Bioethanol a Microbial Biofuel Metabolite; New Insights of Yeasts Metabolic Engineering
Abstract
:1. Introduction
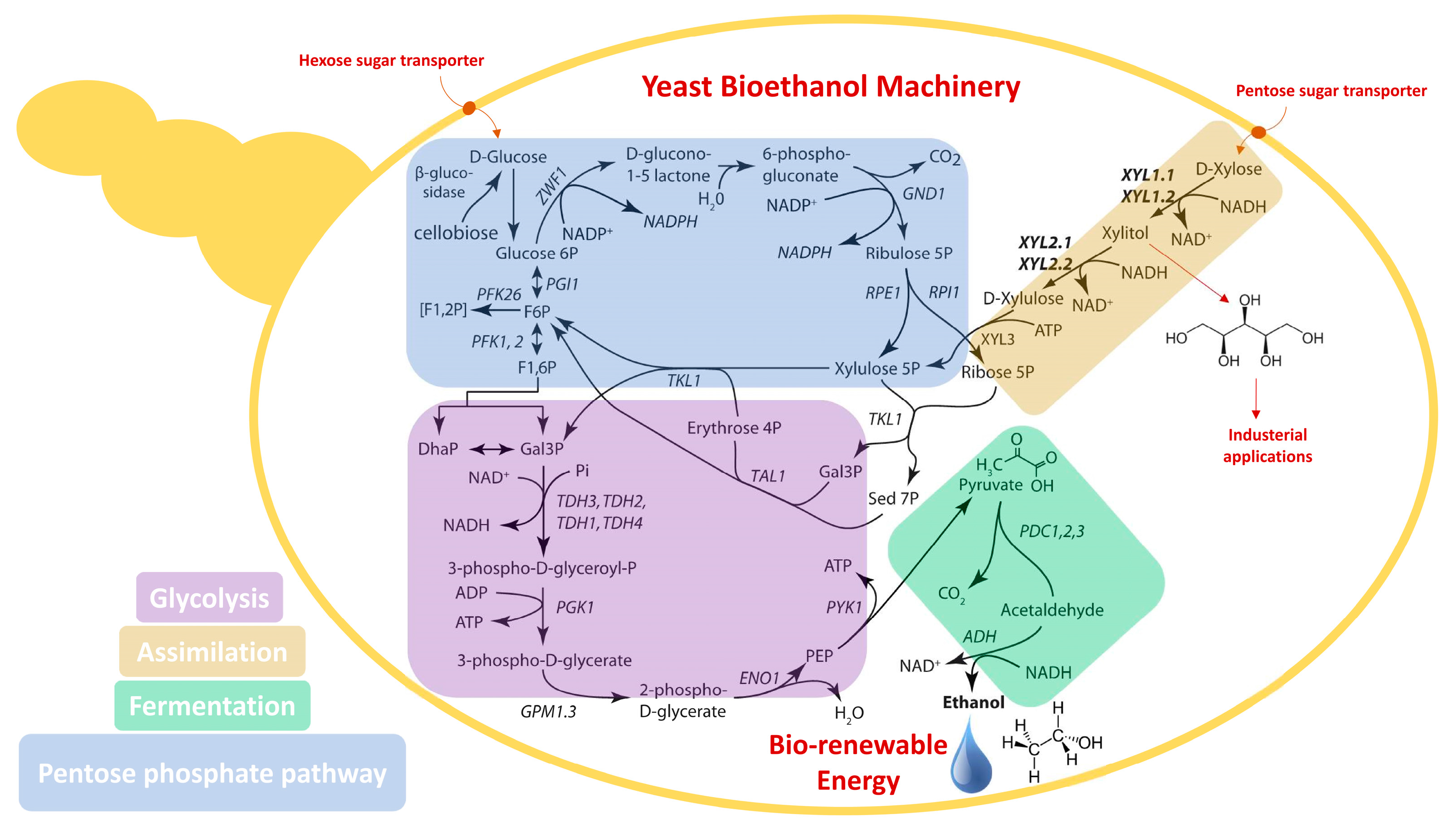
2. New Yeast for Lignocelluloses Bioconversion
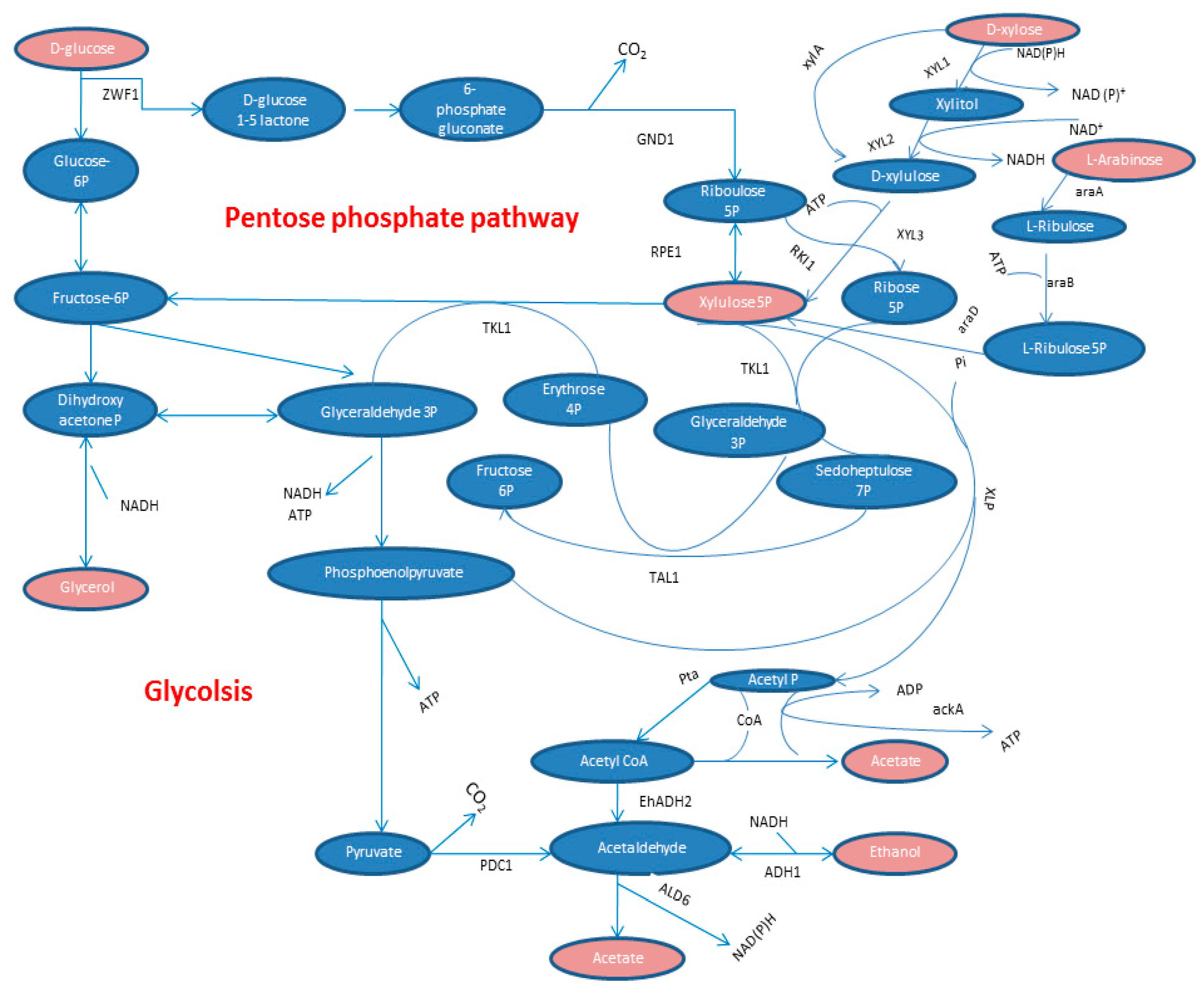
3. Pentose Phosphate Pathway
4. Xylose Isomerase Mechanism
5. Pathway of Xylose Reductase and Xylitol Dehydrogenase
6. Xylose Transport
7. Xylulokinase
8. Xylanase and Cellulose
9. Xylitol Production
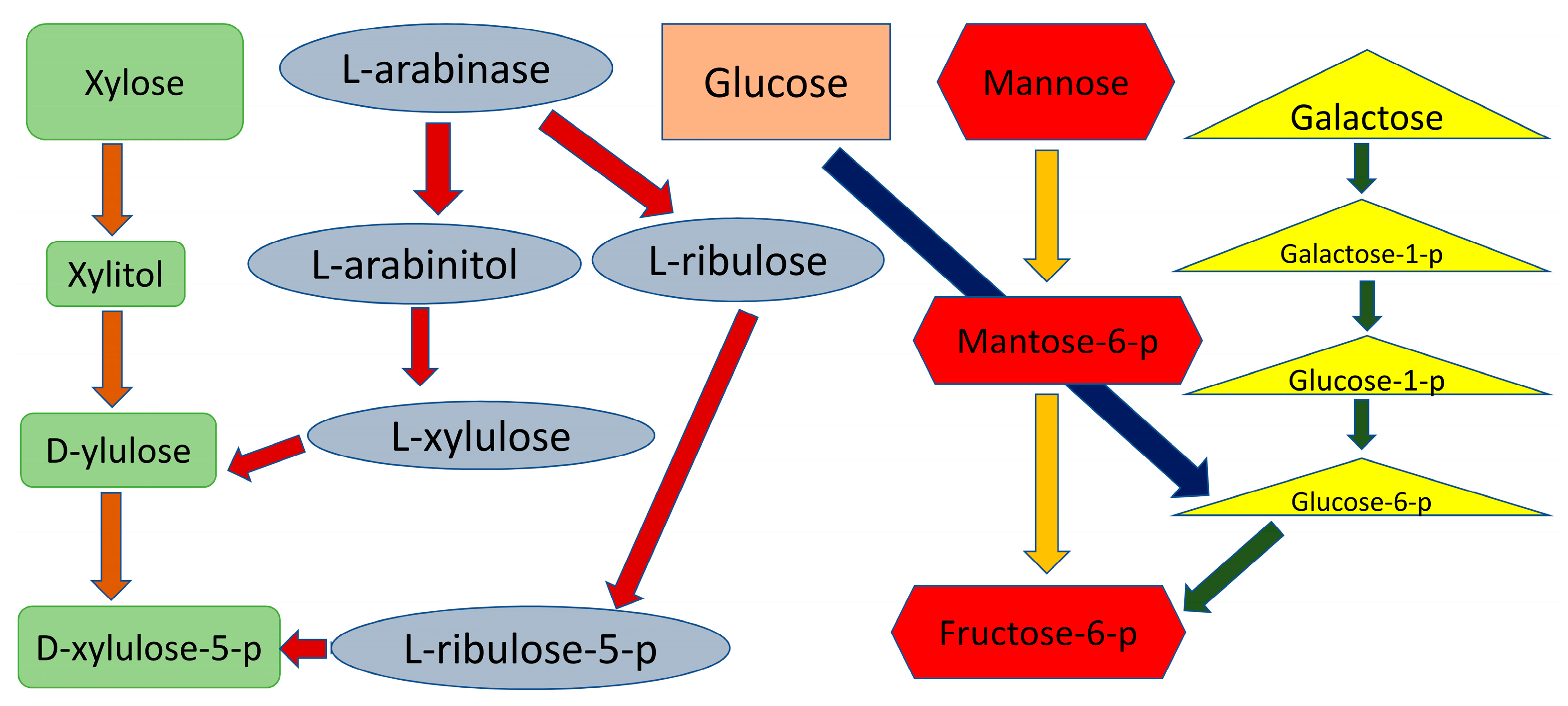
10. Arabinose Utilization
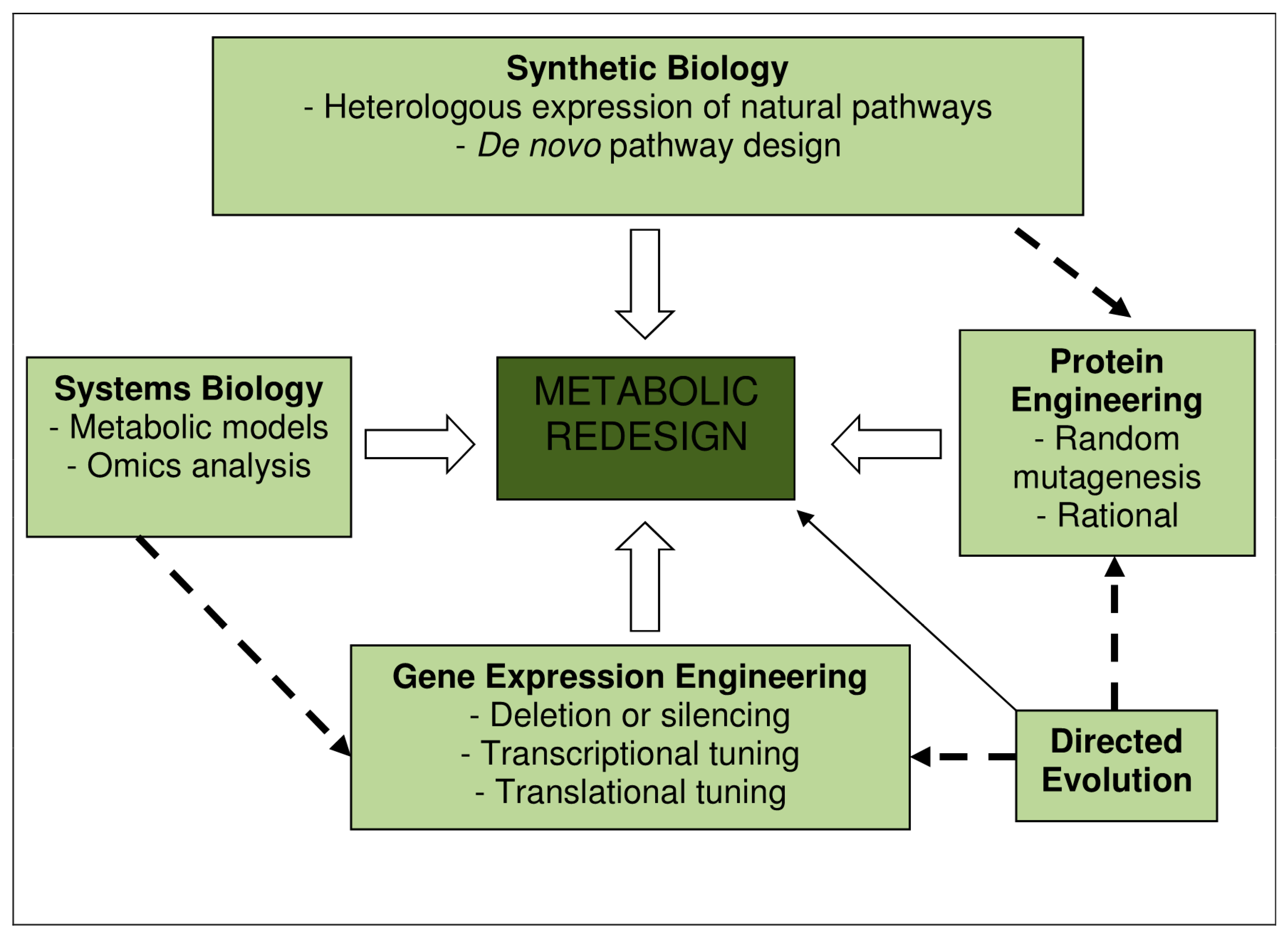
11. Factors Enhancing the Productivity of Bioethanol
12. Factors Prohibiting the Bioethanol Production from Lignocellulose
13. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kiran, B.; Kumar, R.; Deshmukh, D. Perspectives of microalgal biofuels as a renewable source of energy. Energy Convers. Manag. 2014, 88, 1228–1244. [Google Scholar] [CrossRef]
- Xie, D. Integrating Cellular and Bioprocess Engineering in the Non-Conventional Yeast Yarrowia lipolytica for Biodiesel Production. Front. Bioeng. Biotechnol. 2017, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Caspeta, L.; Buijs, N.A.A.; Nielsen, J. The role of biofuels in the future energy supply. Energy Environ. Sci. 2013, 6, 1077–1082. [Google Scholar] [CrossRef]
- Caspeta, L.; Caro-Bermúdez, M.A.; Ponce-Noyola, T.; Martinez, A. Enzymatic hydrolysis at high-solids loadings for the conversion of agave bagasse to fuel ethanol. Appl. Energy 2014, 113, 277–286. [Google Scholar] [CrossRef]
- Rouhollah, H.; Iraj, N.; Giti, E.; Sorah, A. Mixed sugar fermentation by Pichia stipitis, Saccharomyces cerevisiae and an isolated xylose fermenting Kluyveromyces marxinus and their co-culture. Afr. J. Biotechnol. 2007, 6, 1110–1114. [Google Scholar]
- Fromanger, R.; Guillouet, S.E.; Uribelarrea, J.L.; Molina-Jouve, C.; Cameleyre, X. Effect of controlled oxygen limitation on Candida shehatae physiology for ethanol production from xylose and glucose. J. Ind. Microbiol. Biotechnol. 2010, 37, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.; Vanderberghel, L.; Medeiros, A.; Karp, S.; Buckeridge, M.; Ramos, L.P.; Pitarelo, A.P.; Ferreira-Leito, V.; Gottschalk, L.; Ferrara, M.A.; et al. Bioethanol from lignocelluloses: Status and perspectives in Brazil. Bioresour. Technol. 2010, 101, 4820–4825. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Ladisch, M. Features of promising technologies for treatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, J.; Martinez, A.; Ingram, L.O. Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol. Bioeng. 2000, 68, 524–530. [Google Scholar] [CrossRef]
- Fu, N.; Peires, P.; Markham, J.; Bayor, J. A novel co-culture process with Zymomonas mobilis and Pichia stipitis for efficient ethanol production on glucose/xylose mixtures. Enzyme Microb. Technol. 2009, 45, 210–217. [Google Scholar] [CrossRef]
- Van Vleet, J.H.; Jeffries, T.W. Yeast metabolic engineering for hemicellulosic ethanol production. Curr. Opin. Biotechnol. 2009, 20, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Aristidou, A.; Penttila, M. Metabolic engineering applications to renewable resource utilization. Curr. Opin. Biotechnol. 2000, 11, 187–198. [Google Scholar] [CrossRef]
- Van Zyl, W.; Lynd, L.; den Hann, R.; McBride, J. Consolidated bioprocessing for bioethanol production using Saccharomyces cerevisiae. Adv. Biochem. Eng. Biotechnol. 2007, 108, 205–235. [Google Scholar] [PubMed]
- Hahn-Hägerdal, B.; Karhumaa, K.; Fonseca, C.; Martins, S.; Gorwa-Grauslund, M.F. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 2007, 74, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Cate, J.H. Metabolic engineering of yeast for lignocellulosic biofuel production. Curr. Opin. Chem. Biol. 2017, 41, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Van Maris, A.J.; Abbott, D.A.; Bellissimi, E.; van den Brink, J.; Kuyper, M.; Luttik, M.A.; Wisselink, H.W.; Scheffers, W.A.; van Dijken, J.P.; Pronk, J.T. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: Current status. Antonie Van Leeuwenhoek 2006, 90, 391–418. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.; Mast-Gerlach, E.; Popovic, M.K.; Bajpai, R.; Stahl, U. Relevance of microbial coculture fermentations in biotechnology. J. Appl. Microbiol. 2009, 109, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Grootjen, D.R.J.; Vleesenbeek, R.M.; Windmeijer, G.A.; van der Lans, R.G.J.M.; Luyben, K.C.A. A flocculating strain of Pichia stipitis for the conversion of glucose/xylose mixtures. Enzyme Microb. Technol. 1991, 13, 734–739. [Google Scholar] [CrossRef]
- Fu, N.; Peiris, P. Co-fermentation of a mixture of glucose and xylose to ethanol by Zymomonas mobilis and Pachysolen tannophilus. World J. Microbiol. Biotechnol. 2008, 24, 1091–1097. [Google Scholar] [CrossRef]
- Weir, P.M. The ecology of Zymomonas: A review. Folia Microbiol. 2016, 61, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolysates I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.; Ahring, B. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.; Ramrez, J.A.; Garrote, G.; Vzquez, M. Kinetic study of the acid hydrolysis of sugar cane bagasse. J. Food Eng. 2002, 55, 309–318. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hgerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.D.; Panganiban, C.; Mansfield, S.D. Tolerance and adaptation of ethanologenic yeasts to lignocellulosic inhibitory compounds. Biotechnol. Bioeng. 2006, 93, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Skoog, K.; Hähn-Hagerdal, B. Xylose fermentation. Enzyme Microb. Technol. 1988, 10, 66–80. [Google Scholar] [CrossRef]
- Schneider, H. Conversion of Pentoses to Ethanol by Yeasts and Fungi. Crit. Rev. Biotechnol. 1989, 9, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, A. Microbial Pentose Utilization. Adv. Appl. Microbiol. 1993, 39, 91–152. [Google Scholar] [PubMed]
