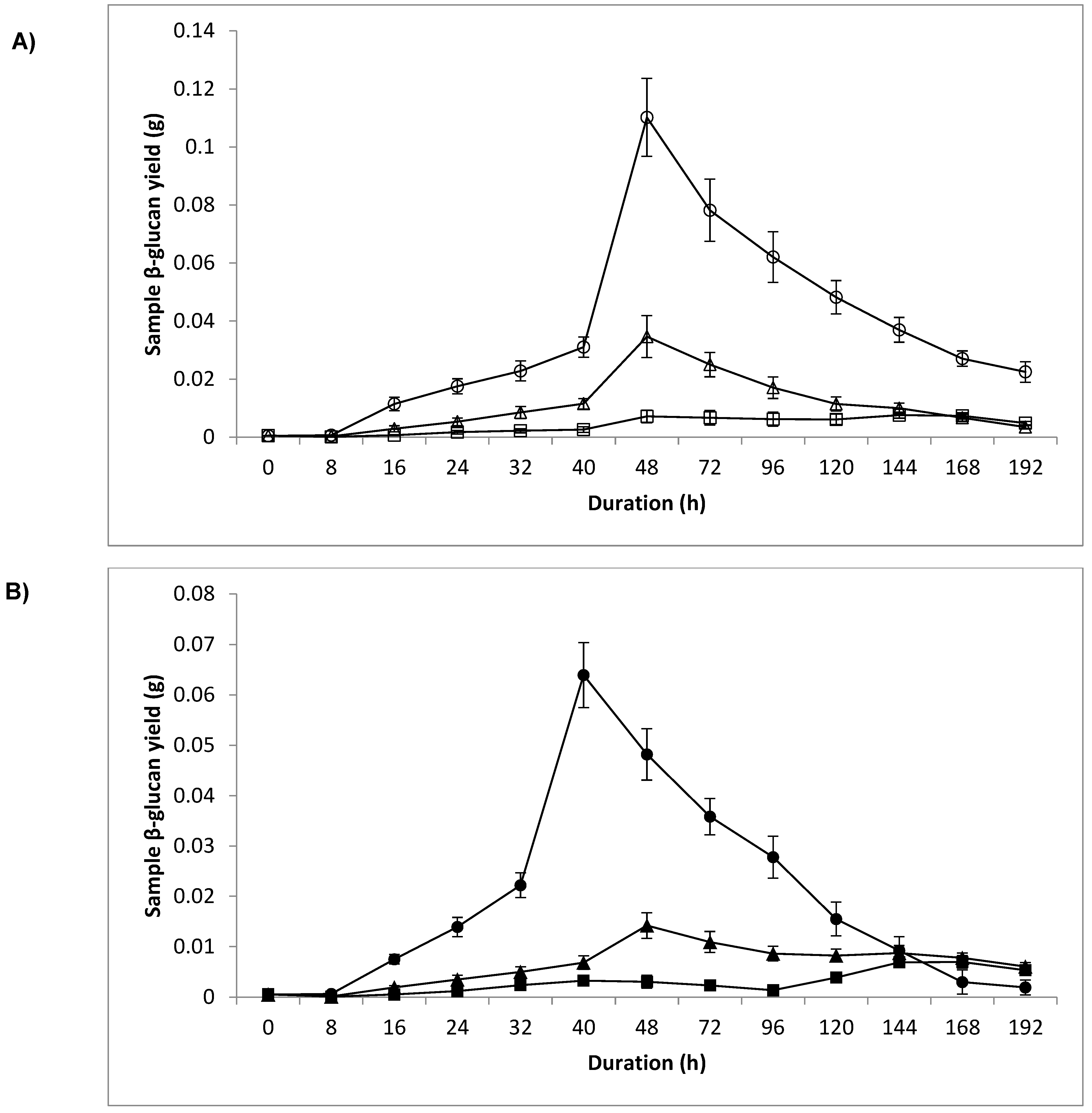
3.4. Dry Weight and Sample β-Glucan Yield
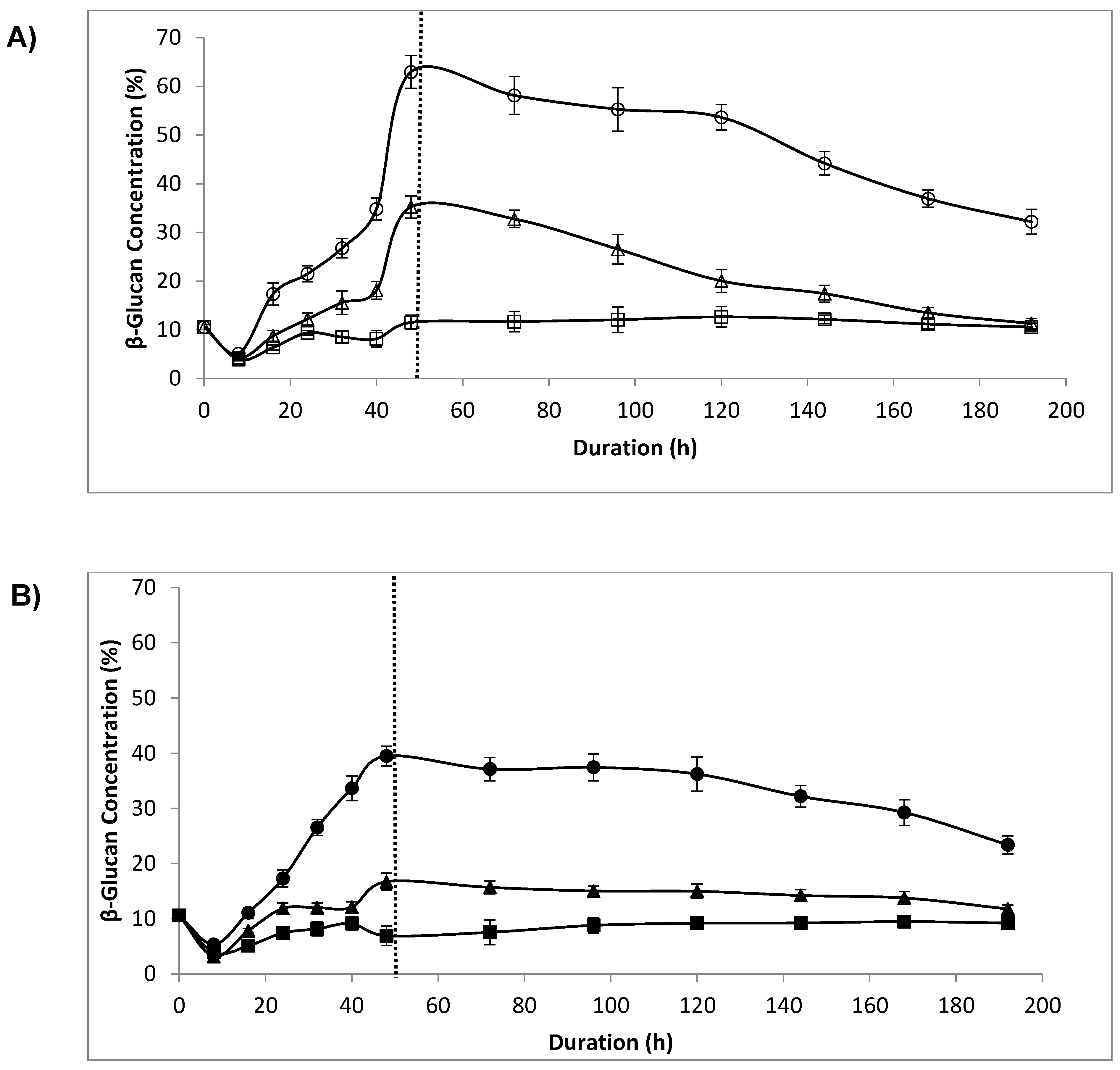
3.5. β-Glucan Concentration
3.6. Cell Wall Treatment with Lytic Enzyme and Sonication
4. Discussion
4.1. Cell Viability
4.2. Optical Density and Cell Count
4.3. Dry Weight and Sample β-Glucan Yield
4.4. β-Glucan Concentration, Lytic Enzyme and Sonication
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Reggiori, F.; Klionsky, D.J. Autophagic processes in yeast: Mechanism, machinery and regulation. Genetics 2013, 194, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Biochemistry of Alcoholic Fermentation and Metabolic Pathways of Wine Yeasts. In Handbook of Enology; John Wiley & Sons, Ltd.: Somerset, NJ, USA, 2006; pp. 53–77. [Google Scholar]
- Branduardi, P.; Porro, D. Yeasts in Biotechnology. In Yeast; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 347–370. [Google Scholar]
- Ballou, C.E. Genetics of Yeast Mannoprotein Biosynthesis. In Fungal Polysaccharides; American Chemical Society: Washington, DC, USA, 1980; Volume 126, pp. 1–14. [Google Scholar]
- François, J.M.; Walther, T.; Parrou, J.L. Genetics and Regulation of Glycogen and Trehalose Metabolism in Saccharomyces cerevisiae. In Microbial Stress Tolerance for Biofuels; Liu, Z.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–55. [Google Scholar]
- Wilson, W.A.; Hughes, W.E.; Tomamichel, W.; Roach, P.J. Increased glycogen storage in yeast results in less branched glycogen. Biochem. Biophys. Res. Commun. 2004, 320, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Arvindekar, A.U.; Patil, N.B. Glycogen—A covalently linked component of the cell wall in Saccharomyces cerevisiae. Yeast 2002, 19, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M. Review: Cell wall assembly in yeast. Yeast 1994, 10, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Van der Rest, M.E.; Kamminga, A.H.; Nakano, A.; Anraku, Y.; Poolman, B.; Konings, W.N. The plasma membrane of Saccharomyces cerevisiae: Structure, function, and biogenesis. Microbiol. Rev. 1995, 59, 304–322. [Google Scholar] [PubMed]
- Rank, G.H.; Robertson, A.J. Protein and lipid composition of the yeast plasma membrane. In Yeast Genetics: Fundamental and Applied Aspects; Spencer, J.F.T., Spencer, D.M., Smith, A.R.W., Eds.; Springer: New York, NY, USA, 1983; pp. 225–241. [Google Scholar]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary yeast β-1,3/1,6-D-glucan. Nutr. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. β glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Varelas, V.; Liouni, M.; Calokerinos, A.C.; Nerantzis, E.T. An evaluation study of different methods for the production of β-D-glucan from yeast biomass. Drug Test. Anal. 2016, 8, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Boorsma, A.; de Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hägerdal, B.; Karhumaa, K.; Larsson, C.U.; Gorwa-Grauslund, M.; Görgens, J.; van Zyl, W.H. Role of cultivation media in the development of yeast strains for large scale industrial use. Microb. Cell Fact. 2005, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Yun, H.S. Production of soluble β-glucan from the cell wall of Saccharomyces cerevisiae. Enzym. Microb. Technol. 2006, 39, 496–500. [Google Scholar] [CrossRef]
- Catley, B.J. Isolation and analysis of cell walls. In Yeast, A Practical Approach; Campbell, I., Duffus, J.H., Eds.; Oxford University Press: London, UK, 1988; pp. 163–183. [Google Scholar]
- McMurrough, I.; Rose, A.H. Effect of growth rate and substrate limitation on the composition and structure of the cell wall of Saccharomyces cerevisiae. Biochem. J. 1967, 105, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Uscanga, B.; François, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Naruemon, M.; Romanee., S.; Cheunjit, P.; Xiao, H.; McLandsborough, L. A.; Pawadee, M. Influence of additives on Saccharomyces cerevisiae β-glucan production. Int. Food Res. J. 2013, 20, 1953–1959. [Google Scholar]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Modifications of Saccharomyces pastorianus cell wall polysaccharides with brewing process. Carbohydr. Polym. 2015, 124, 322–330. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Rodríguez-Peña, J.M.; Bermejo, C.; Nombela, C.; Arroyo, J. The high osmotic response and cell wall integrity pathways cooperate to regulate transcriptional responses to zymolyase-induced cell wall stress in Saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 10901–10911. [Google Scholar] [CrossRef] [PubMed]
- Ene, I.V.; Walker, L.A.; Schiavone, M.; Lee, K.K.; Martin-Yken, H.; Dague, E.; Gow, N.A.R.; Munro, C.A.; Brown, A.J.P. Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. MBio 2015. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, D.J.; van der Merwe, G.K.; van Vuuren, H.J.J. Genome-wide expression analyses: Metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res. 2003, 3, 375–399. [Google Scholar] [CrossRef]