- Maleszka, R.; Schneider, H. Fermentation of d-Xylose, xylitol, and D-xylulose by yeasts. Can. J. Microbiol. 1982, 28, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Slininger, P.J.; Bothast, R.J.; van Cawenberge, J.E.; Kurtzman, C.P. Conversion of d-Xylose to ethanol by the yeast Pachysolen tannophilus. Biotechnol. Bioeng. 1982, 24, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Latimer, L.N.; Dueber, J.E. Iterative Optimization of Xylose Catabolism in Saccharomyces cerevisiae Using Combinatorial Expression Tuning. Biotechnol. Bioeng. 2017, 114, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.E. Toward a science of metabolic engineering. Science 1991, 252, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Stephanopoulos, G.; Vallino, J.J. Network rigidity and metabolic engineering in metabolite overproduction. Science 1991, 252, 1675–1681. [Google Scholar] [CrossRef]
- Stephanopoulos, G.; Nielsen, J.; Aristidou, A. Metabolic Engineering; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Feist, A.M.; Henry, C.S.; Reed, J.L.; Krummenacker, M.; Joyce, A.R.; Karp, P.D.; Broadbelt, L.J.; Hatzimanikatis, V.; Palsson, B.Ø. A genomescale metabolic reconstruction for Escherichia coli K-12 MG1655 that accounts for 1260 ORFs and thermodynamic information. Mol. Syst. Biol. 2007, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Keseler, I.M.; Bonavides-Martínez, C.; Collado-Vides, J.; Gama-Castro, S.; Gunsalus, R.P.; Johnson, D.A.; Krummenacker, M.; Nolan, L.M.; Paley, S.; Paulsen, I.T.; et al. EcoCyc: A comprehensive view of Escherichia coli biology. Nucleic Acids Res. 2009, 37, D464–D470. [Google Scholar] [CrossRef] [PubMed]
- Endy, D. Foundations for engineering biology. Nature 2005, 438, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Piontek, M.; Hagedorn, J.; Hollenberg, C.P.; Gellissen, G.; Strasser, A.W. Two novel expression systems based on the yeasts Schwanniomyces occidentalis and Pichia stipitis. Appl. Microbiol. Biotechnol. 1998, 50, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Bowles, D.J. Coexpression of neighboring genes in the genome of Arabidopsis thaliana. Genome Res. 2004, 14, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.A.; Mitra, R.D.; Hughes, J.D.; Church, G.M. A computational analysis of whole-genome expression data reveals chromosomal domains of gene expression. Nat. Genet. 2000, 26, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Lercher, M.J.; Urrutia, A.O.; Hurst, L.D. Clustering of housekeeping genes provides a unified model of gene order in the human genome. Nat. Genet. 2002, 31, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Lercher, M.J.; Blumenthal, T.; Hurst, L.D. Co. expression of neighboring genes in Caenorhabditis elegans is mostly due to operons and duplicate genes. Genome Res. 2003, 13, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Bakht, S.; Leggett, M.; Maxwell, C.; Melton, R.; Osbourn, A. A gene cluster for secondary metabolism in oat: implications for the evolution of metabolic diversity in plants. Proc. Natl. Acad. Sci. USA. 2004, 101, 8233–8238. [Google Scholar] [CrossRef] [PubMed]
- Hesberg, C.; Hansch, R.; Mendel, R.R.; Bittner, F. Tandem orientation of duplicated xanthine dehydrogenase genes from Arabidopsis thaliana: Differential gene expression and enzyme activities. J. Bio. Chem. 2004, 279, 13547–13554. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Takesawa, T.; Kanzaki, H.; Nakamura, I. Genomic structure and differential expression of two tandem-arranged GSTZ genes in rice. Gene 2004, 335, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Prescott, E.M.; Proudfoot, N.J. Transcriptional collision between convergent genes in budding yeast. Proc. Natl. Acad. Sci. USA 2002, 99, 8796–8801. [Google Scholar] [CrossRef] [PubMed]
- Valerius, O.; Brendel, C.; Duvel, K.; Braus, G.H. Multiple factors prevent transcriptional interference at the yeast ARO4-HIS7 locus. J. Biol. Chem. 2002, 277, 21440–21445. [Google Scholar] [CrossRef] [PubMed]
- Springer, C.; Valerius, O.; Strittmatter, A.; Braus, G.H. The adjacent yeast genes ARO4 and HIS7 carry no intergenic region. J. Biol. Chem. 1997, 272, 26318–26324. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.A.; Laprade, L.; Winston, F. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 2004, 429, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Paro, R. Gene regulation: A reason for reading nonsense. Nature 2004, 429, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Wang, M.; Gutierrez, G. Short-range compositional correlation in the yeast genome depends on transcriptional orientation. Gene 2004, 333, 151–155. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.D. Bioethanol production: Status and prospects. Renew. Energy 1997, 10, 295–302. [Google Scholar] [CrossRef]
- Claassen, P.A.M.; van Lier, J.B.; Lopez Contreras, A.M.; van Niel, E.W.J.; Sijtsma, L.; Stams, A.J.M.; de Vries, S.S.; Weusthuis, R.A. Utilization of biomass for the supply of energy carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Wyman, C.E. Biomass ethanol: Technical progress, opportunities, and commercial challenges. Annu. Rev. Energy Environ. 1999, 24, 189–226. [Google Scholar] [CrossRef]
- Kheshgi, H.S.; Prince, R.C.; Marland, G. The potential of biomass fuels in the context of global climate change: Focus on transportation fuels. Annu. Rev. Energy Environ. 2000, 25, 199–244. [Google Scholar] [CrossRef]
- Roca, C.; Olsson, L. Increasing ethanol productivity during xylose fermentation by cell recycling of recombinant Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2003, 60, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Dien, B.S.; Bothast, R.J. Fuel ethanol production from corn fiber—Current status and technical prospects. Appl. Biochem. Biotechnol. 1998, 70, 115–125. [Google Scholar] [CrossRef]
- Saha, B.C.; Bothast, R.J. Pretreatment and enzymatic saccharification of corn fiber. Appl. Biochem. Biotechnol. 1999, 76, 65–77. [Google Scholar] [CrossRef]
- Hinmann, N.D.; Wright, J.D.; Hoagland, W.; Wyman, C.E. Xylose fermentation—An economic analysis. Appl. Biochem. Biotechnol. 1989, 20, 391–401. [Google Scholar] [CrossRef]
- Olsson, L.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- Smil, V. Crop residues: Agriculture′s largest harvest—Crop residues incorporate more than half of the world agricultural phytomass. Bioscience 1999, 49, 299–308. [Google Scholar] [CrossRef]
- Lynd, L.R. Overview and evaluation of fuel ethanol from cellulosic biomass: Technology, economics, the environment, and policy. Annu. Rev. Energy Environ. 1996, 21, 403–465. [Google Scholar] [CrossRef]
- Pettersen, R.C. The chemical composition of wood. Adv. Chem. Ser. 1984, 1984, 57–126. [Google Scholar]
- Hespell, R.B. Extraction and characterization of hemicelluloses from the corn fiber produced by corn wet-milling processes. J. Agric. Food Chem. 1998, 46, 2615–2619. [Google Scholar] [CrossRef]
- Lawford, H.G.; Rousseau, J.D. Production of ethanol from pulpmill hardwood and softwood spent sulfite liquors by genetically engineered Escherichia coli. Appl. Biochem. Biotechnol. 1993, 39, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F.; Wu, Z.W.; Lee, Y.Y. Shrinking-bed model for percolation process applied to dilute-acid pretreatment hydrolysis of cellulosic biomass. Appl. Biochem. Biotechnol. 1998, 70, 37–49. [Google Scholar] [CrossRef]
- Kim, K.H.; Tucker, M.P.; Keller, F.A.; Aden, A.; Nguyen, Q.A. Continuous countercurrent extraction of hemicellulose from pretreated wood residues. Appl. Biochem. Biotechnol. 2001, 91–93, 253–267. [Google Scholar] [CrossRef]
- Wang, P.Y.; Schneider, H. Growth of yeasts on D-xylulose 1. Can. J. Microbiol. 1980, 26, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Shopsis, C.; Schneider, H. Fermentation of a pentose by yeasts. Biochem. Biophys. Res. Commun. 1980, 94, 248–254. [Google Scholar] [CrossRef]
- Schneider, H.; Wang, P.Y.; Chan, Y.K.; Maleszka, R. Conversion of d-Xylose into ethanol by the yeast Pachysolen tannophilus. Biotechnol. Lett. 1981, 3, 89–92. [Google Scholar] [CrossRef]
- Jeffries, T.W. A comparison of Candida tropicalis and Pachysolen tannophilus for conversion of xylose to ethanol. Biotechnol. Bioeng. Symp. 1982, 12, 103–110. [Google Scholar]
- Toivola, A.; Yarrow, D.; Bosch, E.; van den Dijken, J.P.; van Scheffers, W.A. Alcoholic fermentation of deuterium-xylose by yeasts. Appl. Environ. Microbiol. 1984, 47, 1221–1223. [Google Scholar] [PubMed]
- Du Preez, J.C.; Boschm, M.; Prior, B.A. Xylose fermentation by Candida shehatae and Pichia stipitis—Effects of pH, temperature and substrate concentration. Enzyme Microb. Technol. 1986, 8, 360–364. [Google Scholar] [CrossRef]
- Jeffries, T.W.; Shi, N.Q. Genetic engineering for improved xylose fermentation by yeasts. Adv. Biochem. Eng. Biotechnol. 1999, 65, 117–161. [Google Scholar] [PubMed]
- Hahn-Hägerdal, B.; Wahlbom, C.F.; Gardonyi, M.; Zyl, W.H.; van Cordero Otero, R.R.; Jönsson, L.J. Metabolic engineering of Saccharomyces cerevisiae for xylose utilization. Adv. Biochem. Eng. Biotechnol. 2001, 73, 53–84. [Google Scholar] [PubMed]
- Jeffries, T.W.; Van Vleet, J.R.H. Pichia stipitis genomics, transcriptomics, and gene clusters. FEMS Yeast Res. 2009, 9, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Melake, T.; Passoth, V.; Klinner, U. Characterization of the genetic system of the xylose-fermenting yeast Pichia stipitis. Curr. Microbiol. 1996, 33, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.W.; Jin, Y.S. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl. Microbiol. Biot. 2004, 63, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Yang, V.W.; Marks, J.A.; Davis, B.P.; Jeffries, T.W. High efficiency transformation of Pichia stipitis based on its URA3 gene and a homologous autonomous replication sequence, ARS2. Appl. Environ. Microbiol. 1994, 60, 4245–4254. [Google Scholar]
- Lu, P.; Davis, B.P.; Hendrick, J.; Jeffries, T.W. Cloning and disruption of the beta-isopropylmalate dehydrogenase gene (LEU2) of Pichia stipitis with URA3 and recovery of the double auxotroph. Appl. Microbiol. Biot. 1998, 49, 141–146. [Google Scholar] [CrossRef]
- Laplaza, J.M.; Torres, B.R.; Jin, Y.S.; Jeffries, T.W. Sh ble and Cre adapted for functional genomics and metabolic engineering of Pichia stipitis. Enzyme Microb. Technol. 2006, 38, 741–747. [Google Scholar] [CrossRef]
- Van Dijken, J.P.; van den Bosch, E.; Hermans, J.J.; de Miranda, L.R.; Scheffers, W.A. Alcoholic fermentation by ‘nonfermentative’ yeasts. Yeast 1986, 2, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, J.C.; van Driessel, B.; Prior, B.A. Ethanol tolerance of Pichia stipitis and Candida shehatae strains in fed-batch cultures at controlled low dissolved-oxygen levels. Appl. Microbiol. Biot. 1989, 30, 53–58. [Google Scholar] [CrossRef]
- Nardi, J.B.; Bee, C.M.; Miller, L.A.; Nguyen, N.H.; Suh, S.O.; Blackwell, M. Communities of microbes that inhabit the changing hindgut landscape of a subsocial beetle. Arthropod Struct. Dev. 2006, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.O.; Marshall, C.J.; McHugh, J.V.; Blackwell, M. Wood ingestion by passalid beetles in the presence of xylose fermenting gut yeasts. Mol. Ecol. 2003, 12, 3137–3145. [Google Scholar] [CrossRef] [PubMed]
- Vaughan Martini, A.E. Comparazione dei genomi del lievito Pichia stipitis e de alcune specie imperfette affini. Ann. Fac. Agric. Univ. Perugia 1984, 38, 331–335. [Google Scholar]
- Kurtzman, C.P. Candida shehatae–genetic diversity and phylogenetic relationships with other xylose-fermenting yeasts. Antonie van Leeuwenhoek 1990, 57, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Gupthar, A.S. Theoretical and practical aspects of ploidy estimation in Pichia stipitis. Mycol. Res. 1994, 98, 716–718. [Google Scholar] [CrossRef]
- Hahn-H¨agerdal, B.; Pamment, N. Microbial pentose metabolism. Appl. Biochem. Biotechnol. 2004, 113–116, 1207–1209. [Google Scholar]
- Nigam, J.N. Development of xylose-fermenting yeast Pichia stipitis for ethanol production through adaptation on hardwood hemicellulose acid prehydrolysate. J. Appl. Microbiol. 2001, 90, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Nigam, J.N. Ethanol production from hardwood spent sulfite liquor using an adapted strain of Pichia stipitis. J. Ind. Microbiol. Biot. 2001, 26, 145–150. [Google Scholar] [CrossRef]