- Logothetis, S.; Nerantzis., E.T.; Gioulioti, A.; Kanelis, T.; Tataridis, P.; Walker, G. Influence of sodium chloride on wine yeast fermentation performance. Int. J. Wine Res. 2010, 2, 35–42. [Google Scholar] [CrossRef]
- Ishmayana, S.; Learmonth, R.P.; Kennedy, U.J. Fermentation performance of the yeast Saccharomyces cerevisiae in media with high sugar concentration. In Proceedings of the 2nd International Seminar on Chemistry, Jatinangor, Indonesia, 24–25 November 2011; pp. 379–385. [Google Scholar]
- Lei, H.; Xu, H.; Feng, L.; Yu, Z.; Zhao, H.; Zhao, M. Fermentation performance of lager yeast in high gravity beer fermentations with different sugar supplementations. J. Biosci. Bioeng. 2016, 122, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Novo, M.; Gonzalez, R.; Bertran, E.; Martínez, M.; Yuste, M.; Morales, P. Improved fermentation kinetics by wine yeast strains evolved under ethanol stress. LWT Food Sci. Technol. 2014, 58, 166–172. [Google Scholar] [CrossRef]
- Trainotti, N.; Stambuk, B.U. NaCl stress inhibits maltose fermentation by Saccharomyces cerevisiae. Biotechnol. Lett. 2001, 23, 1703–1707. [Google Scholar] [CrossRef]
- Pratt, P.L.; Bryce, J.H.; Stewart, G.G. The effects of osmotic pressure and ethanol on yeast viability and morphology. J. Inst. Brew. 2003, 109, 218–228. [Google Scholar] [CrossRef]
- Ren, H.; Wang., X.; Liu, D.; Wang, B. A glimpse of the yeast Saccharomyces cerevisiae responses to NaCl stress. Afr. J. Microbiol. Res. 2012, 6, 713–718. [Google Scholar]
- Dhar, R.; Sägesser, R.; Weikert, C.; Yuan, J.; Wagner, A. Adaptation of Saccharomyces cerevisiae to saline stress through laboratory evolution. J. Evol. Biol. 2011, 24, 1135–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilloy, V.; Ortiz-Julien, A.; Dequin, S. Reduction of ethanol yield and improvement of glycerol formation by adaptive evolution of the wine yeast Saccharomyces cerevisiae under hyperosmotic conditions. Appl. Environ. Microbiol. 2014, 80, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, S.; Nerantzis, E.T.; Tataridis, P.; Goulioti, A.; Kannelis, A.; Walker, G.M. Alleviation of stuck wine fermentations using salt-preconditioned yeast. J. Inst. Brew. 2014, 120, 174–182. [Google Scholar] [CrossRef]