- Lee, H.; Biely, P.; Latta, R.K.; Barbosa, M.F.S.; Schneider, H. Utilization of xylan by yeasts and its conversion to ethanol by Pichia stipitis strains. Appl. Environ. Microbiol. 1986, 52, 320–324. [Google Scholar]
- Ozcan, S.; Kotter, P.; Ciriacy, M. Xylan-hydrolyzing enzymes of the yeast Pichia stipitis. Appl. Microbiol. Biot. 1991, 36, 190–195. [Google Scholar] [CrossRef]
- Koivistoinen, O.M.; Hilditch, S.; Voutilainen, S.P.; Boer, H.; Penttila, M.; Richard, P. Identification in the yeast Pichia stipitis of the first l-rhamnose-1-dehydrogenase gene. FEBS J. 2008, 275, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Fuganti, C.; Grasselli, P.; Zucchi, G.; Allegrone, G.; Barbeni, M. On the microbial biogeneration of (R) gamma-jasmolactone. Bioorg. Med. Chem. Lett. 1993, 3, 2777–2780. [Google Scholar] [CrossRef]
- Conceicao, G.J.A.; Moran, P.J.S.; Rodrigues, J.A.R. Highly efficient extractive biocatalysis in the asymmetric reduction of an acyclic enone by the yeast Pichia stipitis. Tetrahedron Asymmetry 2003, 14, 43–45. [Google Scholar] [CrossRef]
- Targonski, Z. Biotransformation of lignin-related aromatic compounds by Pichia stipitis Pignal. Zbl. Mikrobiol. 1992, 147, 244–249. [Google Scholar]
- Kim, M.S.; Chung, Y.S.; Seo, J.H.; Jo, D.H.; Park, Y.H.; Ryu, Y.W. High-yield production of xylitol from xylose by a xylitol dehydrogenase defective mutant of Pichia stipitis. J. Microbiol. Biotechnol. 2001, 11, 564–569. [Google Scholar]
- Ilmen, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttila, M. Efficient production of L-lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Slininger, P.J.; Gorsich, S.W. Enhanced biotransformation of furfural and hydroxymethylfurfural by newly developed ethanologenic yeast strains. Appl. Microbiol. Biot. 2005, 121, 451–460. [Google Scholar]
- Harhangi, H.R.; Akhmanova, A.S.; Emmens, R.; van der Drift, C.; de Laat, W.T.; van Dijken, J.P.; Jetten, M.S.; Pronk, J.T.; Op den Camp, H.J. Xylose metabolism in the anaerobic fungus Piromyces sp strain E2 follows the bacterial pathway. Arch. Microbiol. 2003, 180, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Sonderegger, M.; Jeppsson, M.; Hahn-Hagerdal, B.; Sauer, U. Molecular basis for anaerobic growth of Saccharomyces cerevisiae on xylose, investigated by global gene expression and metabolic flux analysis. Appl. Environ. Microbiol. 2004, 70, 2307–2317. [Google Scholar] [CrossRef]
- Karhumaa, K.; Hahn-Hagerdal, B.; Gorwa-Grauslund, M.F. Investigation of limiting metabolic steps in the utilization of xylose by recombinant Saccharomyces cerevisiae using metabolic engineering. Yeast 2005, 22, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, B.; Boles, E.; Keller, M. Construction and optimization of pentose-fermenting yeast strains for bioethanol production. Zuckerindustrie 2006, 131, 627–631. [Google Scholar]
- Matsushika, A.; Watanabe, S.; Kodaki, T.; Makino, K.; Sawayama, S. Bioethanol production from xylose by recombinant Saccharomyces cerevisiae expressing xylose reductase, NADP(1)-dependent xylitol dehydrogenase, and xylulokinase. J. Biosci. Bioeng. 2008, 105, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Karhumaa, K.; Sanchez, R.G.; Hahn-Hagerdal, B.; Gorwa-Grauslund, M.F. Comparison of the xylose reducta sexylitol dehydrogenase and the xylose isomerase pathways for xylose fermentation by recombinant Saccharomyces cerevisiae. Microb. Cell Fact. 2007, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Weierstall, T.; Hollenberg, C.P.; Boles, E. Cloning and characterization of three genes (SUT1-3) encoding glucose transporters of the yeast Pichia stipitis. Mol. Microbiol. 1999, 31, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Saloheimo, A.; Rauta, J.; Stasyk, O.; Sibirny, A.; Penttilä, M.; Ruohonen, L. Xylose transport studies with xylose-utilizing Saccharomyces cerevisiae strains expressing heterologous and homologous permeases. Appl. Microbiol. Biotechnol. 2007, 74, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Hector, R.E.; Qureshi, N.; Hughes, S.R.; Cotta, M.A. Expression of a heterologous xylose transporter in a Saccharomyces cerevisiae strain engineered to utilize xylose improves aerobic xylose consumption. Appl. Microbiol. Biotechnol. 2008, 80, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.J.; Spencer-Martins, I.; Goncalves, P. The expression in Saccharomyces cerevisiae of a glucose/xylose symporter from Candida intermedia is affected by the presence of a glucose/xylose facilitator. Microbiology 2008, 154, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Laplaza, J.M.; Jeffries, T.W. Saccharomyces cerevisiae engineered for xylose metabolism exhibits a respiratory response. Appl. Environ. Microbiol. 2004, 70, 6816–6825. [Google Scholar] [CrossRef] [PubMed]
- Passoth, V.; Zimmermann, M.; Klinner, U. Peculiarities of the regulation of fermentation and respiration in the crabtreenegative, xylose-fermenting yeast Pichia stipitis. Appl. Biochem. Biotechnol. 1996, 57–58, 201–212. [Google Scholar] [CrossRef]
- Passoth, V.; Cohn, M.; Schafer, B.; Hahn-Hagerdal, B.; Klinner, U. Molecular analysis of the hypoxia induced ADH2- promoter in the respiratory yeast Pichia stipitis. Yeast 2003, 20, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Passoth, V.; Cohn, M.; Schafer, B.; Hahn-Hagerdal, B.; Klinner, U. Analysis of the hypoxia-induced ADH2 promoter of the respiratory yeast Pichia stipitis reveals a new mechanism for sensing of oxygen limitation in yeast. Yeast 2003, 20, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Klinner, U.; Fluthgraf, S.; Freese, S.; Passoth, V. Aerobic induction of respiro-fermentative growth by decreasing oxygen tensions in the respiratory yeast Pichia stipitis. Appl. Microbiol. Biot. 2005, 67, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Auer, L.; Lazuka, A.; Sillam-Dussès, D.; Miambi, E.; O’Donohue, M.; Hernandez-Raquet, G. Uncovering the Potential of Termite Gut Microbiome for Lignocellulose Bioconversion in Anaerobic Batch Bioreactors. Front. Microbiol. 2017, 8, 2623. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Fu, X.; Wang, L.; Alhujaily, A.; Zhang, J.; Ma, F.; Zhang, X.; Yu, H. A novel and efficient fungal delignification strategy based on versatile peroxidase for lignocellulose bioconversion. Biotechnol. Biofuels 2017, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.A.; Columbano, A.; Perra, A. Emerging Role of the Pentose Phosphate Pathway in Hepatocellular Carcinoma. Front. Oncol. 2017, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.W. Engineering yeasts for xylose metabolism. Curr. Opin. Biotechnol. 2006, 17, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Kötter, P.; Ciriacy, M. Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 38, 776–783. [Google Scholar] [CrossRef]
- Metzger, M.H.; Hollenberg, C.P. Isolation and characterization of the Pichia stipitis transketolase gene and expression in a xylose-utilising Saccharomyces cerevisiae transformant. Appl. Microbiol. Biotechnol. 1994, 42, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Walfridsson, M.; Hallborn, J.; Penttilä, M.; Keränen, S.; Hahn-Hägerdal, B. Xylose-metabolizing Saccharomyces cerevisiae strains overexpressing the TKL1 and TAL1 genes encoding the pentose phosphate pathway enzymes transketolase and transaldolase. Appl. Environ. Microbiol. 1995, 61, 4184–4190. [Google Scholar] [PubMed]
- Bao, X.; Gao, D.; Qu, Y.; Wang, Z.; Walfridssion, M.; Hahn-Hägerdal, B. Effect on product formation in recombinant Saccharomyces cerevisiae strains expressing different levels of xylose metabolic genes. Chin. J. Biotechnol. 1997, 13, 225–231. [Google Scholar] [PubMed]
- Meinander, N.Q.; Boels, I.; Hahn-Hägerdal, B. Fermentation of xylose/glucose mixtures by metabolically engineered Saccharomyces cerevisiae strains expressing XYL1 and XYL2 from Pichia stipitis with and without overexpression of TAL1. Bioresour. Technol. 1999, 68, 79–87. [Google Scholar] [CrossRef]
- Hawkins, P.J.; Ordonez, P.A.; Oresnik, I.J. Characterization of mutants that affect the non-oxidative pentose phosphate pathway in Sinorhizobium meliloti. J. Bacteriol. 2017. [CrossRef]
- Lee, M.; Henriëtte, J.; Rozeboom, H.J.; de Waal, P.P.; de Jong, R.M.; Dudek, H.M.; Janssen, D.B. Metal Dependence of the Xylose Isomerase from Piromyces sp. E2 Explored by Activity Profiling and Protein Crystallography. Biochemistry 2017, 56, 5991–6005. [Google Scholar] [PubMed]
- Temer, B.; dos Santos, L.V.; Negri, V.A.; Galhardo, J.P.; Magalhães, P.H.M.; José, J.; Marschalk, C.; Corrêa, T.L.R.; Carazzolle, M.F.; Pereira, G.A.G. Conversion of an inactive xylose isomerase into a functional enzyme by co-expression of GroEL-GroES chaperonins in Saccharomyces cerevisiae. BMC Biotechnol. 2017, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Rangarajan, M.; Hartley, B.S. d-Xylose (D-glucose) isomerase from Arthrobacter strain NRRL B3728. Purification and properties. Biochem. J. 1991, 277, 255–261. [Google Scholar] [PubMed]
- Walfridsson, M.; Bao, X.; Anderlund, M.; Lilius, G.; Bulow, L.; Hahn-Hägerdal, B. Ethanolic fermentation of xylose with Saccharomyces cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active xylose (glucose) isomerase. Appl. Environ. Microbiol. 1996, 62, 4648–4651. [Google Scholar] [PubMed]
- Träff, K.L.; Otero Cordero, R.R.; van Zyl, W.H.; Hahn-Hägerdal, B. Deletion of the GRE3aldose reductase gene and its influence on xylose metabolism in recombinant strains of Saccharomyces cerevisiae expressing the xylA and XKS1 genes. Appl. Environ. Microbiol. 2001, 67, 5668–5674. [Google Scholar] [CrossRef] [PubMed]
- Lönn, A.; Gardonyi, M.; Zyl, W.V.; Hahn-Hägerdal, B.; Otero, R.C. Cold adaptation of xylose isomerase from Thermus thermophilus through random PCR mutagenesis. Gene cloning and protein characterization. Eur. J. Biochem. 2002, 269, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kuyper, M.; Harhangi, H.R.; Stave, A.K.; Winkler, A.A.; Jetten, M.S.M.; De Laat, W.T.A.M.; Den Ridder, J.J.J.; Op den Camp, H.J.M.; Van Dijken, J.P.; Pronk, J.T. High-level functional expression of a fungal xylose isomerase: The key to efficient ethanolic fermentation of xylose by Saccharomyces cerevisiae? FEMS Yeast Res. 2003, 4, 69–78. [Google Scholar] [CrossRef]
- Jeffries, T.W. Emerging technology for fermenting d-Xylose. Trends Biotechnol. 1985, 3, 208–212. [Google Scholar] [CrossRef]
- Verhoeven, M.D.; Lee, M.; Kamoen, L.; van den Broek, M.; Janssen, D.B.; Daran, J.G.; Antonius, J.A.; van Maris, A.J.A.; Pronk, J.T. Mutations in PMR1stimulate xylose isomerase activity and anaerobic growth on xylose of engineered Saccharomyces cerevisiae by influencing manganese homeostasis. Sci. Rep. 2017, 7, 46155. [Google Scholar] [CrossRef] [PubMed]
- Wijsman, M.R.; Bruinenberg, P.M.; Van Dijken, J.P.; Scheffers, W.A. Incapacity for anaerobic growth in xylose-fermenting yeasts. Antonie Van Leeuwenhoek 1985, 51, 563–564. [Google Scholar] [CrossRef]
- Hallborn, J.; Gorwa, M.F.; Meinander, N. The influence of cosubstrate and aeration on xylitol formation by recombinant Saccharomyces cerevisiae expressing the XYL1 gene. Appl. Microbiol. Biotechnol. 1994, 42, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Thestrup, H.N.; Hahn-Hägerdal, B. Xylitol formation and reduction equivalent generation during anaerobic xylose conversion with glucose as cosubstrate in recombinant Saccharomyces cerevisiae expressing the xyl1 gene. Appl. Environ. Microbiol. 1995, 61, 2043–2045. [Google Scholar] [PubMed]
- Dahn, K.M.; Davis, B.P.; Pittman, P.E.; Kenealy, W.R.; Jeffries, T.W. Increased xylose reductase activity in the xylose-fermenting yeast Pichia stipitis by overexpression of XYL1. Appl. Biochem. Biotechnol. 1996, 57–58, 267–276. [Google Scholar] [CrossRef]
- Kötter, P.; Amore, R.; Hollenberg, C.P.; Ciriacy, M. Isolation and characterization of the Pichia stipitis xylitol dehydrogenase gene, XYL2, and construction of a xylose-utilizing Saccharomyces cerevisiae transformant. Curr Genet. 1990, 18, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Amore, R.; Kötter, P.; Kuster, C.; Ciriacy, M.; Hollenberg, C.P. Cloning and expression in Saccharomyces cerevisiae of the NAD(P)H-dependent xylose reductase-encoding gene (XYL1) from the xylose-assimilating yeast Pichia stipitis. Gene 1991, 109, 89–97. [Google Scholar] [CrossRef]