- Prieto, M.A.; Vázquez, J.A.; Murado, M.A. Comparison of several mathematical models for describing the joint effect of temperature and ph on glucanex activity. Biotechnol. Prog. 2012, 28, 372–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnani, M.; Calliari, C.M.; de Macedo Jr, F.C.; Mori, M.P.; de Syllos Cólus, I.M.; Castro-Gomez, R.J.H. Optimized methodology for extraction of (1→3)(1→6)-β-d-glucan from Saccharomyces cerevisiae and in vitro evaluation of the cytotoxicity and genotoxicity of the corresponding carboxymethyl derivative. Carbohydr. Polym. 2009, 78, 658–665. [Google Scholar] [CrossRef]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Application of different methods for the extraction of yeast β-glucan. e-J. Sci. Technol. 2016, 11, 75–89. [Google Scholar]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Valorization of winery spent yeast waste biomass as a new source for the production of β-glucan. Waste Biomass Valori. 2016, 7, 807–817. [Google Scholar] [CrossRef]
- Logothetis, S.; Tataridis, P.; Kanellis, A.; Nerantzis, E.T. The effect of preconditioning cells under osmotic stress on high alcohol production. Zb. Matice Srp. za Prir. Nauke 2013, 405–414. [Google Scholar] [CrossRef]
- Bauer, F.F.; Pretorius, I.S. Yeast stress response and fermentation efficiency: How to survive the making of wine—A Review. S. Afr. J. Enol. Viticult. 2000, 21, 27–51. [Google Scholar]
- Patynowski, R.J.; Jiranek, V.; Markides, A.J. Yeast viability during fermentation and sur lie ageing of a defined medium and subsequent growth of Oenococcus oeni. Aust. J. Grape Wine Res. 2002, 8, 62–69. [Google Scholar] [CrossRef]
- Morris, G.J.; Winters., L.; Coulson, G.E.; Clarke, K.J. Effect of osmotic stress on the ultrastructure and viability of the yeast Saccharomyces cerevisiae. Microbiology 1986, 132, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Naruemon, M.; Romanee., S.; Cheunjit, P.; Xiao, H.; McLandsborough, L.A.; Pawadee, M. Effect of three additives on the cell morphology and β-glucan production in Saccharomyces cerevisiae. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 283–295. [Google Scholar]
- Gilliland, R.B. Determination of yeast viability. J. Inst. Brew. 1959, 65, 424–429. [Google Scholar] [CrossRef]
- Logothetis, S.; Walker, G.; Nerantzis, E.T. Effect of salt hyperosmotic stress on yeast cell viability. Zb. Matice Srp. za Prir. Nauke 2007, 113, 271–284. [Google Scholar] [CrossRef]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Cell walls. In The Yeasts; Rose, A.H.H.J.D., Ed.; Academic Press: London, UK, 1991; Volume 4, pp. 199–277. [Google Scholar]
- Inouhe, M.; Sugo, E.; Tohoyama, H.; Joho, M.; Nevins, D.J. Cell wall metabolism and autolytic activities of the yeast Saccharomyces exiguus. Int. J. Biol. Macromol. 1997, 21, 11–14. [Google Scholar] [CrossRef]