- Walfridsson, M.; Anderlund, M.; Bao, X.; Hahn-Hägerdal, B. Expression of different levels of enzymes from the Pichia stipitis XYL1 and XYL2 genes in Saccharomyces cerevisiae and its effects on product formation during xylose utilisation. Appl. Microbiol. Biotechnol. 1997, 48, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Jeffries, T.W. Changing flux of xylose metabolites by altering expression of xylose reductase and xylitol dehydrogenase in recombinant Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2003, 105–108, 277–285. [Google Scholar] [CrossRef]
- Zong, H.; Zhang, C.; Zhuge, B.; Lu, X.; Fang, H.; Sun, J. Effects of xylitol dehydrogenase (XYL2) on xylose fermentation by engineered Candida glycerinogenes. Biotechnol. Appl. Biochem. 2017, 64, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Park, Y.C.; Yong-Su Jin, Y.S.; Seo, J.H. Construction of efficient xylose-fermenting Saccharomyces cerevisiae through a synthetic isozyme system of xylose reductase from Scheffersomyces stipites. Bioresour. Technol. 2017, 241, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.J.; Gonçalves, P.; Spencer-Martins, I. Two glucose/xylose transporter genes from the yeast Candida intermedia: First molecular characterization of a yeast xylose-H+ symporter. Biochem. J. 2006, 395, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Runquist, D.; Fonseca, C.; Rådstrom, P.; Spencer-Martins, I.; Hahn-Hägerdal, B. Expression of the Gxf1 transporter from Candida intermedia improves fermentation performance in recombinant xylose-utilizing Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2009, 82, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Katahira, S.; Ito, M.; Takema, H.; Fujita, Y.; Tanino, T.; Tanak, T.; Fukuda, H.; Kondo, A. Improvement of ethanol productivity during xylose and glucose co-fermentation by xylose-assimilating S. cerevisiae via expression of glucose transporter Sut1. Enzyme Microb. Technol. 2008, 43, 115–119. [Google Scholar] [CrossRef]
- Boles, E.; Hollenberg, C.P. The molecular genetics of hexose transport in yeasts. FEMS Microbiol. Rev. 1997, 21, 85–111. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Volker, B.; Boles, E.; Fuhrmann, G.F. Characterisation of glucose transport in Saccharomyces cerevisiae with plasma membrane vesicles (countertransport) and intact cells (initial uptake) with single Hxt1, Hxt2, Hxt3, Hxt4, Hxt6, Hxt7 or Gal2 transporters. FEMS Yeast Res. 2002, 2, 539–550. [Google Scholar] [PubMed]
- Meinander, N.Q.; Hahn-Hägerdal, B. Influence of co-substrate concentration on xylose conversion by recombinant, XYL1expressing Saccharomyces cerevisiae: A comparison of different sugars and ethanol as cosubstrates. Appl. Environ. Microbiol. 1997, 63, 1959–1964. [Google Scholar] [PubMed]
- Lee, W.J.; Kim, M.D.; Ryu, Y.W.; Bisson, L.F.; Seo, J.H. Kinetic studies on glucose and xylose transport in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2002, 60, 186–191. [Google Scholar] [PubMed]
- Buziol, S.; Becker, J.; Baumeister, A.; Jung, S.; Mauch, K.; Reuss, M.; Boles, E. Determination of in vivo kinetics of the starvation-induced Hxt5 glucose transporter of Saccharomyces cerevisiae. FEMS Yeast Res. 2002, 2, 283–291. [Google Scholar] [PubMed]
- Jin, Y.S. Metabolic Engineering of Xylose Fermentation in Saccharomyces Cerevisiae. PhD Thesis, University of Wisconsin, Madison, WI, USA, 2002. [Google Scholar]
- Hamacher, T.; Becker, J.; Gardonyi, M.; Hahn-Hägerdal, B.; Boles, E. Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiology 2002, 148, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Colabardini, C.A.; Ries, L.N.A. Brown Functional characterization of a xylose transporter in Aspergillus nidulans. Biotechnol. Biofuels 2014, 7, 46–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rintala, E.; Wiebe, M.G.; Tamminen, A.; Ruohonen, L.; Penttila, M. Transcription of hexose transporters of Saccharomyces cerevisiae is affected by change in oxygen provision. BMC Microbiol. 2008, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, M.; Andersson, J.; Liden, G. Modeling simultaneous glucose and xylose uptake in Saccharomyces cerevisiae from kinetics and gene expression of sugar transporters. Bioprocess Biosyst. Eng. 2008, 31, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Van Maris, A.J.; Winkler, A.A.; Kuyper, M.; de Laat, W.T.; Van Dijken, J.P.; Pronk, J.T. Development of efficient xylose fermentation in Saccharomyces cerevisiae: Xylose isomerase as a key component. Adv. Biochem. Eng. Biotechnol. 2007, 108, 179–204. [Google Scholar] [PubMed]
- Nijland, J.G.; Shin, H.Y.; Boender, L.G.M.; de Waal, P.P.; Klaassen, P.; Driessen, A.J.M. Improved xylose metabolism by a CYC8 mutant of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Ho, N.W. Cloning the yeast xylulokinase gene for the improvement of xylose fermentation. Appl. Biochem. Biotechnol. 1988, 17, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.X.; Ho, N.W. Xylulokinase activity in various yeasts including Saccharomyces cerevisiae containing the cloned xylulokinase gene. Appl. Biochem. Biotechnol. 1990, 24–25, 193–199. [Google Scholar] [CrossRef]
- Ho, N.W.; Tsao, G.T. Recombinant Yeasts for Effective Fermentation of Glucose and Xylose. US Patent 5789210, 8 November 1993. [Google Scholar]
- Rodriguez-Pena, J.M.; Cid, V.J.; Arroyo, J.; Nombela, C. The YGR194c (XKS1) gene encodes the xylulokinase from the budding yeast Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1998, 162, 155–160. [Google Scholar] [CrossRef]
- Eliasson, A.; Christensson, C.; Wahlbom, F.C.; Hahn-hägerdal, B. Anaerobic xylose fermentation by recombinant Saccharomyces cerevisia carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures. Appl. Environ. Microbiol. 2000, 66, 3381–3386. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.H.; Chowdary, G.V.; Reddy, D.S.; Ayyanna, C. Simultaneous saccharification and fermentation of pretreated Antigonum leptopus (Linn) leaves to ethanol. J. Chem. Technol. Biotechnol. 1999, 74, 1055–1060. [Google Scholar] [CrossRef]
- Jin, Y.S.; Ni, H.; Laplaza, J.M.; Jeffries, T.W. Optimal growth and ethanol production from xylose by recombinant Saccharomyces cerevisiae require moderate D-xylulokinase activity. Appl. Environ. Microbiol. 2003, 69, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Toivari, M.H.; Penttilä, M. The role of xylulokinase in Saccharomyces cerevisiae xylulose catabolism. FEMS Microbiol. Lett. 2000, 190, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.; Christensson, C.; Hobley, T.; Hahn-Hägerdal, B. Xylulokinase overexpression in two strains of Saccharomyces cerevisiae also expressing xylose reductase and xylitol dehydrogenase and its effect on fermentation of xylose and lignocellulosic hydrolysate. Appl. Environ. Microbiol. 2001, 67, 4249–4255. [Google Scholar] [CrossRef] [PubMed]
- Toivari, M.H.; Aristidou, A.; Ruohonen, L.; Penttilä, M. Conversion of xylose to ethanol by recombinant Saccharomyces cerevisiae: Importance of xylulokinase (XKS1) and oxygen availability. Metab. Eng. 2001, 3, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Jones, S.; Shi, N.Q.; Jeffries, T.W. Molecular cloning of XYL3 (D-xylulokinase) from Pichia stipitis and characterization of its physiological function. Appl. Environ. Microbiol. 2002, 68, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Sunna, A.; Antranikian, G. Xylanolytic Enzymes from Fungi and Bacteria. Crit. Rev. Biotechnol. 1997, 17, 39–67. [Google Scholar] [CrossRef] [PubMed]
- Morosoli, R.; Zalce, E.; Durand, S. Secretion of a Cryptococcus albidus xylanase in Pichia stipitis resulting in a xylan fermenting transformant. Curr. Genet. 1993, 24, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Den Haan, R.; Van Zyl, W.H. Differential expression of the Trichoderma reesei β-xylanase II (xyn2) gene in the xylose-fermenting yeast Pichia stipitis. Appl. Microbiol. Biotechnol. 2001, 57, 5215–5227. [Google Scholar]
- Basaran, P.; Basaran, N.; Hang, Y.D. Isolation and characterization of Pichia stipitismutants with enhanced xylanase activity. World J. Microbiol. Biotechnol. 2000, 16, 545–550. [Google Scholar] [CrossRef]
- Görgens, J.F.; Passoth, V.; van Zyl, W.H.; Knoetze, J.H.; Hahn-Hagerdahl, B. Amino acid supplementation, controlled oxygen limitation and sequential double induction improve heterologous xylanase production by Pichia stipitis. FEMS Yeast Res. 2005, 5, 677–683. [Google Scholar] [CrossRef] [PubMed]
- La Grange, D.C.; Pretorius, I.S.; Claeyssens, M.; Van Zyl, W.H. Degradation of xylan to d-Xylose by recombinant Saccharomyces cerevisiae coexpressing the Aspergillus niger β-xylosidase (xlnD) and the Trichoderma reesei xylanase II (xyn2) genes. Appl. Environ. Microbiol. 2001, 67, 5512–5519. [Google Scholar] [CrossRef] [PubMed]
- Görgens, J.F.; Pianas, J.; van, W.H.; Knoetze, J.H.; Hahn-Hägerdal, B. Comparison of three expression systems for heterologous xylanase production by S. cerevisiae in defined medium. Yeast 2004, 21, 1205–1217. [Google Scholar] [PubMed]
- Jampala, P.; Tadikamalla, S.; Preethi, M.; Ramanujam, S.; Uppuluri, K.B. Concurrent production of cellulase and xylanase from Trichoderma reesei NCIM 1186: Enhancement of production by desirability-based multi-objective method. Biotech 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, J.C.; Prior, B.A.; Monteiro, A.M.T. The effect of aeration on xylose fermentation by Candida shehatae and Pachysolen tannophilus—A comparative study. Appl. Microbiol. Biotechnol. 1984, 19, 261–266. [Google Scholar]
- Sanchez, S.; Bravo, V.; Castro, E.; Moya, A.J.; Camacho, F. Comparative study of the fermentation of d-glucose/d-Xylose mixtures with Pachysolen tannophilus and Candida shehatae. Bioprocess Eng. 1999, 21, 525–532. [Google Scholar] [CrossRef]
- Vandeska, E.; Amartey, S.; Kuzmanova, S.; Jeffries, T.W. Fed-batch culture for xylitol production by Candida boidinii. Proc. Biochem. 1996, 31, 265–270. [Google Scholar] [CrossRef]
- Winkelhausen, E.; Pittman, P.; Kuzmanova, S.; Jeffries, T.W. Xylitol formation byCandida boidinii in oxygen limited chemostat culture. Biotechnol. Lett. 1996, 18, 753–758. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Da Silva, S.S.; Almeida, E.S.J.B.; Vitolo, M. Xylose reductase activity of Candida guilliermondii during xylitol production by fed-batch fermentation: Selection of process variables. Appl. Biochem. Biotechnol. 2002, 98–100, 875–883. [Google Scholar] [CrossRef]
- Sanchez, S.; Bravo, V.; Castro, E.; Moya, A.J.; Camacho, F. The fermentation of mixtures Of D-glucose and d-Xylose by Candida shehatae, Pichia stipitis or Pachysolen tannophilusto produce ethanol. J. Chem. Technol. Biotechnol. 2002, 77, 641–648. [Google Scholar] [CrossRef]
- Cho, J.Y.; Jeffries, T.W. Pichia stipitis genes for alcohol dehydrogenase with fermentative and respiratory functions. Appl. Environ. Microbiol. 1998, 64, 1350–1358. [Google Scholar] [PubMed]
- Cho, J.Y.; Jeffries, T.W. Transcriptional control of ADH genes in the xylose-fermenting yeast Pichia stipitis. Appl. Environ. Microbiol. 1999, 65, 2363–2368. [Google Scholar] [PubMed]
- Kim, Y.S.; Kim, S.Y.; Kim, J.H.; Kim, S.C. Xylitol production using recombinantSaccharomyces cerevisiae containing multiple xylose reductase genes at chromosomal delta-sequences. J. Biotechnol. 1999, 67, 159–171. [Google Scholar] [CrossRef]
- Jeppsson, M.; Johansson, B.; Hahn-Hägerdal, B.; Gorwa-Grauslund, M.F. Reduced oxidative pentose phosphate pathway flux in recombinant xylose-utilizing Saccharomyces cerevisiae strains improves the ethanol yield from xylose. Appl. Environ. Microbiol. 2002, 68, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Verho, R.; Putkonen, M.; Londesborough, J.; Penttilä, M. Production of ethanol from L-arabinose by Saccharomyces cerevisiae containing a fungal L-arabinose pathway. FEMS Yeast Res. 2003, 3, 185–189. [Google Scholar] [CrossRef]