- Shimoi, H.; Kitagaki, H.; Ohmori, H.; Iimura, Y.; Ito, K. Sed1p is a major cell wall protein of Saccharomyces cerevisiae in the stationary phase and is involved in lytic enzyme resistance. J. Bacteriol. 1998, 180, 3381–3387. [Google Scholar] [PubMed]
- De Nobel, H.; Ruiz, C.; Martin, H.; Morris, W.; Brul, S.; Molina, M.; Klis, F.M. Cell wall perturbation in yeast results in dual phosphorylation of the slt2/mpk1 map kinase and in an slt2-mediated increase in fks2–lacz expression, glucanase resistance and thermotolerance. Microbiology 2000, 146, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Rep, M.; Krantz, M.; Thevelein, J.M.; Hohmann, S. The transcriptional response of Saccharomyces cerevisiae to osmotic shock: Hot1p and msn2p/msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 2000, 275, 8290–8300. [Google Scholar] [CrossRef] [PubMed]
- O′Rourke, S.M.; Herskowitz, I. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell 2004, 15, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Chang, J.E.; Yun, H.S. Estimation of soluble-glucan content of yeast cell wall by the sensitivity to glucanex® 200g treatment. Enzym. Microb. Technol. 2004, 35, 672–677. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.A.; Sun, D.W.; Han, Z. Disruption and protein release by ultrasonication of yeast cells. Innov. Food Sci. Emerg. Technol. 2013, 18, 132–137. [Google Scholar] [CrossRef]
- Apar, D.K.; Ozmek, B. Protein releasing kinetics of bakers’ yeast cells by ultrasound. Chem. Biochem. Eng. Q. 2008, 22, 113–118. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varelas, V.; Sotiropoulou, E.; Karambini, X.; Liouni, M.; Nerantzis, E.T. Impact of Glucose Concentration and NaCl Osmotic Stress on Yeast Cell Wall β-d-Glucan Formation during Anaerobic Fermentation Process. Fermentation 2017, 3, 44. https://doi.org/10.3390/fermentation3030044
Varelas V, Sotiropoulou E, Karambini X, Liouni M, Nerantzis ET. Impact of Glucose Concentration and NaCl Osmotic Stress on Yeast Cell Wall β-d-Glucan Formation during Anaerobic Fermentation Process. Fermentation. 2017; 3(3):44. https://doi.org/10.3390/fermentation3030044
Chicago/Turabian StyleVarelas, Vassileios, Evangelia Sotiropoulou, Xara Karambini, Maria Liouni, and Elias T. Nerantzis. 2017. "Impact of Glucose Concentration and NaCl Osmotic Stress on Yeast Cell Wall β-d-Glucan Formation during Anaerobic Fermentation Process" Fermentation 3, no. 3: 44. https://doi.org/10.3390/fermentation3030044