- Nissen, T.L.; Schulze, U.; Nielsen, J.; Villadsen, J. Flux distributions in anaerobic, glucose-limited continuous cultures of Saccharomyces cerevisiae. Microbiology 1997, 143, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Erlemann, P.; Buithanh, N.A.; Dellweg, H. Xylose fermentation by yeasts 4. Purification and kinetic studies of xylose reductase from Pichia stipitis. Appl. Microbiol. Biotechnol. 1988, 29, 148–154. [Google Scholar]
- Michal, G. Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology; Wiley: New York, NY, USA, 1999; pp. 189–200. [Google Scholar]
- Anushree, K.; Anand, G. Xylitol production by Saccharomyces cerevisiae overexpressing different xylose reductases using non-detoxified hemicellulosic hydrolysate of corncob. Biotech 2016, 6, 127. [Google Scholar] [CrossRef]
- Park, N.H.; Yoshida, S.; Takakashi, A.; Kawabata, Y.; Sun, H.J.; Kusakabe, I. A new method for the preparation of crystalline L-arabinose from arabinoxylan by enzymatic hydrolysis and selective fermentation with yeast. Biotechnol. Lett. 2001, 23, 411–416. [Google Scholar] [CrossRef]
- Dien, B.S.; Kurtzman, C.P.; Saha, B.C.; Bothast, R.J. Screening for l-arabinose fermenting yeasts. Appl. Biochem. Biotechnol. 1996, 57–58, 233–242. [Google Scholar] [CrossRef]
- Shi, N.Q.; Prahl, K.; Hendrick, J.; Cruz, J.; Lu, P.; Cho, J.Y.; Jones, S.; Jeffries, T. Characterization and complementation of a Pichia stipitis mutant unable to grow on d-xylose or l-arabinose. Appl. Biochem. Biotechnol. 2000, 848, 201–216. [Google Scholar] [CrossRef]
- Witteveen, C.F.B.; Busink, R.; Van de Vondervoort, P.; Dijkema, C.; Swart, K.; Visser, J. L-Arabinose and d-Xylose Catabolism in Aspergillus niger. J. Gen. Microbiol. 1989, 135, 2163–2171. [Google Scholar] [CrossRef]
- Rees, D.A. Polysaccharide Shapes. Outline Studies of Biology; Wiley: New York, NY, USA, 1977; pp. 62–73. [Google Scholar]
- Lucas, C.; Van Uden, N. Transport of hemicellulose monomers in the xylose-fermenting yeast Candida shehatae. Appl. Microbiol. Biotechnol. 1986, 23, 491–495. [Google Scholar] [CrossRef]
- Hallborn, J.; Walfridsson, M.; Penttilä, M.; Keranen, S.; Hahn-Hägerdal, B. A short-chain dehydrogenase gene from Pichia stipitis having d-arabinitol dehydrogenase activity. Yeast 1995, 11, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Londesborough, J.; Putkonen, M.; Kalkkinen, N.; Penttilä, M. Cloning and expression of a fungal l-arabinitol 4-dehydrogenase gene. J. Biol. Chem. 2001, 276, 40631–40637. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Putkonen, M.; Väänänen, R.; Londesborough, J.; Penttilä, M. The missing link in the fungal l-arabinose catabolic pathway, identification of the l-xylulose reductase gene. Biochemistry 2002, 41, 6432–6643. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Boles, E. A modified Saccharomyces cerevisiae strain that consumes l-arabinose and produces ethanol. Appl. Environ. Microbiol. 2003, 69, 4144–4150. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Qiu, C.; Wang, S.; Shen, Y.; Du, B.; Ding, Y.; Bao, X. Coutilization of D-Glucose, d-Xylose, and l-Arabinose in Saccharomyces cerevisiae by Coexpressing the Metabolic Pathways and Evolutionary Engineering. Biomed. Res. Int. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Boyce, A.N. Bioethanol production from fermentable sugar juice. Sci. World J. 2014, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Field, S.; Ryden, P.; Wilson, D.; James, S.; Roberts, I.; Richardson, D.; Waldron, K.W.; Clarke, T.A. Identification of furfural resistant strains of Saccharomyces cerevisiae and Saccharomyces paradoxus from a collection of environmental and industrial isolates. Biotechnol. Biofuels 2015, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MarelneCot, M.; Loret, M.O.; Francois, J. Physiological behavior of Saccharomyces cerevisiaein aerated fed-batch fermentation for high level production of bioethanol. FEMS Yeast Res. 2007, 7, 22–32. [Google Scholar]
- Liu, R.; Shen, F. Impacts of main factors on bioethanol fermentation from stalk juice of sweet sorghum by immobilized Saccharomyces cerevisiae (CICC 1308). Bioresour. Technol. 2008, 99, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Phisalaphong, M.; Srirattana, N.; Tanthapanichakoon, W. Mathematical modeling to investigate temperature effect on kinetic parameters of ethanol fermentation. J. Biochem. Eng. 2006, 28, 36–43. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Li, C.; Sakakibara, K.; Tanaka, S.; Kong, H. Factors affecting ethanol fermentation using Saccharomy cescerevisiae BY4742. Biomass Bioenergy 2012, 47, 395–401. [Google Scholar] [CrossRef]
- Staniszewski, M.; Kujawski, W.; Lewandowska, M. Ethanol production from whey in bioreactor with co-immobilized enzyme and yeast cells followed by pervaporative recovery of product—Kinetic model predictions. J. Food Eng. 2007, 82, 618–625. [Google Scholar] [CrossRef]
- Laopaiboon, L.; Thanonkeo, P.; Jaisil, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice in batch and fed-batch fermentations by Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 1497–1501. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar]
- Larsson, S.; Quintana-Sáinz, A.; Reimann, A.; Nilvebrant, N.-O.; Jönsson, L. Influence of lignocellulose-derived aromatic compounds on oxygen-limited growth and ethanolic fermentation by Saccharomyces cerevisiae. In Twenty-First Symposium on Biotechnology for Fuels and Chemicals; Finkelstein, M., Davison, B., Eds.; Humana Press: Fort Collins, CO, USA, 2000; pp. 617–632. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, K.A.; El-Ghwas, D.E.; Easa, S.M.; Abdelwahab Hassan, M.I. Bioethanol a Microbial Biofuel Metabolite; New Insights of Yeasts Metabolic Engineering. Fermentation 2018, 4, 16. https://doi.org/10.3390/fermentation4010016
Selim KA, El-Ghwas DE, Easa SM, Abdelwahab Hassan MI. Bioethanol a Microbial Biofuel Metabolite; New Insights of Yeasts Metabolic Engineering. Fermentation. 2018; 4(1):16. https://doi.org/10.3390/fermentation4010016
Chicago/Turabian StyleSelim, Khaled A., Dina E. El-Ghwas, Saadia M. Easa, and Mohamed I. Abdelwahab Hassan. 2018. "Bioethanol a Microbial Biofuel Metabolite; New Insights of Yeasts Metabolic Engineering" Fermentation 4, no. 1: 16. https://doi.org/10.3390/fermentation4010016





